Introduction
Hearing loss is a widespread chronic condition affecting over 25 million people aged 12 years or older in the United States. It may affect 1 in 5 children, temporarily or permanently, by the age of 18.[1][2] Despite the high prevalence of hearing loss, many adults do not receive appropriate or timely evaluation and treatment for their hearing concerns.[3] Appropriate treatment for hearing loss depends greatly on the pathophysiology and the severity of the condition, and a detailed hearing assessment provides the healthcare team with the necessary diagnostic information to guide treatment. While Weber and Rinne tuning fork tests are useful for clinical screening and identifying the type of hearing loss, they may miss nuances such as mild hearing loss, bilateral hearing loss, or mixed conductive and sensorineural hearing loss.[4][5] Pure-tone (isolated frequency) audiometry evaluation over the range of frequencies important for everyday listening can determine the degree, configuration, and type of hearing loss in a manner detailed enough to assist the healthcare team in determining the etiology and prognosis for the hearing loss as well as the optimal treatment strategy.[6]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
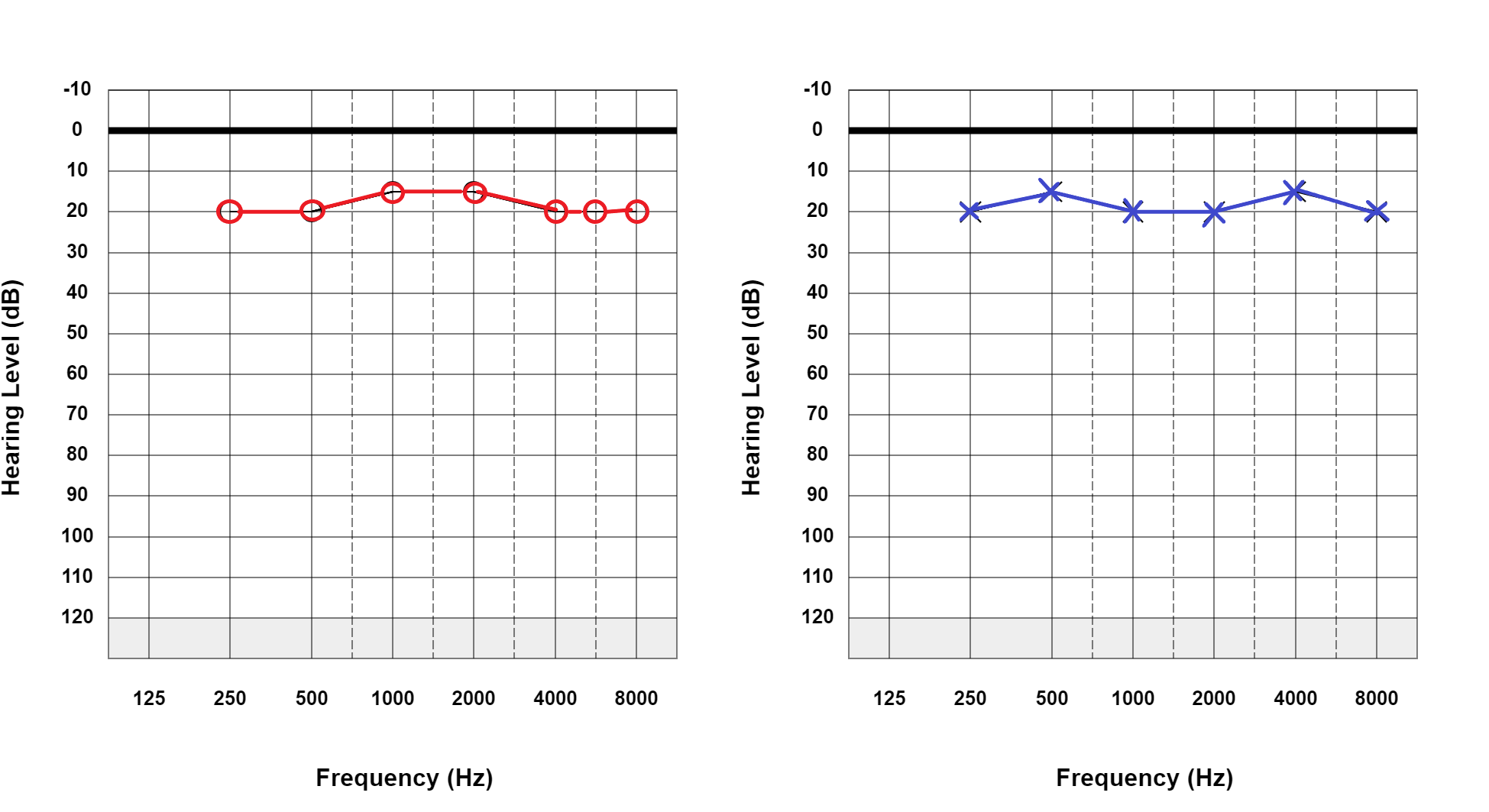
Pure-tone testing assesses the auditory system through 2 pathways. In air conduction testing, sound waves enter the external auditory canal and pass through the tympanic membrane, ossicular chain, and cochlea to reach the cochlear nerve (part of cranial nerve VIII) and then travel through the brainstem to the auditory cortex. In bone conduction testing, sound waves are introduced directly to the cochlea through the vibration of a bone conduction oscillator on the mastoid process.[7] See Graph. Normal Hearing Thresholds.
