Introduction
Retinal detachment (RD) is defined as the separation of the neurosensory retina (NSR) from the underlying retinal pigment epithelium (RPE) layer. Rhegmatogenous RD (RRD) is characterized by the passage of fluid from the vitreous cavity into the potential space between the NSR and the RPE through a full-thickness retinal break. It causes a sudden painless loss of vision. Its incidence is reportedly around 6.3 to 17.9 per 100,000 population.[1]
The history of successful management of RRD starts with Jules Gonin. RRD was considered an untreatable disease until Jules Gonin proved that it is caused by retinal break(s) and not vice versa. He also developed the first successful surgery for RRD, i.e., “Ignipuncture.” The surgery included break(s) localization, subretinal fluid (SRF) drainage through a 2 to 3 mm long scleral incision constructed beneath the retinal break, and direct thermocautery to the breakthrough of the drainage sclerostomy.[2][3] He also established the principles of RRD surgery that are valid even today. The three principles of RRD surgery are:
- Accurate identification and localization of retinal break(s)
- Creation of chorioretinal adhesion at the site of retinal break(s)
- Creation of appositional closure between the retinal break(s) and the RPE
Ernst Custodis performed the first scleral buckling (SB) surgery in 1949. He used a polyviol exoplant and did not drain SRF. Lincoff (1965) made multiple modifications to the Custodis procedure. He introduced using a silicone sponge instead of a polyviol exoplant, cryotherapy (for creating chorioretinal adhesions) instead of diathermy, and a spatula needle (for scleral suturing). He also did not drain SRF in almost any case.[4]
The surgical approaches for the treatment of RRD include pneumatic retinopexy, SB, and pars plana vitrectomy (PPV). Vitrectomy has gained popularity worldwide over the last few decades due to its better ergonomics.[5] This has led to a drastic reduction in the number of SB procedures. Even the vitreoretinal training programs have shifted their focus away from teaching SB. This dangerous trend can potentially make SB a “dead art.” It is imperative to mention that the anatomical and functional outcomes of SB for the management of primary RRD are almost comparable to PPV.[5][6][7][8] SB continues to be a great tool in the armamentarium of a vitreoretinal surgeon.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
It is essential to discuss the relevant anatomy to understand SB.
i) Tenon’s capsule is a layer of the fascia that envelops the globe. It extends from the limbus to the optic nerve. The extraocular muscles pierce the fascia and get covered by a glove-like sleeve of fascia that extends anteriorly untll the site of insertion. These sleeves connect to the intermuscular septum, which is another layer of fascia present between the recti insertions. The intermuscular septum has to be divided and stripped off to ensure easy passage of the exoplants and scleral suture placement. However, care is necessary to avoid damage to the recti and their ligaments. It must be remembered that these ligaments are functionally important for muscle action.
ii) Ora serrata inside the eye corresponds to the spiral of Tillaux outside, i.e., site of recti insertion.
iii) The vitreous base inside the eye lies around 2-3mm chord length posterior to the spiral of Tillaux outside.
iv) The superior oblique (SO) muscle travels under the superior rectus (SR) muscle and gets inserted 12-14mm posterior to the limbus. One vortex vein is usually present under the temporal edge of the SO insertion. The surgeon must be cautious while hooking the SR muscle to prevent engaging and/or damaging the SO tendon and the vortex vein.
v) The inferior oblique (IO) muscle passes under the lateral rectus (LR) muscle. The surgeon must be cautious while hooking the LR muscle to prevent engaging and/or damaging the IO tendon. The LR muscle can be hooked from the superior side to reduce the chances of inadvertently engaging and/or damaging the IO muscle.
vi) The scleral thickness varies according to the location. It is the thinnest, just behind the recti insertions. The thickness at the eyeball equator (5mm chord length posterior to the spiral of Tillaux) is approximately 1 mm.
vii) The anterior ciliary arteries exit the recti near their respective insertions. These vessels contribute to the formation of several vascular plexuses, which are responsible for the blood supply of the anterior segment of the eye. The scleral suture should be placed at least 1mm posterior to the spiral of Tillaux to prevent the exoplant from rubbing against the muscle at the site of their insertion, which can cause damage to these vessels and the anterior segment ischemia.
viii) There are seven vortex veins with at least one in each quadrant. They take a 2 to 4 mm intrascleral course in the posterior direction before exiting the sclera around 14 to 18 mm posterior to the limbus. Four of these vortex veins are present on either side of the vertical recti, i.e., 1,5,7 and 11 clock hours. Care must be exercised to prevent accidental damage to the vortex vein. These include hooking the muscles in a pre-equatorial plane, draining the SRF near the horizontal recti, and avoiding placement of scleral sutures in the region of the intrascleral portion of the vortex veins. The suture can be straddled if a vortex vein comes in the path of the suture.
