Introduction
Electrocochleography (ECochG) is a testing procedure that enables the clinician to assess cochlear electrical potentials. While it can be challenging to perform and has been largely replaced by more convenient tests, it continues to be used in several clinical scenarios for diagnostic and intraoperative purposes. In recent years, advances in technology and a greater understanding of cochlear physiology have further enhanced the utility of ECochG, particularly in monitoring the functional status of the cochlea during cochlear implantation surgeries.
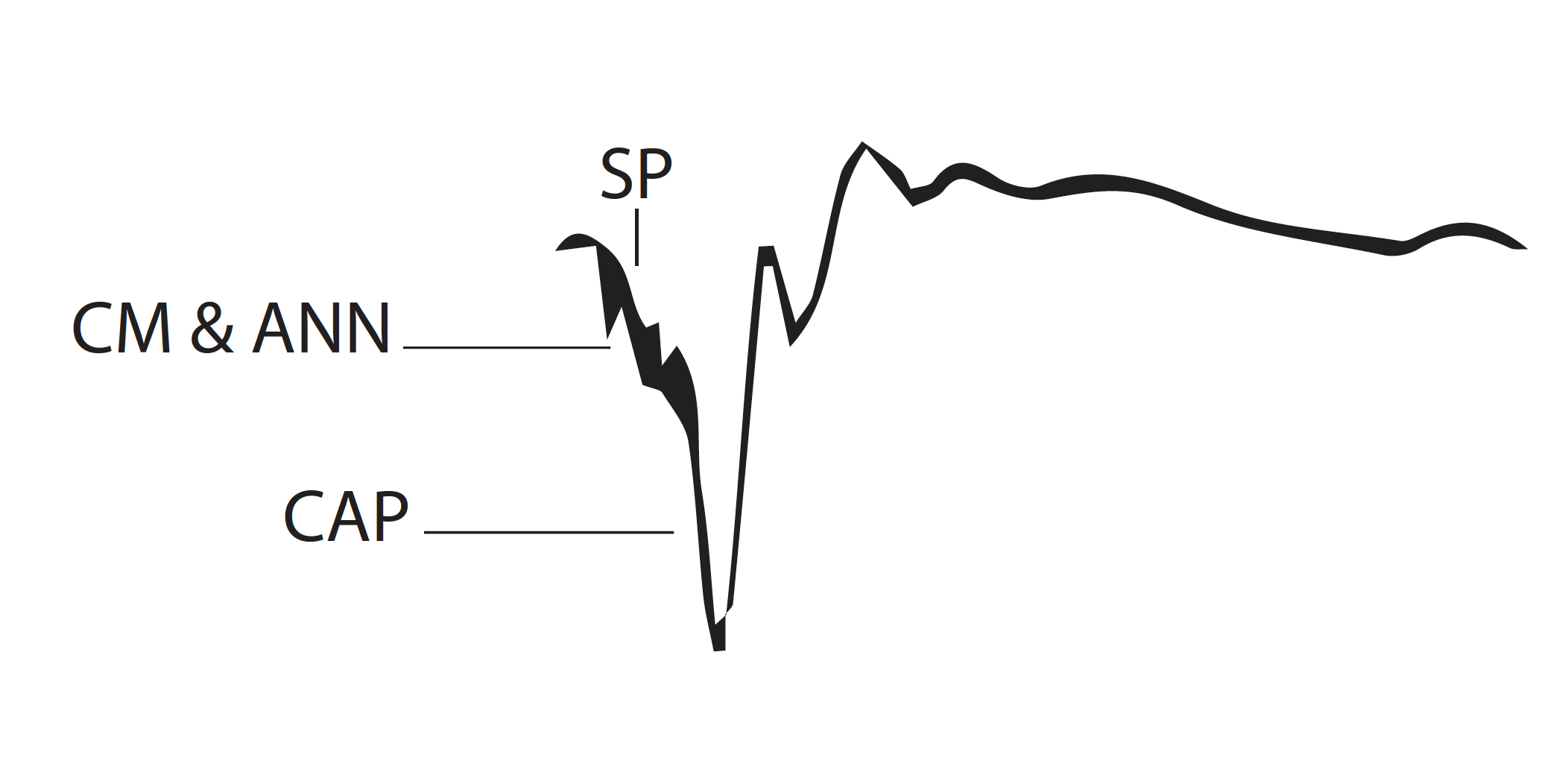
ECochG comprises 3 potentials: the cochlear microphonic (CM), the action potential (AP), and the summation potential (SP) (see Image. Electrocochleography Diaphragm).[1] The CM is an alternating current resembling the waveform of the stimulus. It is primarily generated by the outer hair cells. The quality of the CM recording is highly variable and may be easily confused with stimulus artifacts.[1] Production of an artifact is much less likely when the stimulation source is intracochlear (ie, via a cochlear implant), and the CM may demonstrate post-implantation changes after insertion. Furthermore, the real-time feedback offered by ECochG during cochlear implantation has been used to ensure optimal placement of the implant electrodes, minimize trauma to the cochlear structures, and preserve residual hearing.