By utilizing both methods of pure-tone testing, hearing loss is determined to be conductive (involving the pinna through the ossicular chain), sensorineural (involving the cochlea to the auditory cortex), or mixed (both conductive and sensorineural involvement).[8] For some patients, a false "conductive" component may appear in the presence of "third-window" pathologies like semicircular canal dehiscence because of changes in sound transmission from the dehiscence.[9] Third window phenomena are referred to as such because they function as third connections between the inner and middle ears when counted along with the oval and round windows.
Indications
Pure-tone evaluation should be performed for patients concerned about abnormal auditory perception, ear trauma, or otologic disease.[10] This includes but is not limited to, subjective hearing loss, tinnitus, hyperacusis, ototoxic monitoring protocols, dizziness or vertigo, middle ear dysfunction, traumatic brain injury or temporal bone fracture, loud noise exposure, blast injury, failed hearing screening, speech delay in children, or conditions with risk factors for hearing loss.[11][12]
Contraindications
A reliable pure-tone evaluation cannot be completed if a patient cannot provide reliable behavioral responses.[13] This is particularly true of children under 6 months of age but is also seen in patients with developmental delays or neurological impairment. Behavioral pure-tone testing may also be contraindicated if unreliable responses are given by patients who are malingering or who have factitious disorders.[14] In these circumstances, objective electrophysiologic or otoacoustic emission testing instead of behavioral responses is necessary to build a complete clinical picture.[15]
Equipment
Pure-tone evaluation is typically performed in a sound-treated booth to reduce the impact of external sounds, with the booth environment and electroacoustic equipment calibrated to American National Standards Institute (ANSI) standards to optimize inter-test and intra-test reliability. Audiometers generate the sounds presented for testing, controlling the pitch, loudness, transducer type, and ear of presentation.[16] Various transducers may be used depending on the auditory pathway being assessed and the anatomical limitations of the ears. Standard electroacoustic transducers for pure-tone audiometry are supra-aural headphones, insert earphones, circumaural headphones, and bone conduction oscillators.[17][18] Speakers aligned at 90 or 45 degrees from the testing seat are used for children who cannot tolerate headphones. The presented tones are warbled in a sound-field environment to avoid standing waves from a single pure-tone frequency.[19] Sound field testing evaluates the composite best hearing ear, which means that the threshold recorded is the quietest sound audible by either ear at each frequency presented. A normal sound-field audiogram, therefore, does not indicate normal hearing across all tested frequencies in both ears but rather that at least 1 of the ears could hear the tone normally at each tested frequency. On the other hand, because recorded thresholds in sound-field audiometry indicate the acuity of the better-hearing ear, if a hearing loss is encountered, that elevated threshold represents the hearing loss in the better-hearing ear; there may or may not be worse hearing in the other ear. For this reason, individual ear testing to rule out unilateral hearing loss cannot be obtained with sound-field audiometry.
Regardless of the method of presenting the tones, patient responses are typically given with a button, a hand raise, or a verbal acknowledgment, with adaptations made for children unable to give a deliberate response with reliability.[20][21] These adaptation techniques are visual reinforcement audiometry and conditioned play audiometry, which use structured play-like stimuli-and-response with toys to obtain more reliable information.[22] Pure-tone screenings, such as in schools or inpatient hospital environments, are performed with calibrated portable audiometers that may test air and bone conduction or air conduction only, depending on the model.[23] Screenings assess for awareness of a minimum response level at a limited set of frequencies to determine if further diagnostic evaluation is warranted.[24] Standard pure-tone assessment with a portable audiometer using a precise transducer set-up and performed in a sufficiently quiet environment can obtain comparable threshold accuracy to sound booth assessments.[16]
Personnel
When pure-tone testing is indicated for evaluation of a specific complaint, the procedure is typically performed by an audiologist; however, screening audiometry (as is commonly used for school-aged children and military personnel) may be performed using automated testing equipment by an audiology technician.
Preparation
Patients are seated in a sound booth for diagnostic pure-tone testing and counseled regarding the testing method and expected response. Parents are instructed on how behavioral testing occurs with young children, typically with the patient seated in the parent's lap. A young child may receive basic instructions to assist in understanding and comfort with the test procedure. The audiologist places appropriate transducers on the patient's head or ears before closing the sound booth door and relocating to an adjacent booth to control the audiometer. The audiologist can observe the patient through an insulated window and hear feedback from the patient via a microphone inside the booth.