Before understanding the principles of SB, let us first understand the pathophysiology of retinal break formation. Retinal breaks form due to the traction caused by vitreous on the retina. This traction results from a combination of forces, including the gravitational forces, inertial forces exerted by the vitreous during the ocular movements and blunt trauma, and contraction of the vitreous gel at sites of vitreoretinal adhesions secondary to posterior vitreous detachment (PVD). The sum of these forces acts either perpendicular or oblique to the retinal surface and leads to a retinal tear if this force exceeds the forces responsible for promoting retinal attachment. Once a break is created, the fluid currents guide fluid from the vitreous cavity into the subretinal space via the retinal tears.
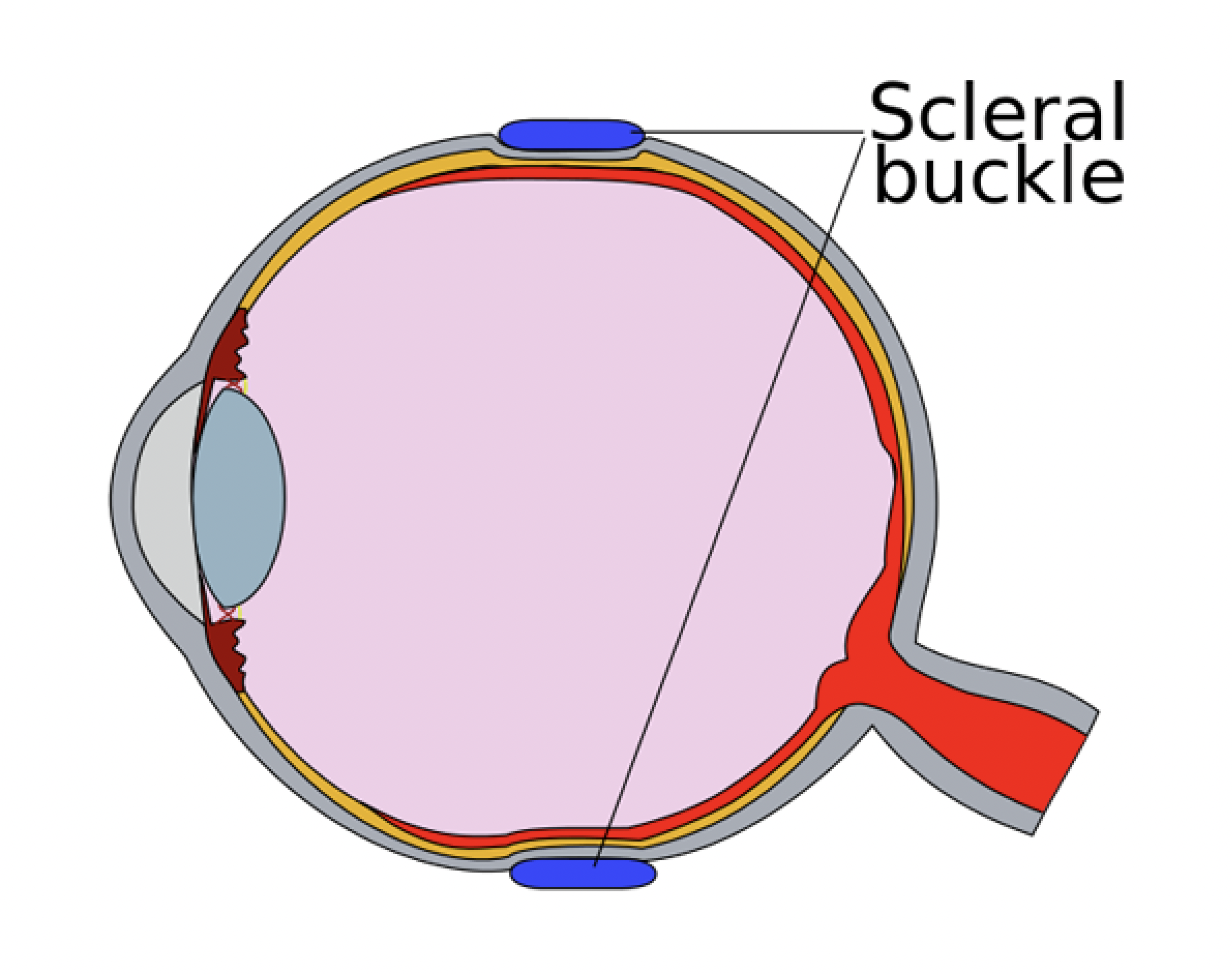
The term “buckle” literally means “deformation of a structure under stress.” SB produces indentation of the eyeball, thus deforming the inner scleral surface from concave to convex. This modifies the direction of the tractional forces responsible for break formation as well as disrupts the flow of fluid through the retinal break. Hence, the equilibrium responsible for maintaining retinal attachment is re-established. In addition, the 360-degree circumferential band creates a new ora serrata with reduced vitreous base diameter, which results in reduced traction (Hook’s law) and lesser chances of new break formation.