Action potentials represent the compound (ie, summed) response of clusters of nerve fibers firing in response to the frequency of a given stimulus. The AP initiates at the onset of the stimulus and is mainly produced by the nerve fibers in the basal turn of the cochlea that respond to high-frequency sounds, especially in response to transient stimuli.[2] APs are generally interpreted using 2 negative peaks, N1 and N2, which correlate to auditory brainstem response (ABR) waves I and II.[1][3][4] The salient features of N1 and N2 are their magnitude and latency, with magnitude referring to the number and intensity of nerve fibers firing. In contrast, latency is the time that elapses between the stimulus and the appearance of the N1 peak, similar to absolute latency in ABR recordings.
The SP is a direct current potential resulting from signal transduction initiated by the vibration of the basilar membrane. The SP is clinically most important concerning the AP, as the SP:AP amplitude ratio is often used to diagnose patients with Ménière disease (MD) or endolymphatic hydrops (ELH).[1]
The auditory nerve also produces a potential called the auditory nerve neurophonic (ANN). Its frequency varies with stimulus frequency due to phase-locking, particularly with lower-frequency stimuli. When the ANN is decreased or absent, it strongly indicates auditory neuropathy.
Procedures
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Procedures
Two general approaches to ECochG recording are employed: transtympanic (TT) and extratympanic (ET). TT ECochG provides more reliable recordings yet is also more invasive because it requires a needle electrode to pass through the tympanic membrane (TM) and be positioned directly on the cochlear promontory. Due to the invasive nature of this method, it has largely been superseded in the clinical context by extratympanic (or tympanic) ECochG, as patients much better tolerate this method.[5][6] ET ECochG utilizes a foam rubber or similar insert with an electrode to detect ECochG responses (see Image. Electrocochleography Tracing).
Notably, with the advent of modern cochlear implantation techniques, TT ECochG has seen renewed interest, as it offers superior accuracy during intraoperative monitoring, allowing surgeons to ensure that the implantation is causing minimal trauma to the cochlea.[7]
While TT ECochG has significant accuracy benefits over ET approaches due to the proximity of the recording electrodes to the signal-generating anatomical structures, it requires physician monitoring and patient cooperation and is challenging to perform in most clinical settings.[1] TT ECochG remains useful in certain intraoperative situations and is generally considered a more accurate recording method than ET ECochG. Nevertheless, TT ECochG has certain limitations beyond patient tolerance. Firstly, the needle risks penetration of an abnormally positioned round window in cochlear malformations.[8] Secondly, the high impedance factor of a needle may exclude lower action potential frequencies. TT ECochG is particularly helpful in the intraoperative setting because it provides precise neuromonitoring without concern for patient compliance. Local or topical anesthetics may improve patient tolerance in clinical settings. Three primary methods of stimulus are presented: clicks, tone bursts, and chirps.[6] Click stimuli assess a large population of neurons with a single, brief stimulus, while tonal stimuli are more precisely aligned with audiometric thresholds, especially at 1,000 Hz, 2,000 Hz, and 4,000 Hz; chirp usage is less common. The application of each method remains controversial and is dependent on clinician preference.
ET ECochG monitoring may be performed non-invasively and typically painlessly through in-ear inserts. While not as precise as TT ECochG due to the distance of the electrode from the cochlea, ET ECochG provides generally accurate data while being much more easily tolerated by patients. Insert earphones may still be somewhat uncomfortable, particularly if there is close proximity to the TM. ET ECochG provides a much more easily tolerated screening examination for patients with suspected MD/ELH.