Technique or Treatment
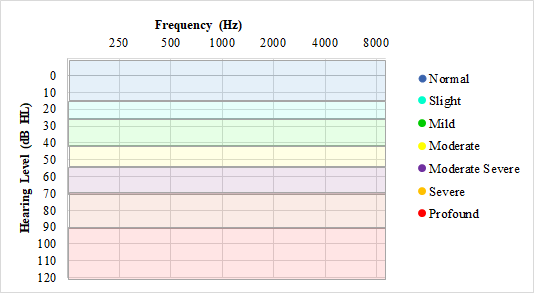
Results are recorded on a graph known as the audiogram, which logarithmically plots sound frequency in cycles per second (Hertz or Hz) from low pitch to high pitch along the X-axis and loudness in decibels of hearing level (dB HL) from soft to loud along a reversed Y-axis.[25] Decibels of hearing level differ from decibels of sound pressure level because the use of hearing level reflects that normal human ears hear better in the middle-frequency ranges than at the higher and lower frequencies, thus normalizing the scale using an isophonic curve. For example, a 0 dB hearing level (normal hearing) at 500 Hz is equivalent to a 13.5 dB sound pressure level, according to ANSI S3.6-1996. At 1000 Hz, 0 dB HL equals 7.5 dB SPL, and at 2000 Hz, 0 dB HL equals 9 dB SPL. Down at 125 Hz, however, 0 dB HL equals 45 dB SPL, and up at 8000 Hz, 0 dB HL is 15.5 dB SPL. See Graph. Normal Hearing Ranges for Audiograms.
A standard audiogram graphs pitch in octaves and most inter-octaves from 250 Hz to 8000 Hz; the full testable range includes 125 Hz and ultrahigh frequencies of 9, 10, 11.2, 12.5, 14, 16, and 20 kHz.[20][26] A common method of summarizing standard pure-tone audiometric findings is using the pure-tone average (PTA), the arithmetic mean of each ear's thresholds at 500, 1000, and 2000 Hz. The PTA is often reported in the research literature as an outcome measure. Still, it is important to remember that it does not reflect hearing in the lowest and highest frequencies, which may be affected by certain exposures, such as loud noises and ototoxins. Ultra-high frequencies are typically tested for ototoxic monitoring protocols or if the standard audiogram does not reflect the patient's reported auditory concerns.[27][28]
Pure-tone sounds are presented through the appropriate transducers to the better-hearing ear, if 1 exists, starting at 1000 Hz. Initially, the tone is presented at a level estimated to be easily audible to confirm the patient understands the task of perception and response. If no response occurs, the volume is increased in 20 dB increments until the patient does respond or reinstruction is deemed necessary. Once the initial response is obtained, a threshold search is performed by descending 10 dB in volume for every response to a presentation and ascending 5 dB for every presentation without a response.[29] A threshold is recorded as the softest ascending presentation a patient responds to at least 50% of the time (determined by a minimum of 2 out of 3 responses and a maximum of 2 out of 4 responses).[10]
The standard testing order is 1000, 2000, 3000, 4000, 6000, and 8000 Hz, then repeating 1000 Hz to assess intra-test reliability and account for practice effects before proceeding to 500 and 250 Hz. If a patient demonstrates many false positives (responding without stimuli) or false negatives (not responding to stimuli that should be audible), reinstruction may be warranted. Patients with constant tinnitus may have difficulty distinguishing the pure-tone stimuli from their tinnitus, and modification of the tone stimuli to a warbled tone or a series of 2 or 3 pulses may be done to improve response accuracy.[20] Hearing loss is generally categorized by the degree of difficulty detecting sound in 5 dB increments, from normal hearing sensitivity at ≤25 dB HL to mild (26 to 40 dB HL), moderate (41 to 55 dB HL), moderately severe (61 to 70 dB HL), severe (71 to 90 dB HL), or profound (91+ dB HL) hearing loss.[8] The pediatric cutoff for normal hearing sensitivity is ≤15 dB HL, with 20 and 25 dB HL categorized as slight hearing loss due to the higher need for sound access for appropriate development in children.[30]
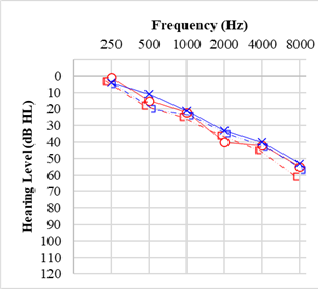
Once air conduction and bone conduction thresholds are determined, hearing loss is categorized as sensorineural if the frequencies falling outside normal limits have air conduction and bone conduction thresholds within 10 dB of each other. The hearing loss is considered conductive if bone conduction is within normal limits, but at least 1 frequency has a difference between bone conduction and air conduction of ≥15 dB; the loss is described as "mixed" if there are some sensorineural loss frequencies and some conductive loss frequencies, or if the hearing loss has both conductive and sensorineural aspects in 1 or more frequencies.[31] The configuration of a hearing loss refers to the shape of the plotted audiogram, as some configurations are strongly correlated with specific causes of hearing loss, such as sloping high-frequency loss with presbycusis, notching mid-frequency loss with genetic causes or low-frequency hearing loss rising to normal hearing with cochlear hydrops (see Graph. Audiogram for Presbycusis).[8]
Due to how sound transmits through the skull, a sufficiently loud presentation of air conduction or bone conduction to 1 ear may cross to the cochlea of the other ear to elicit a response, which may confuse audiometry results.[32] The volume required before this crossover between ears is called interaural attenuation and varies between individuals and transducers.[33] When an asymmetry between ears is present, or a conductive component is suspected, masking noise is introduced to the non-test ear (NTE) via air conduction to artificially raise that ear's threshold and prevent a response from that ear.[34] The minimum volume possible to overcome interaural attenuation is used to calculate when masking is necessary by comparing the questionable threshold of the test ear to the bone conduction threshold of the NTE for that frequency. For bone conduction testing, the interaural attenuation is 0 dB, the supra-aural headphone interaural attenuation is 40 dB, and the insert earphone interaural attenuation is 55 dB. A complete pure-tone assessment includes both bone and air conduction for the left and right ear, with masking completed as appropriate.