Indications
SB serves best in the following situations:
- Young patients
- Phakic eyes
- High myopia
- Absence of a PVD
- The absence of advanced proliferative vitreoretinopathy (PVR) changes. RRD with PVR grade C1 can be treated with SB
- RRD secondary to breaks anterior to the equator
- RRD secondary to retinal dialysis[9]
Contraindications
The various contraindications for SB include:
- Scleromalacia or severe scleral thinning
- Advanced PVR changes
- Dense vitreous hemorrhage or significant cataract which precludes fundus view
- Previous glaucoma surgery
- RRD secondary a giant retinal tear
- RRD secondary to retinal breaks posterior to the equator
Equipment
It is of paramount importance to know about the cryotherapy machine, the various silicon buckles, and the suture material used during SB surgery.
1. Chorioretinal adhesion, for the treatment of retinal break(s), can be achieved with either diathermy, cryotherapy, or laser photocoagulation.
i) Diathermy: Heat is generated by delivering a current via sclera at a frequency of 13.56 MHz. However, it cannot be used on the intact sclera as this can cause scleral shrinkage and necrosis. As scleral dissection is needed before applying diathermy, it is used with scleral implants.
ii) Laser photocoagulation can produce chorioretinal adhesions only in the presence of close contact between the NSR and the RPE. It is therefore used for treating retinal breaks in the attached retina only.
iii) Cryotherapy is the best modality for treating retinal break(s) during SB surgery. It is based on the Joule-Thompson principle, which states there is a sudden temperature drop when a gas is allowed to expand through a narrow aperture. This sudden temperature drop is used to produce chorioretinal adhesions. The intracellular water freezes to form intracellular crystals during the freezing phase, which causes mechanical injury. During the thawing phase, water and electrolytes get separated and cause a change in the intracellular pH, leading to the rupture of the cell membranes. The most commonly used gas for cryotherapy is nitrous oxide. It achieves a temperature of -89 degrees C when used at 600 psi pressure.
The cryotherapy machine consists of the following:
- Cryoprobe. The pressurized gas is made to expand through a small aperture near the tip of the probe. This expansion causes a sudden fall in the temperature, thus leading to the cryo effect. The tip of the probe is used to indent the sclera. The cryoprobe can be either straight or curved.
- A pedal to activate the cryoprobe
- The panel has sites for attaching the input and output tubings for the cryoprobe and a gauge to display the pressure that is built inside the cryoprobe
- Nitrous oxide cylinder, which is blue in color
The surgeon should always ensure that the cryoprobe is working and adequate pressure is being built before starting surgery.
2. The buckles used to produce scleral indent can be placed either on the intact sclera (exoplants) or inside a partial-thickness scleral tunnel made after scleral dissection (implants). As scleral implants are now of purely historical interest, we will describe only the exoplants.
The exoplants used currently are made of modified, cross-linked polydimethylsiloxane. The material offers multiple advantages, including water insolubility, low toxicity, high elasticity, and biological inactivity (neither supporting bacterial growth nor carcinogenic). The silicone explants can be either solid or spongy in consistency. The sponge explants contain closed air cells within their substance.
