Introduction
Bone conduction refers to the phenomenon in which vibrations are transmitted through the bones of the skull to the cochlea and the associated sensorineural structures, resulting in the perception of sound. Bone conduction is in contrast to the route of sound transmission known as air conduction, in which sound is transmitted in the air through the ear canal to the ossicles of the middle ear (malleus, incus, stapes) via the tympanic membrane, thus stimulating the sensorineural organs of the inner ear.[1]
Multiple mechanisms are involved in bone conduction sound transmission, including the inertial force affecting cochlear fluids and middle ear ossicles, pressure changes in the ear canal, and pressure changes transmitted through a third window of the cochlea (which is a pathologic, abnormal structure).[2] Ultimately, air conduction and bone conduction cause a vibration of the cochlea's basilar membrane, a structure attached medially to the osseous spiral lamina, resulting in cochlear nerve stimulation.[3] Methods for testing bone conduction have existed since the 19th century. Early methods involved tuning forks and including the Weber and Rinne tests, which are still used today.[4] Modern bone conduction evaluation is frequently performed as a component of audiometry testing, especially when it is clinically useful to distinguish between sensorineural and conductive hearing loss. Bone conduction evaluation methods involve using specialized equipment, including an oscillator, to produce vibrations at predetermined frequencies and amplitudes.[5]
Procedures
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Procedures
Tuning Fork Tests
The Rinne and Weber tests are designed to stimulate bone conduction and are used as a part of the initial evaluation of hearing loss. They are screening tests and are not considered to replace formal audiometry.[6] These tests are performed using a tuning fork with a frequency of 256 Hz, 512 Hz, or 1024 Hz, with 512 Hz being the most commonly used frequency; 128 Hz tuning forks are not used for the Rinne and Weber tests but are instead used in neurological evaluations.[7]
The Weber test is performed by placing the vibrating tuning fork equidistant from both ears on either the vertex, forehead, bridge of the nose, or chin and asking the patient if the sound is heard loudest in 1 ear or equally in both ears.[8] In patients with normal hearing or symmetrical conductive hearing loss, the Weber test does not demonstrate lateralization (ie, the sound of the tuning fork is heard equally in both ears). In patients with unilateral sensorineural hearing loss, the Weber test lateralizes to the unaffected ear (ie, the sound of the tuning fork is heard louder in the better ear). In patients with unilateral conductive hearing loss, the Weber test lateralizes to the affected ear (ie, the sound of the tuning fork is heard in the worse ear).
The Rinne test differentiates between sound transmission by air conduction and bone conduction. The Rinne test is performed by placing the vibrating tuning fork onto the mastoid process and then asking the patient to report when they can no longer hear the sound. Once the patient can no longer hear the sound, the vibrating tuning fork is immediately moved adjacent to the ear canal about 3 cm from the ear, and the patient is again asked to report when they can no longer hear the sound. A normal finding – a “positive Rinne test” – indicates air conduction is perceived more than bone conduction. In this case, the patient can hear the tuning fork adjacent to the ear canal longer than when they heard the sound over their mastoid process. An abnormal finding of the Rinne test – termed a “negative Rinne test” – suggests bone conduction is perceived more than air conduction. In this case, the patient cannot hear or can only faintly hear the tuning fork after it is moved from the mastoid process to the air adjacent to the ear canal.[9]
Pure Tone Audiometry
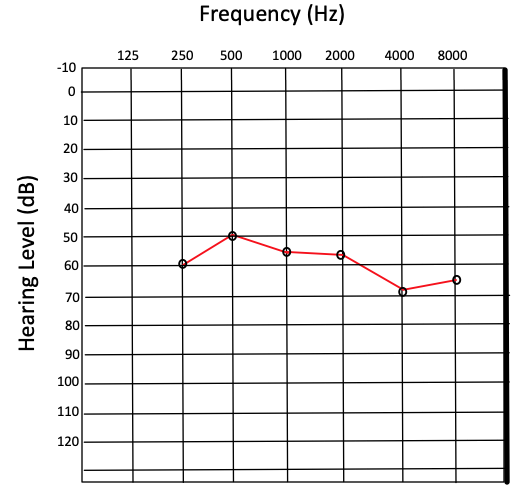