Complications
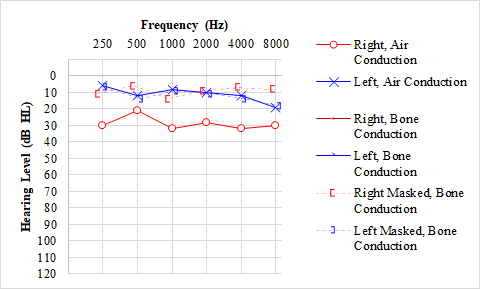
Multiple factors can affect the outcomes or accuracy of pure-tone evaluation. Pure-tone thresholds can vary slightly with different transducers and transducer placement or in patient alertness and attention variations. In adults, standard diagnostic test-retest variability is typically ±5 dB across frequencies.[20] The pediatric population's test-retest variability may be as great as +/-10 dB.[35] A masking dilemma occurs when the amount of masking noise necessary to prevent sound crossover of a tone to the NTE is loud enough to cross back to the test ear and falsely elevate the evaluated threshold.[36] This occurs most commonly with severe bilateral conductive hearing loss.[37] See Graph. Conductive Hearing Loss.
Otalgia, particularly of the pinna or auditory canal, may prevent the appropriate placement of headphones for air conduction. Supra-aural headphones are necessary in cases of atresia, but when stenotic or partially occluded canals are involved, the pressure they apply may cause canal obstruction and introduce a false conductive component.[38] Insert earphones can prevent external auditory canals from collapsing during testing and reduce the incidence of masking dilemmas.[39] Additionally, due to the differences in calibration between inserted earphones and headphones, the larger cavity created by tympanostomy tubes can cause low-frequency thresholds to be falsely elevated when using inserted earphones.[40]
Behavioral complications include false positive responses, which can result from confusing tinnitus with the presented tone, responding to a regular rhythm of tone presentation, or over-eagerness to "do well" on the evaluation, particularly when a "passing score" is required for employment qualification. False negatives may occur when a patient does not provide consistent responses to audible tones due to a lack of understanding of testing procedures, a deficit in central processing, or a non-organic deficit caused by malingering or factitious disorders.[41] A Stenger test may be used to assess for malingering in a unilateral or asymmetric hearing loss by observing how the patient responds to a pure tone presented simultaneously to both ears when 1 tone is easily audible in the better-hearing ear but is quieter than the apparent threshold in the ear with greater hearing loss.[42]
If the patient responds because they hear the quiet tone in the better-hearing ear, the Stenger test is negative, and the hearing loss may be considered accurate. If the patient does not respond, which typically occurs because the patient deliberately does not respond to the louder tone in the tested ear and does not realize a quieter tone in the NTE, the Stenger is positive and indicates at least some degree of exaggeration in the responses. Stenger testing has a high sensitivity for detecting unilateral non-organic hearing loss.[43]
Clinical Significance
Pure-tone audiometry is the standard gold method of determining the type, degree, and configuration of hearing loss due to its widespread availability, inter-test reliability, and relative ease of execution. Pure-tone audiometry results provide context for diagnosis, reassurance, monitoring, or further investigation of ear concerns. Repeated assessments track changes in hearing as an indicator of changes in ear health over time. When a sudden change in hearing is reported, immediate pure-tone evaluation is warranted to determine whether a significant hearing loss has occurred and if it meets the criteria for sudden sensorineural hearing loss (SSNHL). Identification of SSNHL and rapid initiation of therapy is critical due to the limited time window for SSNHL treatment to be effective.[44] In SSNHL, a hearing asymmetry is considered significant if there is a ≥30 dB difference over 3 consecutive frequencies.[45] Outside of sudden hearing loss, a significant threshold shift, according to the American Academy of Otolaryngology-Head and Neck Surgery, is ≥10 dB increase in the PTA, or at 3000, 4000, and 6000 Hz, in either ear; the Occupational Safety and Health Administration standards for a shift for noise-related hearing loss are an average of ≥10 dB threshold increases for 2, 3, and 4 kHz.[46]
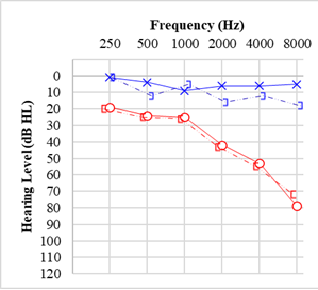