The exoplants can be classified into four types:
i) Bands and strips: They are used for encircling the eyeball all around, thus supporting the vitreous base. The most commonly used bands include band numbers 240 and 42. The 240 band is flat on both sides and measures 2.5mm in width and 0.6mm in height. The 42 band has one side flat (shiny looking) and the other side convex (dull looking) and measures 4.0mm in width and 1.25mm in height. While suturing, care must be taken that the flat side is placed towards the sclera.
ii) Implants and Wedges: They need scleral dissection for implantation and are rarely used now.
iii) Tires: They are used as a circumferential segmental buckle to support the break(s). The radius of curvature of these tires is similar to that of the globe. One of their sides has a groove that measures 2.5 mm. It is provided for fixing the 240 band for simultaneous 360-degree encirclage. The other side of these tires can be either convex or concave. The convex ones are used to manage RRD secondary to retinal dialysis as the convex curvature supports the vitreous base. The most commonly used tire is number 286, measuring 7.0 mm in width. The concave ones can further be of two types, i.e., symmetrical and asymmetrical. The symmetrical tires have their groove in the center and are used to manage RRD secondary to lattice(s) and atrophic hole(s). The most commonly used symmetrical tire is number 277, which measures 7.0mm in width. On the contrary, the groove in asymmetrical tires does not pass through the center. They are used to manage RRD secondary to horse-shoe tears (HST). The most commonly used asymmetrical tire is number 276, measuring 7.0mm in width. They are placed such that the slender side is positioned anteriorly under the muscle insertion while the hefty side is positioned posteriorly. This arrangement ensures that the anterior slender side of the tire doesn’t budge against the muscles, thus reducing the chances of anterior segment ischemia, while the posterior, hefty side supports the break(s).
iv) Sponges: Currently, they are mainly used as radial buckles to support posterior break(s). However, they can also be used as circumferential buckles. They are cylindrical in shape and need to be cut lengthwise into two halves with equal thickness, and the convex side is placed towards the globe to produce a high indentation. As the air cells get exposed to the surface after the sponge is cut, they can absorb fluid and harbor bacteria, which in turn can cause infection.
3. Sutures: The most commonly used suture for SB procedure is a 5.0 polybutylate-coated, braided, polyester suture with a 3/8 circle spatulated needle. The flat top and bottom of the needle ensuring that it dissects the lamellar structure of the sclera while passing. Half circle needle can be used to place sutures in the extreme posterior sclera.
Personnel
The surgical procedure is complicated and associated with a long learning curve. It is usually performed by ophthalmologists trained in performing vitreoretinal surgeries. Sagong et al. reported that a surgical experience of around 30 cases is required to achieve stable clinical results.[10] As it requires a lot of practice to master the skill, wet lab models have been prepared to practice various steps of the surgery. Pujari et al. described a model using mannequin-mounted goat eyes to practice suture placement.[11]
Preparation
The surgery is mostly performed under peribulbar anesthesia. A 50 to 50 mixture of lidocaine and bupivacaine, mixed with hyaluronidase, is administered to achieve excellent anesthesia and akinesia. General anesthesia can be used for young and uncooperative patients.
The patient’s head is placed with the neck slightly extended to achieve good surgical access. The skin in and around the orbit is cleaned with a 10% povidone-iodine solution. Topical 5% povidone-iodine drops are then instilled in the conjunctival sac. The skin is completely dried, and a sterile self-adhesive drape is placed.
Technique or Treatment
The surgical success depends on the accurate identification and localization of all the retinal breaks. A thorough pre-operative attempt should be made to search for all the breaks as any untreated break(s) can lead to surgical failure. It is reported that around 50% of the detachments have more than one break.
Lincoff established a set of rules for identifying the primary break(s) based on the topography of the detachment.[12][13]
- The primary break(s) in a total bullous RRD should be located between 11 to 1’o clock.
- The primary break(s) in a superior RRD with one side below the horizontal meridian should be present within 1.5 clock hours from 12’o clock on the same side. It should be remembered that inferior breaks never lead to bullous RD. The break in a bullous inferior RD should be located in the superior half with fluid tracking down from the peripheral retina. The track can be made prominent by asking the patient to lie with their head in a hyperextended position.
- The primary break(s) in a temporal/ nasal RD should be located within 1.5 clock hours from the highest point of detachment.
- The primary break(s) in a superior RD with another bullous component should be located at the edge of the bullous part.
- The primary break(s) in an inferior symmetrical RD should be located at 6’o clock.