Indications
Historically, ECochG was largely utilized for diagnosing and monitoring MD/ELH. As briefly mentioned above, the primary measurements used for interpretation are the SP and the AP, which will be discussed more thoroughly below. Patients for whom ECochG should be considered as a potential diagnostic tool are mainly those with symptoms consistent with MD/ELH. Patients with severe vertigo, nausea/vomiting, tinnitus, disequilibrium, aural fullness, and similar related symptoms. Criteria for the diagnosis of MD were standardized in 1995 by the American Academy of Otolaryngology-Head and Neck Surgery; the Gibson 10-point score may also be used to assess the likelihood of MD/ELH and may be further utilized to determine the efficacy of ECochG.[9][1][10] ECochG is also employed to monitor patient progress in response to treatment.
Other indications for ECochG include intraoperative nerve monitoring and identification of the first wave of the ABR in patients with extremely poor hearing or a diagnosis of “hidden hearing loss” (auditory neuropathy).[11] In cases of auditory neuropathy, the AP amplitude will be reduced, as will the ANN. While ABR may not be as precise as ECochG, it is more easily obtainable and reliable and does not require the same level of patient cooperation.[1] Hence, ECochG, while valuable in specific instances, may not be a patient first-choice assessment tool, depending on the clinical scenario. More recent data have also suggested that ECochG may help predict MD, diagnose superior semicircular canal dehiscence, and monitor cochlear function intraoperatively during its repair.[12]
Potential Diagnosis
The primary condition diagnosed and monitored by ECochG is MD/ELH. Intraoperative nerve monitoring also remains an important indication for ECochG, particularly in removing cerebellopontine angle tumors. In the context of cochlear implantation, ECochG provides valuable real-time feedback regarding the functional integrity of the cochlea, assisting surgeons in making critical intraoperative decisions. ECochG may also be used to estimate hearing thresholds, but more convenient testing methods, such as ABR, have largely been replaced in this indication. While the reproducibility, specificity, and sensitivity of ET recordings remain poor, an SP:AP ratio ≥0.45 is nevertheless a significant diagnostic indicator of MD.[11] As there is substantial variability with both recordings, particularly with the SP, the SP:AP ratio must be interpreted cautiously and in the appropriate clinical context.
ECochG monitoring via ET electrode may be used to assess whether the electrode has traversed the cochlea's basilar membrane during cochlear implant insertion and to assess residual hearing. However, correlations between ECochG and pure-tone audiograms have demonstrated significant variability concerning postoperative results.[13]
Normal and Critical Findings
Sound is transmitted from the tympanic membrane to the inner ear via ossicular chain movement. The vibration of the stapes footplate on the oval window creates a hydromechanical wave of perilymph in the cochlea, which generates vibration at different points on the basilar membrane, depending on the frequency of the sound stimulus. Basilar membrane movement causes the corresponding movement of hair cell stereocilia, thereby opening ion channels and converting mechanical energy into electrical voltage. This voltage elicits neurotransmitter release in the spiral ganglion and results in the perception of sound. The CM represents the alternating current voltage caused by the outer hair cells' potassium and calcium ion influx in response to an auditory stimulus.