The American Speech-Language-Hearing Association considers a threshold shift significant for ototoxicity monitoring when there is a ≥20 dB threshold increase at a single frequency, ≥10 dB at 2 adjacent frequencies, or loss of response at 3 or more consecutive previously-obtained frequencies in the ultra-high frequency range where maximum volume outputs are limited.[47] Hearing asymmetry is a common symptom of retrocochlear pathologies, such as vestibular schwannoma (see Graph. Audiogram for Vestibular Schwannoma/Acoustic Neuroma).[48] The definition of a significant hearing asymmetry varies throughout the literature, but an asymmetry of ≥20 dB at 2 consecutive frequencies or ≥15 dB at 2 frequencies between 2,000 and 8,000 Hz should raise suspicion for retrocochlear pathology.[49]
Aside from hearing deficits, pure-tone audiometry is utilized in the test battery to assess loudness discomfort levels, indicating hyperacusis when the discomfort threshold is ≤85 dB HL or softer.[50][51] Pure-tone thresholds also help determine the etiology of tinnitus, and pure-tone presentations are also used as part of pitch and loudness matching assessment for characterizing tinnitus handicaps and guiding auditory-based treatment for tinnitus.[52] It is important to remember that pure-tone audiometry does not provide a complete diagnostic picture for auditory neuropathy or central auditory processing concerns, such as difficulty understanding speech in noise, which requires speech audiometry or other objective tests to assess accurately.[53][54] Central auditory processing disorder testing may be performed on patients of school age or older with sufficient attention spans for extended assessment.[55]
Enhancing Healthcare Team Outcomes
Hearing loss is the third most common chronic health condition in aging adults and affects 1 in 5 children to some degree by the age of 18. Relationships between the severity of hearing loss and the severity of ear disease, as well as the relationships between hearing loss and communication, social, and educational deficits in children, make catching hearing loss early a critical healthcare task. Pure-tone audiometry is a low-risk, low-cost procedure but can provide crucial information regarding differential diagnosis, symptom etiology or pathophysiology, and guidance for appropriate treatment. Any time a hearing loss or abnormal auditory perception is reported as a symptom, a referral to audiology for testing that includes pure-tone evaluation should be part of the evaluation process.[1][8] If a concern about hearing is not addressed, negative outcomes range from developmental delays to poorer quality of life to debilitating disability.[56][57][58]
The close correlation between ear health and hearing abnormalities makes coordination between otolaryngologists and audiologists critical. Pure-tone testing is also key for pre-and post-treatment evaluation when outcome measures include the preservation or improvement of hearing as a significant indicator of efficacy, such as for idiopathic sudden sensorineural hearing loss, and otologic surgeries, including tympanoplasty, stapedotomy, semicircular canal dehiscence resurfacing, or mastoidectomy.[59][60][61] Due to the myriad causes of hearing loss, care coordination among healthcare providers relying on pure-tone audiometry may include general practitioners, pediatricians, otolaryngologists, oncologists, neurologists, speech-language pathologists, and audiologists. Inter-professional communication and care coordination are essential for evidence-based, timely management of patients and optimal treatment outcomes.k
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Lieu JEC, Kenna M, Anne S, Davidson L. Hearing Loss in Children: A Review. JAMA. 2020 Dec 1:324(21):2195-2205. doi: 10.1001/jama.2020.17647. Epub [PubMed PMID: 33258894]
Goman AM, Lin FR. Prevalence of Hearing Loss by Severity in the United States. American journal of public health. 2016 Oct:106(10):1820-2. doi: 10.2105/AJPH.2016.303299. Epub 2016 Aug 23 [PubMed PMID: 27552261]
Mahboubi H, Lin HW, Bhattacharyya N. Prevalence, Characteristics, and Treatment Patterns of Hearing Difficulty in the United States. JAMA otolaryngology-- head & neck surgery. 2018 Jan 1:144(1):65-70. doi: 10.1001/jamaoto.2017.2223. Epub [PubMed PMID: 29167904]
Chole RA,Cook GB, The Rinne test for conductive deafness. A critical reappraisal. Archives of otolaryngology--head & neck surgery. 1988 Apr [PubMed PMID: 3348896]
Kelly EA, Li B, Adams ME. Diagnostic Accuracy of Tuning Fork Tests for Hearing Loss: A Systematic Review. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2018 Aug:159(2):220-230. doi: 10.1177/0194599818770405. Epub 2018 Apr 17 [PubMed PMID: 29661046]
Level 1 (high-level) evidenceMusiek FE, Shinn J, Chermak GD, Bamiou DE. Perspectives on the Pure-Tone Audiogram. Journal of the American Academy of Audiology. 2017 Jul/Aug:28(7):655-671. doi: 10.3766/jaaa.16061. Epub [PubMed PMID: 28722648]