Pure tone audiometry (PTA) is performed by producing a tone with a controlled frequency and recording the lowest sound intensity in decibels (dB), at which the tone can be perceived half of the time. This value is referred to as the threshold and is used to compare a patient’s hearing acuity with an average population. Thresholds are measured for frequencies ranging from 250 to 8000 hertz (Hz). The results are recorded on a graph known as an audiogram, which ordinarily displays hearing threshold level on the y-axis and frequency on the x-axis (see Image. Audiogram Results Showing Severe Hearing Loss).[10]
Although audiometry is sometimes performed solely using earphones, which rely largely on AC, bone conduction can also be evaluated during audiometry and is an essential component of a true comprehensive audiogram. This is accomplished by positioning an oscillator on the patient’s mastoid process and measuring threshold values for the same frequencies used in air conduction testing. In this manner, bone conduction can be compared with air conduction graphically, illustrating what is referred to as an air-bone gap to document differences in air conduction vs bone conduction. Frequently, results from the right ear are plotted as an “O” for air conduction thresholds and an “<” for bone conduction thresholds, while results from the left ear are plotted as an “X” for air conduction thresholds and an “>” for bone conduction thresholds.[11] Often, the non-test ear is intentionally exposed to a masking frequency that is out of phase from the tone generated by the oscillator in the test ear, thus creating a noise-canceling effect. This masking technique is intended to prevent the oscillator's sound from being perceived by the non-test ear. This would skew test results, especially if the non-test ear has better acuity – and thus a lower threshold – at certain frequencies than the test ear.[12]
Auditory Brainstem Response
PTA is a behavioral hearing test because it is based on the subjective perception of tones reported by the patient. Auditory brainstem response (ABR), also referred to as brainstem auditory evoked potentials (BAEP), on the other hand, is a method that relies solely on objective measures to evaluate the function of auditory pathways to the level of the mesencephalon of the brainstem.[13] ABR testing was developed in the 1970s and is used to diagnose and study sensorineural patterns of hearing loss.[14] Electrical signals produced by hair cell vibration ascend to the auditory cortex via several nuclei, including the cochlear nuclei, the medial geniculate nuclei, the lateral lemniscus, the inferior colliculi, and the superior olivary complex. Potentials generated by these signals are recorded by electrodes placed on the surface of the scalp, ears, and forehead.[15] The readings in ABR testing consist of a series of positive wave peaks with negative troughs between them. These peaks are labeled I-VII and normally correspond with a detected stimulus in the brainstem nuclei 10 milliseconds after a test sound is produced. From this data, predicted thresholds can be calculated and compared at varying frequencies similar to PTA.[16]
As an objective testing method, ABR testing has the advantage of being useful in evaluating hearing in infants, young children, and other patients with limited ability to communicate. As with PTA, ABR testing is usually performed with earphones, thus involving air conduction through transforming mechanical vibration in the ear canal into bioelectrical signals via cochlear hair cells. However, because air conduction and bone conduction result in stimulation of the cochlear nerve (cranial nerve VIII), bone conduction can also be evaluated in the stimulation of ABR. Evaluating bone conduction in this manner is accomplished by using an oscillator in contact with the mastoid process or other bony structures of the skull.[17] In comparing PTA with ABR testing in the context of bone conduction stimulation, a difference in threshold level of 18 to 28 dB (decibels) can be expected in individuals with normal hearing, with ABR registering at a higher threshold than PTA.[18][19]
Indications
While the Rinne and Weber tuning fork tests can be useful in the preliminary evaluation of hearing loss, they are not usually performed as standalone tests due to variable reports of sensitivity and specificity and limited diagnostic utility in quantifying the degree of any hearing loss detected.[20] Tuning fork tests are a helpful clinical tool for their convenience and ease of use, but audiometry is the gold standard for formal hearing loss evaluation. For this reason, tuning fork tests are mainly indicated in the setting of suspected hearing loss in a communicative patient as an initial clinical workup to guide further auditory testing or in certain post-operative situations to confirm patent auditory neural pathways.[21]