The SP represents the sum of transduction processes in the cochlea, from the inner hair cells, outer hair cells, and auditory nerve. The SP magnitude corresponds to the amount of distortion in these processes. Consequently, the SP may be enlarged in situations with increased distortion, such as in ELH, where pressure is increased relative to patients with normal inner ear and temporal bone anatomy. The AP, specifically its N1 peak, has 2 main characteristics that are clinically relevant: latency and magnitude. Latency describes the time duration from stimulus to N1 peak, while magnitude correlates with the number of firing nerve fibers. Thus, while the SP magnitude increases with distortion in the transduction process, the AP will remain relatively constant. This results in an increased SP:AP ratio, which is pathognomonic for ELH.[1] The AP also has a second peak (N2), which corresponds to ABR Wave II, although there is currently no known clinical significance associated with the N2 peak of the AP.[1]
SP and AP amplitudes depend on the stimulus's intensity, whether click or tonal stimuli are used, and the method of stimulus presentation. Measurements may be recorded using baseline reference or "peak-to-peak" amplitudes, which may improve consistency due to variability in baseline levels from ET recordings. The SP:AP ratio is considered to be abnormal if it is greater than 0.45, although there is some variability and debate regarding the precise cutoff, particularly if using the ET method, as this typically results in a lower SP:AP ratio when compared to TT ECochG.[14][15][16]
Interfering Factors
Interfering factors in ECochG primarily include a lack of patient cooperation and stimulus artifacts. Artifact is most pronounced in ET recordings, given the greater distance of the stimulus from the cochlea and subsequently reduced amplitude of responses, which enables more minute electrical artifacts to affect the recording.[17] Additionally, light and other electrical equipment in testing rooms may contribute to artifacts due to interference. Grounding electrodes, reducing or removing unnecessary electrical equipment, using tubal insert transducers, placing electrodes on the TM, and purpose-built audiological testing facilities may all help reduce electrical interference.[17]
Complications
As TT ECochG may be uncomfortable and poorly tolerated for patients in the awake setting, it typically requires sedation or anesthesia. Thus, the inherent risks of sedation or anesthesia should be discussed with patients if this method is employed instead of ET ECochG. Of primary concern with TT ECochG is accidental puncturing of the round window, which may result in perilymph leakage into the middle ear and create a potential for hearing loss. ET ECochG is very well tolerated, with no commonly reported complications.[18]
Patient Safety and Education
ECochG is a safe and either non-invasive or minimally-invasive procedure that may diagnose MD/ELH, characterize its severity, or be utilized intraoperatively for neuromonitoring. Patients should be cautioned that the interpretation of results obtained is somewhat subjective and that the procedure may not provide conclusive diagnostic evidence, particularly in the setting of significant artifacts. Patients should also be informed of alternative testing methods, including ABR, which may be tolerated or interpreted more quickly and may be more appropriate for certain patients either instead of or in addition to ECochG.[18]