Level 3 (low-level) evidenceDavies RA. Audiometry and other hearing tests. Handbook of clinical neurology. 2016:137():157-76. doi: 10.1016/B978-0-444-63437-5.00011-X. Epub [PubMed PMID: 27638069]
Michels TC,Duffy MT,Rogers DJ, Hearing Loss in Adults: Differential Diagnosis and Treatment. American family physician. 2019 Jul 15; [PubMed PMID: 31305044]
Castellucci A, Piras G, Del Vecchio V, Crocetta FM, Maiolo V, Ferri GG, Ghidini A, Brandolini C. The effect of superior canal dehiscence size and location on audiometric measurements, vestibular-evoked myogenic potentials and video-head impulse testing. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2021 Apr:278(4):997-1015. doi: 10.1007/s00405-020-06169-3. Epub 2020 Jun 26 [PubMed PMID: 32592013]
Walker HK, Hall WD, Hurst JW, Saunders AZ, Stein AV, Shuster NL. Audiometry. Clinical Methods: The History, Physical, and Laboratory Examinations. 1990:(): [PubMed PMID: 21250083]
Douniadakis DE, Kalli KI, Psarommatis IM, Tsakanikos MD, Apostolopoulos NK. Incidence of hearing loss among children presented with speech-language delay. Scandinavian audiology. Supplementum. 2001:(52):204-5 [PubMed PMID: 11318469]
Casazza G,Meier JD, Evaluation and management of syndromic congenital hearing loss. Current opinion in otolaryngology [PubMed PMID: 28697000]
Level 3 (low-level) evidenceTrudeau S, Anne S, Otteson T, Hopkins B, Georgopoulos R, Wentland C. Diagnosis and patterns of hearing loss in children with severe developmental delay. American journal of otolaryngology. 2021 May-Jun:42(3):102923. doi: 10.1016/j.amjoto.2021.102923. Epub 2021 Jan 13 [PubMed PMID: 33486206]
Wilson WR, Richardson MA. Behavioral audiometry. Otolaryngologic clinics of North America. 1991 Apr:24(2):285-97 [PubMed PMID: 1857613]
Suppiej A, Rizzardi E, Zanardo V, Franzoi M, Ermani M, Orzan E. Reliability of hearing screening in high-risk neonates: comparative study of otoacoustic emission, automated and conventional auditory brainstem response. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2007 Apr:118(4):869-76 [PubMed PMID: 17317296]
Level 2 (mid-level) evidenceMaclennan-Smith F, Swanepoel de W, Hall JW 3rd. Validity of diagnostic pure-tone audiometry without a sound-treated environment in older adults. International journal of audiology. 2013 Feb:52(2):66-73. doi: 10.3109/14992027.2012.736692. Epub 2012 Nov 11 [PubMed PMID: 23140522]
Luks SB, Borton TE, Nolen BL. Insert earphones for audiometry: applications, advantages, and limitations. ENTechnology. 1989 Sep:():21-2, 24, 28-30 [PubMed PMID: 2692634]
Valente M, Valente M, Goebel J. High-frequency thresholds: circumaural earphone versus insert earphone. Journal of the American Academy of Audiology. 1992 Nov:3(6):410-8 [PubMed PMID: 1486204]
Walker G, Dillon H, Byrne D. Sound field audiometry: recommended stimuli and procedures. Ear and hearing. 1984 Jan-Feb:5(1):13-21 [PubMed PMID: 6706021]
DiGiovanni JJ, Repka JN. Response method in audiometry. American journal of audiology. 2007 Dec:16(2):145-8 [PubMed PMID: 18056882]
Level 1 (high-level) evidenceTharpe AM, Bess FH, Sladen DP, Schissel H, Couch S, Schery T. Auditory characteristics of children with autism. Ear and hearing. 2006 Aug:27(4):430-41 [PubMed PMID: 16825892]
Level 2 (mid-level) evidenceNightengale EE, Wolter-Warmerdam K, Yoon PJ, Daniels D, Hickey F. Behavioral Audiology Procedures in Children With Down Syndrome. American journal of audiology. 2020 Sep 3:29(3):356-364. doi: 10.1044/2020_AJA-19-00076. Epub 2020 Jun 15 [PubMed PMID: 32539476]
Walker JJ, Cleveland LM, Davis JL, Seales JS. Audiometry screening and interpretation. American family physician. 2013 Jan 1:87(1):41-7 [PubMed PMID: 23317024]
Halloran DR, Hardin JM, Wall TC. Validity of pure-tone hearing screening at well-child visits. Archives of pediatrics & adolescent medicine. 2009 Feb:163(2):158-63. doi: 10.1001/archpediatrics.2008.526. Epub [PubMed PMID: 19188648]
Salmon MK, Brant J, Hohman MH, Leibowitz D. Audiogram Interpretation. StatPearls. 2024 Jan:(): [PubMed PMID: 35201707]
Abu-Eta R, Gavriel H, Pitaro J. Extended High Frequency Audiometry for Revealing Sudden Sensory Neural Hearing Loss in Acute Tinnitus Patients. International archives of otorhinolaryngology. 2021 Jul:25(3):e413-e415. doi: 10.1055/s-0040-1713921. Epub 2020 Sep 30 [PubMed PMID: 34377177]
Ganesan P, Schmiedge J, Manchaiah V, Swapna S, Dhandayutham S, Kothandaraman PP. Ototoxicity: A Challenge in Diagnosis and Treatment. Journal of audiology & otology. 2018 Apr:22(2):59-68. doi: 10.7874/jao.2017.00360. Epub 2018 Feb 26 [PubMed PMID: 29471610]