An indication for PTA is suspected hearing loss in a patient who can participate in behavioral hearing testing. However, according to U.S. Preventive Services Task Force recommendations, PTA is not recommended as a screening protocol for asymptomatic adult patients due to insufficient evidence to assess the harms and benefits of screening.[22] In contrast, ABR testing as part of newborn hearing screening is universally recommended. Early diagnosis and intervention in several treatable causes of hearing loss must encourage proper language development as the child grows.[23] Testing ABR by bone conduction stimulation is beneficial for differentiating between sensorineural and conductive hearing loss in infants with suspected or diagnosed hearing loss.[24]
Potential Diagnosis
Hearing loss is generally categorized as sensorineural, conductive, or mixed. Sensorineural hearing loss (SNHL) occurs with dysfunction within either the cochlea or any component of the neural pathways leading to and including the auditory cortex. Conductive hearing loss occurs due to disruption of sound conduction through the middle ear, the external ear, or both. Mixed hearing loss occurs due to both SNHL and conductive hearing loss.[25] Bone conduction evaluation is useful in differentiating between these three categories of hearing loss in patients whose diagnoses are uncertain, who require assessment of treatment progression, or who are predisposed to developing hearing loss and thus require periodic screening. Appropriate bone conduction evaluation methods (PTA, ABR, or tuning fork tests) vary depending on the patient’s age and ability to communicate, the suspected mechanism of hearing loss, and the degree of diagnostic certainty required. Of note, some diagnoses may require further tests, including tympanometry, computed tomography, and otoacoustic emissions, none directly involving bone conduction evaluation.[26]
Sensorineural Hearing Loss
Common causes of SNHL include:
- Presbycusis
- Chronic metabolic and autoimmune conditions - eg, diabetes
- Infection - eg, meningitis, viral encephalitis
- Syndromic and nonsyndromic congenital disorders
- Ototoxic medications - eg, platinum-based chemotherapeutic agents, loop diuretics, aminoglycoside antibiotics
- Neoplasm - eg, vestibular schwannoma
- Head trauma
- Noise-induced hearing loss[27][28]
Conductive Hearing Loss
Common causes of conductive hearing loss include:
- Congenital anatomic abnormalities - ie, defects occurring anywhere from the pinna to the footplate of the stapes
- Cholesteatoma
- Obstruction - eg, earwax, foreign body
- Tympanic membrane perforation - eg, barotrauma
- Acute otitis media
- Otitis media with effusion
- Scarring of the middle ear - often related to recurrent infection or trauma
- Otosclerosis
- Ossicular discontinuity
- Neoplasm[29][30][31][32]
Normal and Critical Findings
For results and interpretation of tuning fork tests, see the “Procedures” section. Notably, tuning fork tests are no longer used as definitive diagnostic tools. Instead, tuning fork tests are considered a convenient preliminary clinical test.[6] A normal finding in ABR testing with bone conduction stimulation is considered thresholds of approximately 0 to 15 dB in infants. A normal finding in PTA with bone conduction stimulation is thresholds of 0 to 25 dB in adults. Degrees of hearing impairment are classified by ranges of hearing thresholds above these values. According to the WHO’s Grades of Hearing Impairment, thresholds are categorized as slight (26 to 40 dB), moderate (41 to 60 dB), severe (61 to 80 dB), and profound (>80 dB) impairment.[33]