Clinical Significance
ECochG is a helpful tool for evaluating inner ear fluid distortion and increased pressure in the MD/ELH setting. It additionally may be used to help assess hearing thresholds and auditory neuropathy, as ECochG has been demonstrated to correlate with pure-tone thresholds, albeit with some variability. Recently, other testing methods, such as ABR, have largely replaced ECochG in specific clinical settings due to patient tolerance and ease of use. ECochG has become less commonly utilized in the evaluation of MD/ELH. However, with the growing number of cochlear implantations performed, the role of ECochG in intraoperative neuromonitoring has become increasingly significant. ECochG facilitates optimal electrode placement, potentially reducing postoperative complications and ensuring the best possible outcomes for the patient.[19]
Media
(Click Image to Enlarge)
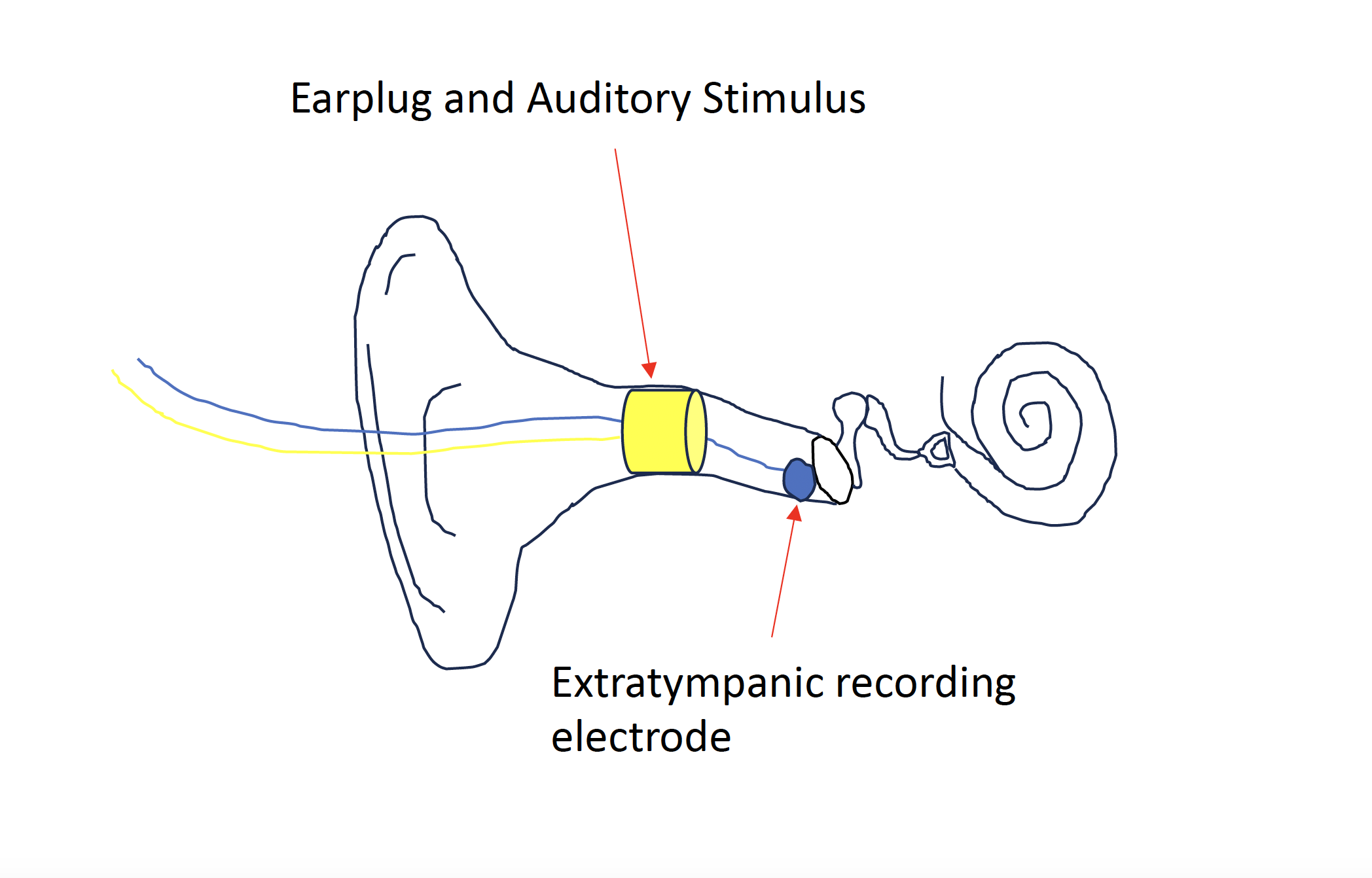
Electrocochleography Diaphragm. An earplug and auditory stimulus are placed within the external auditory canal to isolate and deliver an auditory stimulus. An extra-tympanic recording electrode adjacent to the tympanic membrane is also placed which captures cochlear responses.
Contributed by Douglas Totten, MD, MBA
(Click Image to Enlarge)
References
Gibson WP. The Clinical Uses of Electrocochleography. Frontiers in neuroscience. 2017:11():274. doi: 10.3389/fnins.2017.00274. Epub 2017 May 19 [PubMed PMID: 28634435]
Kiang NY, Pfeiffer RR, Warr WB, Backus AS. Stimulus coding in the cochlear nucleus. Transactions of the American Otological Society. 1965:53():35-58 [PubMed PMID: 5834666]
Level 3 (low-level) evidenceGrant KJ, Mepani AM, Wu P, Hancock KE, de Gruttola V, Liberman MC, Maison SF. Electrophysiological markers of cochlear function correlate with hearing-in-noise performance among audiometrically normal subjects. Journal of neurophysiology. 2020 Aug 1:124(2):418-431. doi: 10.1152/jn.00016.2020. Epub 2020 Jul 8 [PubMed PMID: 32639924]
Vasilkov V, Liberman MC, Maison SF. Isolating auditory-nerve contributions to electrocochleography by high-pass filtering: A better biomarker for cochlear nerve degeneration? JASA express letters. 2023 Feb:3(2):024401. doi: 10.1121/10.0017328. Epub [PubMed PMID: 36858988]
Level 3 (low-level) evidenceFerraro JA, Ferguson R. Tympanic ECochG and conventional ABR: a combined approach for the identification of wave I and the I-V interwave interval. Ear and hearing. 1989 Jun:10(3):161-6 [PubMed PMID: 2744251]
Coraci LM, Beynon AJ. Use of an Extra-Tympanic Membrane Electrode to Record Cochlear Microphonics with Click, Tone Burst and Chirp Stimuli. Audiology research. 2021 Mar 1:11(1):89-99. doi: 10.3390/audiolres11010010. Epub 2021 Mar 1 [PubMed PMID: 33804370]
Cumpston E, Chen P. Implantable Hearing Devices. StatPearls. 2023 Jan:(): [PubMed PMID: 35201706]
Schuerch K, Wimmer W, Rummel C, Caversaccio MD, Weder S. Objective evaluation of intracochlear electrocochleography: repeatability, thresholds, and tonotopic patterns. Frontiers in neurology. 2023:14():1181539. doi: 10.3389/fneur.2023.1181539. Epub 2023 Aug 8 [PubMed PMID: 37621854]