- The primary break(s) in an asymmetric inferior RD should be located within 1.5 clock hours from the highest point of RD.
- RD localized to the posterior pole in high myopic eyes with posterior staphyloma is usually caused by a macular hole.
The surgical steps include:
1. Conjunctival peritomy: A 360-degree circumferential peritomy is performed with the help of non-toothed conjunctival forceps and a blunt-tipped Wescott scissor. It can be performed either at the limbus or 2 to 3 mm away from it. Two radial incisions are usually given at 3 and 9’o clock to prevent the conjunctiva from tearing while retracting it to get adequate scleral exposure. Blunt-tipped Steven’s tenotomy scissors are then inserted in the subtenon’s space between two adjacent recti in all four quadrants and spread wide open (without cutting) to strip the intermuscular septum.
2. Muscle bridling: Traction sutures are placed on all four recti to move and stabilize the eyeball for performing the various surgical maneuvers. This is performed by placing a muscle hook on the sclera posterior to the muscle insertion, passing it under the muscle in a circumferential fashion, and then bringing it anteriorly to engage the muscle. It should be ensured that no resistance is felt while hooking the muscle. Any resistance suggests that the hook is not in the correct plane. In this case, the hook should be removed and re-inserted; otherwise, the muscle can get split. A braided 2.0 silk suture is used to bridle the muscles. Care must be exercised to avoid muscle injury and scleral perforation while passing the suture under the muscle. This can be achieved with the help of a modified muscle hook with a threading eyelet at its tip or passing an inverse suture in a tangential manner (instead of radial). The suture is then thrown into two knots. One knot is tied close to the muscle and used for stabilizing the muscle, while the other is tied close to the open end of the suture and used to pull the traction suture. The surgeon should ensure that the full thickness of muscle is included in the traction suture as incompletely included muscle can get ruptured while pulling the traction suture. The attachments of the tenon’s capsule to the recti and the sclera are then stripped off gently.
The usual sequence for muscle bridling is the medial rectus (MR), followed by the inferior rectus (IR), the LR, and finally, the SR muscle. Both the horizontal can be pulled inferiorly to get a better exposure while bridling the SR muscle. The heart rate should constantly be monitored while pulling the recti as it can trigger the oculocardiac reflex, leading to bradycardia. Any fall in the heart rate warrants immediate muscle release and intravenous atropine if bradycardia persists.[14]
3. Inspection: The eyeball should be inspected for the presence of any areas of scleral thinning or anomalous vortex veins.
4. Break(s) localization: Indirect ophthalmoscopy is performed to localize the previously identified retinal breaks. The breaks are then marked on the scleral surface with the help of gentle diathermy, heated needle head, marker pen, cryoprobe, or other specially designed localizers. A small break is marked with a single spot in the center, while a large HST is marked with three spots, i.e.one at the posterior margin of the break and the other two at each end of the horns. Retinal dialysis is marked with one spot at each end and one at the posterior extent of its mid-point.
The presence of bullous RD can make precise identification of the anteroposterior location of break(s) difficult. Peyman et al. proposed that SRF drainage can make break localization easier. This technique is called as D-ACE sequence (Drainage-Air, Cryo, Encirclage).[15]
5. Treatment of retinal breaks: The cryo-spots should be given contiguously all around the retinal break(s). An immediate tissue reaction is seen on applying cryotherapy in the form of retinal whitening, which progressively expands outwards in all directions. The treatment is continued for 2 or 3 seconds after a distinct whitening of the NSR is observed. In the case of a bullous RD, an orangish hue within the indented choroid can be taken as the endpoint. The surgeon should ensure that the scleral indentation produced around the break is due to the cryoprobe tip and not the shaft, as improper technique can lead to inadvertent posterior freeze. The shaft of the cryoprobe can be covered with a sleeve to prevent inadvertent cryo-treatment to the adjacent areas.
The tip of the cryoprobe should be allowed to thaw completely before attempting removal to avoid complications like choroidal hemorrhage or scleral avulsion. Care must be exercised to avoid damaging the vortex veins while moving the probe from one point to another. Excess cryotherapy should be avoided as it can cause RPE pigment dispersion, leading to ERM formation and proliferative vitreoretinopathy (PVR) changes.[16][17]
It should be remembered that the breaks in the attached retina should be treated first. While treating a break, its anterior margin should be treated first. Suspicious lesions should always be treated. In fact, retinal breaks tend to become prominent after cryo-treatment as the red-colored breaks become better visible in the presence of white background.