Fabijańska A, Smurzyński J, Hatzopoulos S, Kochanek K, Bartnik G, Raj-Koziak D, Mazzoli M, Skarżyński PH, Jędrzejczak WW, Szkiełkowska A, Skarżyński H. The relationship between distortion product otoacoustic emissions and extended high-frequency audiometry in tinnitus patients. Part 1: normally hearing patients with unilateral tinnitus. Medical science monitor : international medical journal of experimental and clinical research. 2012 Dec:18(12):CR765-70 [PubMed PMID: 23197241]
Level 2 (mid-level) evidencePoling GL, Kunnel TJ, Dhar S. Comparing the Accuracy and Speed of Manual and Tracking Methods of Measuring Hearing Thresholds. Ear and hearing. 2016 Sep-Oct:37(5):e336-40. doi: 10.1097/AUD.0000000000000317. Epub [PubMed PMID: 27232075]
Cone BK, Wake M, Tobin S, Poulakis Z, Rickards FW. Slight-mild sensorineural hearing loss in children: audiometric, clinical, and risk factor profiles. Ear and hearing. 2010 Apr:31(2):202-12. doi: 10.1097/AUD.0b013e3181c62263. Epub [PubMed PMID: 20054279]
Level 2 (mid-level) evidenceOntario Health (Quality). Implantable Devices for Single-Sided Deafness and Conductive or Mixed Hearing Loss: A Health Technology Assessment. Ontario health technology assessment series. 2020:20(1):1-165 [PubMed PMID: 32194878]
Level 2 (mid-level) evidenceLIDEN G, NILSSON G, ANDERSON H. Masking in clinical audiometry. Acta oto-laryngologica. 1959 Mar-Apr:50(2):125-36 [PubMed PMID: 13636845]
Gumus NM, Gumus M, Unsal S, Yuksel M, Gunduz M. Examination of Insert Ear Interaural Attenuation (IA)Values in Audiological Evaluations. Clinical and investigative medicine. Medecine clinique et experimentale. 2016 Dec 1:39(6):27507 [PubMed PMID: 27917798]
. Recommendations for masking in pure tone threshold audiometry. British Society of Audiology. British journal of audiology. 1986 Nov:20(4):307-14 [PubMed PMID: 3790774]
Beahan N, Kei J, Driscoll C, Charles B, Khan A. High-frequency pure-tone audiometry in children: a test-retest reliability study relative to ototoxic criteria. Ear and hearing. 2012 Jan-Feb:33(1):104-11. doi: 10.1097/AUD.0b013e318228a77d. Epub [PubMed PMID: 21760512]
Seneviratne S, McNeill C, Greenberg SL, Kong J. Naunton's Masking Dilemma Revisited. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2019 Jan:40(1):e1-e6. doi: 10.1097/MAO.0000000000002043. Epub [PubMed PMID: 30531635]
Lenhardt ML, Goldstein BA, Shulman A. Binaural hearing, atresia, and the masking dilemma. The international tinnitus journal. 2006:12(2):96-100 [PubMed PMID: 17260874]
Randolph LJ, Schow RL. Threshold inaccuracies in an elderly clinical population: ear canal collapse as a possible cause. Journal of speech and hearing research. 1983 Mar:26(1):54-8 [PubMed PMID: 6865382]
Clemis JD, Ballad WJ, Killion MC. Clinical use of an insert earphone. The Annals of otology, rhinology, and laryngology. 1986 Sep-Oct:95(5 Pt 1):520-4 [PubMed PMID: 3767222]
Tokar-Prejna S, Meinzen-Derr J. Relationship between transducer type and low-frequency hearing loss for patients with ventilation tubes. International journal of pediatric otorhinolaryngology. 2006 Jun:70(6):1063-7 [PubMed PMID: 16364457]
Level 2 (mid-level) evidenceSchmidt CM, Am Zehnhoff-Dinnesen A, Deuster D. [Nonorganic (functional) hearing loss in children]. HNO. 2013 Feb:61(2):136-41. doi: 10.1007/s00106-012-2504-3. Epub [PubMed PMID: 22534679]
Level 1 (high-level) evidenceDurmaz A, Karahatay S, Satar B, Birkent H, Hidir Y. Efficiency of Stenger test in confirming profound, unilateral pseudohypacusis. The Journal of laryngology and otology. 2009 Aug:123(8):840-4. doi: 10.1017/S0022215109004769. Epub 2009 Mar 19 [PubMed PMID: 19296863]
Arslan HH, Edizer DT, Cebeci S, Erdal M. Diagnostic utility of Stenger test: reappraisal of its value. The international tinnitus journal. 2014:19(1):57-62. doi: 10.5935/0946-5448.20140008. Epub [PubMed PMID: 27186834]
Stachler RJ, Chandrasekhar SS, Archer SM, Rosenfeld RM, Schwartz SR, Barrs DM, Brown SR, Fife TD, Ford P, Ganiats TG, Hollingsworth DB, Lewandowski CA, Montano JJ, Saunders JE, Tucci DL, Valente M, Warren BE, Yaremchuk KL, Robertson PJ, American Academy of Otolaryngology-Head and Neck Surgery. Clinical practice guideline: sudden hearing loss. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2012 Mar:146(3 Suppl):S1-35. doi: 10.1177/0194599812436449. Epub [PubMed PMID: 22383545]
Level 1 (high-level) evidenceSkarżyńska MB, Kołodziejak A, Gos E, Sanfis MD, Skarżyński PH. Effectiveness of Various Treatments for Sudden Sensorineural Hearing Loss-A Retrospective Study. Life (Basel, Switzerland). 2022 Jan 10:12(1):. doi: 10.3390/life12010096. Epub 2022 Jan 10 [PubMed PMID: 35054488]