Specific patterns on audiograms can be recognized as indicative of particular causes of hearing loss. It is often diagnostically beneficial to distinguish between SNHL, conductive hearing loss, and mixed hearing loss by calculating air-bone gaps. For instance, an air-bone gap is often measurable in patients with tympanic membrane perforation, especially at lower frequencies. This finding indicates that bone conduction is better than air conduction at those frequencies, as expected in a patient whose tympanic membrane is not functionally contributing to conductive hearing due to injury.[34] Another example of a critical finding on audiogram includes bilateral downward sloping high-frequency threshold increases with no significant air-bone gap, which is consistent with presbycusis, a leading cause of SNHL. This finding is due to selective high-frequency hearing loss in the aging inner ear.[35]
In patients with bilateral noise-induced SNHL, as is often observed in patients who work in loud environments without adequate hearing protection, there is no significant air-bone gap, and a threshold increase spanning from approximately 3,000 Hz to 4,000 Hz is characteristically observed. A final critical finding includes a large unilateral threshold increase centered at approximately 1,000 Hz with no air-bone gap. This finding is consistent with unilateral SNHL, which often requires further workup to rule out causes such as a vestibular schwannoma or other mass-occupying lesions.[36] ABR testing may provide additional diagnostic information relating to retrocochlear causes of unilateral SNHL, though magnetic resonance imaging is the gold standard in this situation.[37]
Interfering Factors
Factors that may interfere with the successful and accurate evaluation of bone conduction are numerous and complex. Some evidence suggests that manual (also known as traditional) PTA methods) air-bone gaps are more susceptible to tester bias effects than those obtained via automated PTA methods. This evidence is based on large datasets showing lower air-bone gap variability in manual versus automated PTA. It is hypothesized to be associated with prior knowledge of hearing loss characteristics.[38] Other studies suggest that manual and automated PTA methods produce sufficiently similar results, indicating that either method is acceptable, provided their calculated threshold differences fall within approximately 5 dB of each other.[39][40] It has been hypothesized that frequencies above 4,000 Hz are prone to error from false air-bone gaps during PTA.[41] Occlusion of the ear canal during bone conduction evaluation does not appear to have a significant corrective effect on these false air-bone gaps.[42] However, several studies have suggested that adjusting the reference equivalent threshold force level, a value used in determining bone conduction thresholds, by approximately 14 dB proves to be effective at correcting for false air-bone gaps at these frequencies.[43]
Patient Safety and Education
To ensure accurate assessment and treatment of hearing loss, trained professionals must evaluate patients. Hearing loss can vary in presentation depending on social and environmental factors; thus, a thorough history is warranted in hearing evaluation. Patients regularly exposed to loud occupational and recreational noise may benefit from audiometric screening (often mandated annually for workplace-related noise exposure) in addition to using effective ear protection.[44][45] Some studies suggest that before undergoing PTA, patients should avoid exposure to loud noises (including those louder than a household vacuum cleaner) for at least 14 hours to prevent temporary threshold shifts, which may confound test results. Parents and caretakers of pediatric patients should be educated on the necessity of newborn hearing screening and at well-child visits. Effective follow-up is vital in the early detection and treatment of pediatric hearing loss.[46] Regarding bone conduction evaluation specifically, patients, especially children, should be made aware that the vibration of an oscillator is nonpainful and does not cause harm despite an unusual sensation. With the increasing use of bone-conduction hearing devices, patients with hearing loss should be sufficiently educated on audiometric testing results and counseled in choosing an appropriate amplification device.[47]
Clinical Significance