Monsell EM. New and revised reporting guidelines from the Committee on Hearing and Equilibrium. American Academy of Otolaryngology-Head and Neck Surgery Foundation, Inc. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1995 Sep:113(3):176-8 [PubMed PMID: 7675474]
Lopez-Escamez JA, Carey J, Chung WH, Goebel JA, Magnusson M, Mandalà M, Newman-Toker DE, Strupp M, Suzuki M, Trabalzini F, Bisdorff A, Classification Committee of the Barany Society, Japan Society for Equilibrium Research, European Academy of Otology and Neurotology (EAONO), Equilibrium Committee of the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS), Korean Balance Society. Diagnostic criteria for Menière's disease. Journal of vestibular research : equilibrium & orientation. 2015:25(1):1-7. doi: 10.3233/VES-150549. Epub [PubMed PMID: 25882471]
Goodman SS, Lichtenhan JT, Jennings SG. Minimum Detectable Differences in Electrocochleography Measurements: Bayesian-Based Predictions. Journal of the Association for Research in Otolaryngology : JARO. 2023 Apr:24(2):217-237. doi: 10.1007/s10162-023-00888-0. Epub 2023 Feb 16 [PubMed PMID: 36795197]
Ferraro JA, Kileny PR, Grasel SS. Electrocochleography: New Uses for an Old Test and Normative Values. American journal of audiology. 2019 Oct 16:28(3S):783-795. doi: 10.1044/2019_AJA-HEAL18-18-0190. Epub 2019 Oct 16 [PubMed PMID: 32271120]
Dietz A, Linder P, Iso-Mustajärvi M. A State-of-the-Art Method for Preserving Residual Hearing During Cochlear Implant Surgery. Journal of visualized experiments : JoVE. 2023 May 26:(195):. doi: 10.3791/64021. Epub 2023 May 26 [PubMed PMID: 37306464]
Lefler SM, Kaf WA, Ferraro JA. Comparing Simultaneous Electrocochleography and Auditory Brainstem Response Measurements Using Three Different Extratympanic Electrodes. Journal of the American Academy of Audiology. 2021 Jun:32(6):339-346. doi: 10.1055/s-0041-1727273. Epub 2021 Jun 3 [PubMed PMID: 34082461]
Cumpston E, Chen P. Submandibular Excision. StatPearls. 2023 Jan:(): [PubMed PMID: 33760499]
Hornibrook J. Electrocochleography (EcochG) for the diagnosis of cochlear endolymphatic hydrops. Journal of neurology. 2023 May:270(5):2789. doi: 10.1007/s00415-023-11571-y. Epub 2023 Jan 16 [PubMed PMID: 36645486]
Simpson MJ, Jennings SG, Margolis RH. Techniques for Obtaining High-quality Recordings in Electrocochleography. Frontiers in systems neuroscience. 2020:14():18. doi: 10.3389/fnsys.2020.00018. Epub 2020 Apr 15 [PubMed PMID: 32351368]
Level 2 (mid-level) evidenceImmordino A, Sireci F, Lorusso F, La Gumina R, Montalbano C, Alfarghal M, Immordino P, Dispenza F. Diagnostic Role of Combined Electrocochleography and Pure-Tone Audiometry Monitoring During Dehydrating Test in Ménière's Disease: A Case Series. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2023 Aug 1:44(7):718-724. doi: 10.1097/MAO.0000000000003942. Epub 2023 Jul 1 [PubMed PMID: 37400265]
Level 2 (mid-level) evidenceHarris MS, Koka K, Riggs WJ, Saleh S, Holder JT, Dwyer RT, Prentiss S, Lefler S, Kozlowski K, Hiss MM, Ortmann AJ, Nelson-Bakkum E, Büchner A, Salcher R, Harvey SA, Hoffer ME, Bohorquez JE, Alzhrani F, Alshihri R, Fida A, Danner CJ, Friedland DR, Seidman MD, Lenarz T, Telischi FF, Labadie RF, Buchman CA, Adunka OF. Can Electrocochleography Help Preserve Hearing After Cochlear Implantation With Full Electrode Insertion? Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2022 Aug 1:43(7):789-796. doi: 10.1097/MAO.0000000000003588. Epub 2022 Jul 19 [PubMed PMID: 35861647]