Level 2 (mid-level) evidenceLane CL, Dobie RA, Crawford DR, Morgan MS. Standard threshold shift criteria. An investigation of the most reliable indicator of noise-induced hearing loss. Journal of occupational medicine. : official publication of the Industrial Medical Association. 1985 Jan:27(1):34-42 [PubMed PMID: 3968596]
Schlauch RS, Carney E. A multinomial model for identifying significant pure-tone threshold shifts. Journal of speech, language, and hearing research : JSLHR. 2007 Dec:50(6):1391-403 [PubMed PMID: 18055764]
Durakovic N, Valente M, Goebel JA, Wick CC. What defines asymmetric sensorineural hearing loss? The Laryngoscope. 2019 May:129(5):1023-1024. doi: 10.1002/lary.27504. Epub 2018 Nov 8 [PubMed PMID: 30408187]
Gimsing S. Vestibular schwannoma: when to look for it? The Journal of laryngology and otology. 2010 Mar:124(3):258-64. doi: 10.1017/S0022215109991423. Epub 2009 Nov 19 [PubMed PMID: 19922702]
Knobel KA, Sanchez TG. [Loudness discomfort level in normal hearing individuals]. Pro-fono : revista de atualizacao cientifica. 2006 Jan-Apr:18(1):31-40 [PubMed PMID: 16625869]
Sherlock LP, Formby C. Estimates of loudness, loudness discomfort, and the auditory dynamic range: normative estimates, comparison of procedures, and test-retest reliability. Journal of the American Academy of Audiology. 2005 Feb:16(2):85-100 [PubMed PMID: 15807048]
Level 2 (mid-level) evidenceNascimento IDP, Almeida AA, Diniz J Junior, Martins ML, Freitas TMMWC, Rosa MRDD. Tinnitus evaluation: relationship between pitch matching and loudness, visual analog scale and tinnitus handicap inventory. Brazilian journal of otorhinolaryngology. 2019 Sep-Oct:85(5):611-616. doi: 10.1016/j.bjorl.2018.05.006. Epub 2018 Jun 21 [PubMed PMID: 29983341]
Vermiglio AJ, Soli SD, Fang X. An Argument for Self-Report as a Reference Standard in Audiology. Journal of the American Academy of Audiology. 2018 Mar:29(3):206-222. doi: 10.3766/jaaa.16128. Epub [PubMed PMID: 29488871]
Vermiglio AJ, Soli SD, Freed DJ, Fisher LM. The relationship between high-frequency pure-tone hearing loss, hearing in noise test (HINT) thresholds, and the articulation index. Journal of the American Academy of Audiology. 2012 Nov-Dec:23(10):779-88. doi: 10.3766/jaaa.23.10.4. Epub [PubMed PMID: 23169195]
Moore DR, Sieswerda SL, Grainger MM, Bowling A, Smith N, Perdew A, Eichert S, Alston S, Hilbert LW, Summers L, Lin L, Hunter LL. Referral and Diagnosis of Developmental Auditory Processing Disorder in a Large, United States Hospital-Based Audiology Service. Journal of the American Academy of Audiology. 2018 May:29(5):364-377. doi: 10.3766/jaaa.16130. Epub [PubMed PMID: 29708487]
Wei J, Hu Y, Zhang L, Hao Q, Yang R, Lu H, Zhang X, Chandrasekar EK. Hearing Impairment, Mild Cognitive Impairment, and Dementia: A Meta-Analysis of Cohort Studies. Dementia and geriatric cognitive disorders extra. 2017 Sep-Dec:7(3):440-452. doi: 10.1159/000485178. Epub 2017 Dec 21 [PubMed PMID: 29430246]
Level 1 (high-level) evidenceCunningham LL, Tucci DL. Hearing Loss in Adults. The New England journal of medicine. 2017 Dec 21:377(25):2465-2473. doi: 10.1056/NEJMra1616601. Epub [PubMed PMID: 29262274]
Schönweiler R, Ptok M, Radü HJ. A cross-sectional study of speech- and language-abilities of children with normal hearing, mild fluctuating conductive hearing loss, or moderate to profound sensoneurinal hearing loss. International journal of pediatric otorhinolaryngology. 1998 Aug 1:44(3):251-8 [PubMed PMID: 9780071]
Level 2 (mid-level) evidenceMcClellan J, Nguyen A, Hamilton B, Jethanamest D, Hullar TE, Gupta S. Stapes Surgery Outcomes in Patients With Concurrent Otosclerosis and Superior Semicircular Canal Dehiscence. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2020 Aug:41(7):912-915. doi: 10.1097/MAO.0000000000002673. Epub [PubMed PMID: 32472923]
Vlastarakos PV, Proikas K, Tavoulari E, Kikidis D, Maragoudakis P, Nikolopoulos TP. Efficacy assessment and complications of surgical management for superior semicircular canal dehiscence: a meta-analysis of published interventional studies. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2009 Feb:266(2):177-86. doi: 10.1007/s00405-008-0840-4. Epub 2008 Oct 25 [PubMed PMID: 18953551]
Level 3 (low-level) evidenceStevens SM, Walters ZA, Babo K, Peddireddy N, Tawfik KO, Samy RN. Canal reconstruction mastoidectomy: Outcomes comparison following primary versus secondary surgery. The Laryngoscope. 2019 Nov:129(11):2580-2587. doi: 10.1002/lary.27558. Epub 2019 Mar 18 [PubMed PMID: 30883762]