Evaluating bone conduction is useful as a component of audiometry in various patient populations and clinical settings. Screening for hearing loss using audiometry in preschool and school-age children is an accurate and beneficial tool for guiding early diagnosis and intervention.[48] Notably, bone conduction evaluation is an integral factor in the selection and operation of bone-conduction hearing devices, which have significantly improved the quality of life in pediatric patients with various causes of hearing loss.[49][50] Age-related hearing loss is significantly associated with cognitive impairment and dementia in adults.[51] While this association remains poorly understood and further research is warranted, the decreasing cost and widespread availability of hearing evaluation and hearing aid devices make them a useful clinical tool for improving the quality of life of older patients with suspected hearing loss.[52] More broadly, due to the association of hearing loss with increased disease burden and hospitalization in older adults, PTA with air conduction and bone conduction evaluation can provide clinically relevant information for improving patient outcomes.[53][54]
Media
References
Stenfelt S. Inner ear contribution to bone conduction hearing in the human. Hearing research. 2015 Nov:329():41-51. doi: 10.1016/j.heares.2014.12.003. Epub 2014 Dec 18 [PubMed PMID: 25528492]
Stenfelt S. Acoustic and physiologic aspects of bone conduction hearing. Advances in oto-rhino-laryngology. 2011:71():10-21. doi: 10.1159/000323574. Epub 2011 Mar 8 [PubMed PMID: 21389700]
Level 3 (low-level) evidenceDauman R. Bone conduction: an explanation for this phenomenon comprising complex mechanisms. European annals of otorhinolaryngology, head and neck diseases. 2013 Sep:130(4):209-13. doi: 10.1016/j.anorl.2012.11.002. Epub 2013 Jun 3 [PubMed PMID: 23743177]
Level 3 (low-level) evidenceReiss M, [Tuning-fork tests--outdated?]. Fortschritte der Medizin. 1999 Apr 30; [PubMed PMID: 10361362]
Walker JJ, Cleveland LM, Davis JL, Seales JS. Audiometry screening and interpretation. American family physician. 2013 Jan 1:87(1):41-7 [PubMed PMID: 23317024]
Browning GG, Swan IR. Sensitivity and specificity of Rinne tuning fork test. BMJ (Clinical research ed.). 1988 Nov 26:297(6660):1381-2 [PubMed PMID: 3146371]
Wahid NWB, Hogan CJ, Attia M. Weber Test. StatPearls. 2024 Jan:(): [PubMed PMID: 30252391]
Recommended procedure for Rinne and Weber tuning-fork tests. British Society of Audiology. British journal of audiology. 1987 Aug; [PubMed PMID: 3620757]
Kong EL, Fowler JB. Rinne Test. StatPearls. 2024 Jan:(): [PubMed PMID: 28613725]
Walker HK, Hall WD, Hurst JW, Saunders AZ, Stein AV, Shuster NL. Audiometry. Clinical Methods: The History, Physical, and Laboratory Examinations. 1990:(): [PubMed PMID: 21250083]
Davies RA. Audiometry and other hearing tests. Handbook of clinical neurology. 2016:137():157-76. doi: 10.1016/B978-0-444-63437-5.00011-X. Epub [PubMed PMID: 27638069]
McDermott JC,Fausti SA,Henry JA,Frey RH, Effects of contralateral masking on high-frequency bone-conduction thresholds. Audiology : official organ of the International Society of Audiology. 1990; [PubMed PMID: 2275644]
Bargen GA. Chirp-Evoked Auditory Brainstem Response in Children: A Review. American journal of audiology. 2015 Dec:24(4):573-83. doi: 10.1044/2015_AJA-15-0016. Epub [PubMed PMID: 26649461]
Young A, Cornejo J, Spinner A. Auditory Brainstem Response. StatPearls. 2024 Jan:(): [PubMed PMID: 33231991]
Felix RA 2nd, Gourévitch B, Portfors CV. Subcortical pathways: Towards a better understanding of auditory disorders. Hearing research. 2018 May:362():48-60. doi: 10.1016/j.heares.2018.01.008. Epub 2018 Jan 31 [PubMed PMID: 29395615]
Level 3 (low-level) evidenceBiacabe B,Chevallier JM,Avan P,Bonfils P, Functional anatomy of auditory brainstem nuclei: application to the anatomical basis of brainstem auditory evoked potentials. Auris, nasus, larynx. 2001 Jan; [PubMed PMID: 11137368]
Seo YJ, Kwak C, Kim S, Park YA, Park KH, Han W. Update on Bone-Conduction Auditory Brainstem Responses: A Review. Journal of audiology & otology. 2018 Apr:22(2):53-58. doi: 10.7874/jao.2017.00346. Epub 2018 Feb 26 [PubMed PMID: 29471611]
Kim Y, Han W, Park S, You S, Kwak C, Seo Y, Lee J. Better Understanding of Direct Bone-Conduction Measurement: Comparison with Frequency-Specific Bone-Conduction Tones and Brainstem Responses. Journal of audiology & otology. 2020 Apr:24(2):85-90. doi: 10.7874/jao.2019.00360. Epub 2019 Nov 22 [PubMed PMID: 31747742]
Level 3 (low-level) evidenceMuchnik C, Neeman RK, Hildesheimer M. Auditory brainstem response to bone-conducted clicks in adults and infants with normal hearing and conductive hearing loss. Scandinavian audiology. 1995:24(3):185-91 [PubMed PMID: 8552978]
Kelly EA, Li B, Adams ME. Diagnostic Accuracy of Tuning Fork Tests for Hearing Loss: A Systematic Review. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2018 Aug:159(2):220-230. doi: 10.1177/0194599818770405. Epub 2018 Apr 17 [PubMed PMID: 29661046]
Level 1 (high-level) evidenceBayoumy AB, de Ru JA. Sudden deafness and tuning fork tests: towards optimal utilisation. Practical neurology. 2020 Feb:20(1):66-68. doi: 10.1136/practneurol-2019-002350. Epub 2019 Aug 23 [PubMed PMID: 31444233]
US Preventive Services Task Force, Krist AH, Davidson KW, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Epling JW Jr, Kubik M, Li L, Ogedegbe G, Pbert L, Silverstein M, Stevermer J, Tseng CW, Wong JB. Screening for Hearing Loss in Older Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2021 Mar 23:325(12):1196-1201. doi: 10.1001/jama.2021.2566. Epub [PubMed PMID: 33755083]
American Academy of Pediatrics, Joint Committee on Infant Hearing. Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007 Oct:120(4):898-921 [PubMed PMID: 17908777]
Foxe JJ, Stapells DR. Normal infant and adult auditory brainstem responses to bone-conducted tones. Audiology : official organ of the International Society of Audiology. 1993:32(2):95-109 [PubMed PMID: 8476354]
Anastasiadou S, Al Khalili Y. Hearing Loss. StatPearls. 2024 Jan:(): [PubMed PMID: 31194463]
Tanna RJ, Lin JW, De Jesus O. Sensorineural Hearing Loss. StatPearls. 2024 Jan:(): [PubMed PMID: 33351419]
Chau JK, Lin JR, Atashband S, Irvine RA, Westerberg BD. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. The Laryngoscope. 2010 May:120(5):1011-21. doi: 10.1002/lary.20873. Epub [PubMed PMID: 20422698]
Level 1 (high-level) evidenceKuhn M, Heman-Ackah SE, Shaikh JA, Roehm PC. Sudden sensorineural hearing loss: a review of diagnosis, treatment, and prognosis. Trends in amplification. 2011 Sep:15(3):91-105. doi: 10.1177/1084713811408349. Epub 2011 May 22 [PubMed PMID: 21606048]
Isaacson JE, Vora NM. Differential diagnosis and treatment of hearing loss. American family physician. 2003 Sep 15:68(6):1125-32 [PubMed PMID: 14524400]
Kuo CL, Shiao AS, Yung M, Sakagami M, Sudhoff H, Wang CH, Hsu CH, Lien CF. Updates and knowledge gaps in cholesteatoma research. BioMed research international. 2015:2015():854024. doi: 10.1155/2015/854024. Epub 2015 Mar 18 [PubMed PMID: 25866816]
Quesnel AM, Ishai R, McKenna MJ. Otosclerosis: Temporal Bone Pathology. Otolaryngologic clinics of North America. 2018 Apr:51(2):291-303. doi: 10.1016/j.otc.2017.11.001. Epub 2018 Feb 3 [PubMed PMID: 29397947]
Mills R, Hathorn I. Aetiology and pathology of otitis media with effusion in adult life. The Journal of laryngology and otology. 2016 May:130(5):418-24. doi: 10.1017/S0022215116000943. Epub 2016 Mar 15 [PubMed PMID: 26976514]
Olusanya BO, Davis AC, Hoffman HJ. Hearing loss grades and the International classification of functioning, disability and health. Bulletin of the World Health Organization. 2019 Oct 1:97(10):725-728. doi: 10.2471/BLT.19.230367. Epub 2019 Sep 3 [PubMed PMID: 31656340]
Orji FT, Agu CC. Patterns of hearing loss in tympanic membrane perforation resulting from physical blow to the ear: a prospective controlled cohort study. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2009 Dec:34(6):526-32. doi: 10.1111/j.1749-4486.2009.02035.x. Epub [PubMed PMID: 20070761]
Level 2 (mid-level) evidenceSchuknecht HF, Gacek MR. Cochlear pathology in presbycusis. The Annals of otology, rhinology, and laryngology. 1993 Jan:102(1 Pt 2):1-16 [PubMed PMID: 8420477]
Kim SH, Lee SH, Choi SK, Lim YJ, Na SY, Yeo SG. Audiologic evaluation of vestibular schwannoma and other cerebellopontine angle tumors. Acta oto-laryngologica. 2016:136(2):149-53. doi: 10.3109/00016489.2015.1100326. Epub 2015 Oct 19 [PubMed PMID: 26479426]
Peterein JL, Neely JG. Auditory brainstem response testing in neurodiagnosis: structure versus function. Journal of the American Academy of Audiology. 2012 Apr:23(4):269-275. doi: 10.3766/jaaa.23.4.5. Epub [PubMed PMID: 22463940]
Level 3 (low-level) evidenceMargolis RH, Wilson RH, Popelka GR, Eikelboom RH, Swanepoel de W, Saly GL. Distribution Characteristics of Air-Bone Gaps: Evidence of Bias in Manual Audiometry. Ear and hearing. 2016 Mar-Apr:37(2):177-88. doi: 10.1097/AUD.0000000000000246. Epub [PubMed PMID: 26627469]
Swanepoel de W, Biagio L. Validity of diagnostic computer-based air and forehead bone conduction audiometry. Journal of occupational and environmental hygiene. 2011 Apr:8(4):210-4. doi: 10.1080/15459624.2011.559417. Epub [PubMed PMID: 21391065]
Shojaeemend H, Ayatollahi H. Automated Audiometry: A Review of the Implementation and Evaluation Methods. Healthcare informatics research. 2018 Oct:24(4):263-275. doi: 10.4258/hir.2018.24.4.263. Epub 2018 Oct 31 [PubMed PMID: 30443414]
Lightfoot GR, Hughes JB. Bone conduction errors at high frequencies: implications for clinical and medico-legal practice. The Journal of laryngology and otology. 1993 Apr:107(4):305-8 [PubMed PMID: 8320514]
Tate Maltby M, Gaszczyk D. Is it necessary to occlude the ear in bone-conduction testing at 4 kHz, in order to prevent air-borne radiation affecting the results? International journal of audiology. 2015:54(12):918-23. doi: 10.3109/14992027.2015.1086029. Epub 2015 Oct 8 [PubMed PMID: 26446950]
Margolis RH, Eikelboom RH, Johnson C, Ginter SM, Swanepoel de W, Moore BC. False air-bone gaps at 4 kHz in listeners with normal hearing and sensorineural hearing loss. International journal of audiology. 2013 Aug:52(8):526-32. doi: 10.3109/14992027.2013.792437. Epub 2013 May 29 [PubMed PMID: 23713469]
Level 2 (mid-level) evidenceChung JH, Des Roches CM, Meunier J, Eavey RD. Evaluation of noise-induced hearing loss in young people using a web-based survey technique. Pediatrics. 2005 Apr:115(4):861-7 [PubMed PMID: 15805356]
Level 3 (low-level) evidenceRabinowitz PM. Noise-induced hearing loss. American family physician. 2000 May 1:61(9):2749-56, 2759-60 [PubMed PMID: 10821155]
Level 3 (low-level) evidenceHalloran DR, Hardin JM, Wall TC. Validity of pure-tone hearing screening at well-child visits. Archives of pediatrics & adolescent medicine. 2009 Feb:163(2):158-63. doi: 10.1001/archpediatrics.2008.526. Epub [PubMed PMID: 19188648]
Ellsperman SE, Nairn EM, Stucken EZ. Review of Bone Conduction Hearing Devices. Audiology research. 2021 May 18:11(2):207-219. doi: 10.3390/audiolres11020019. Epub 2021 May 18 [PubMed PMID: 34069846]
Prieve BA, Schooling T, Venediktov R, Franceschini N. An Evidence-Based Systematic Review on the Diagnostic Accuracy of Hearing Screening Instruments for Preschool- and School-Age Children. American journal of audiology. 2015 Jun:24(2):250-67. doi: 10.1044/2015_AJA-14-0065. Epub [PubMed PMID: 25760393]
Level 1 (high-level) evidenceCywka KB, Król B, Skarżyński PH. Effectiveness of Bone Conduction Hearing Aids in Young Children with Congenital Aural Atresia and Microtia. Medical science monitor : international medical journal of experimental and clinical research. 2021 Sep 25:27():e933915. doi: 10.12659/MSM.933915. Epub 2021 Sep 25 [PubMed PMID: 34561413]
Polonenko MJ, Carinci L, Gordon KA, Papsin BC, Cushing SL. Hearing Benefit and Rated Satisfaction in Children with Unilateral Conductive Hearing Loss Using a Transcutaneous Magnetic-Coupled Bone-Conduction Hearing Aid. Journal of the American Academy of Audiology. 2016 Nov/Dec:27(10):790-804 [PubMed PMID: 27885975]
Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of Age-Related Hearing Loss With Cognitive Function, Cognitive Impairment, and Dementia: A Systematic Review and Meta-analysis. JAMA otolaryngology-- head & neck surgery. 2018 Feb 1:144(2):115-126. doi: 10.1001/jamaoto.2017.2513. Epub [PubMed PMID: 29222544]
Level 1 (high-level) evidenceTsakiropoulou E, Konstantinidis I, Vital I, Konstantinidou S, Kotsani A. Hearing aids: quality of life and socio-economic aspects. Hippokratia. 2007 Oct:11(4):183-6 [PubMed PMID: 19582191]
Level 2 (mid-level) evidenceGenther DJ, Frick KD, Chen D, Betz J, Lin FR. Association of hearing loss with hospitalization and burden of disease in older adults. JAMA. 2013 Jun 12:309(22):2322-4. doi: 10.1001/jama.2013.5912. Epub [PubMed PMID: 23757078]
Lin FR, Ferrucci L. Hearing loss and falls among older adults in the United States. Archives of internal medicine. 2012 Feb 27:172(4):369-71. doi: 10.1001/archinternmed.2011.728. Epub [PubMed PMID: 22371929]
Level 3 (low-level) evidence