6. Placement of the exoplants: Exoplants should be placed to support the retinal breaks. The tires should extend 30 degrees or 1 clock hour on either side of the tear and 1 or 2 mm beyond the posterior margin of the posterior-most break.
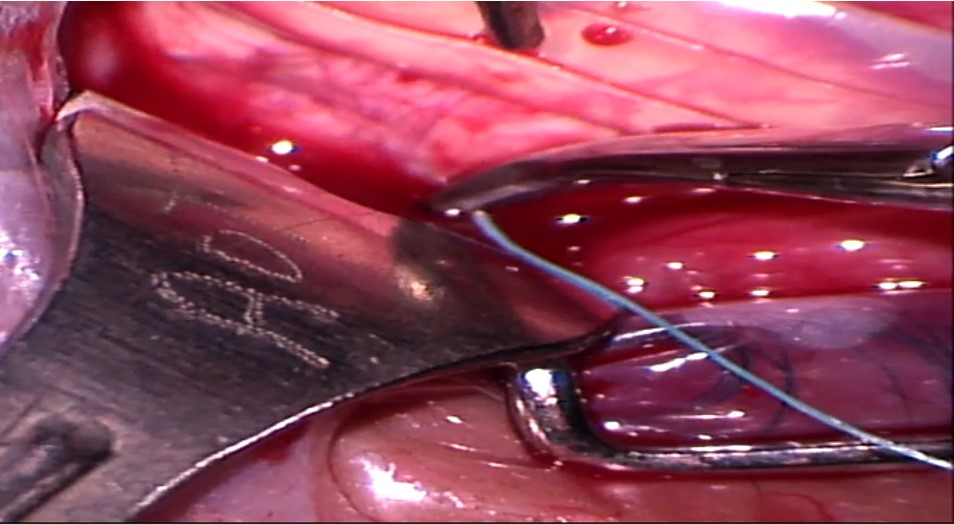
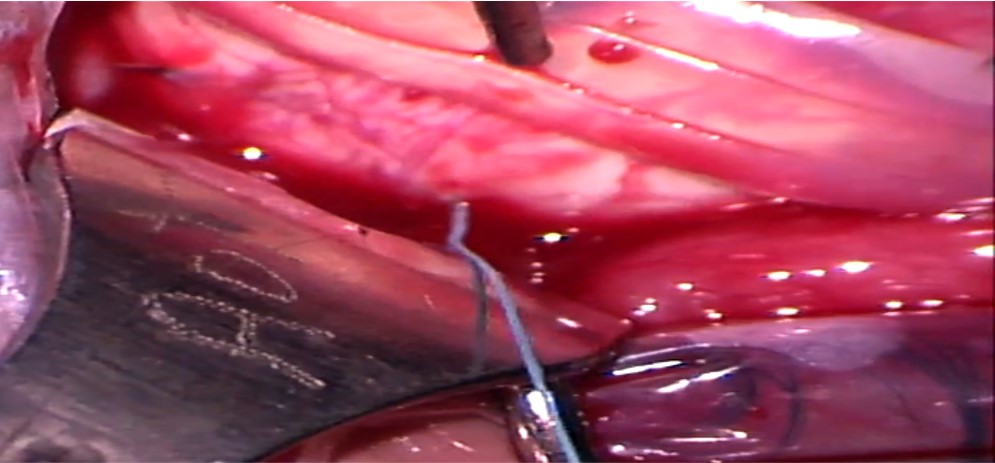
7. Placement of scleral sutures: The sutures can be placed either before or after placing the explants. Adequate exposure of the scleral surface is extremely crucial for placing the scleral sutured. This is achieved by pulling the traction sutures attached to the adjacent recti at an obtuse angle as well as retracting the conjunctiva. For example, the SR is pulled nasally, and LR is pulled inferiorly to place a suture in the supero-temporal quadrant. Laxity of the traction sutures while suturing can lead to scleral perforation.
Mattress sutures with an intrascleral length of 4 to 5 mm are placed parallel to the long axis of the explant in a square configuration at approximately half the scleral thickness. Irregular or nonparallel sutures produce a lesser indentation effect. The tip of the needle is placed on the scleral surface such that its tangent is parallel to the scleral surface. Gentle downward pressure is applied to create a small scleral indent, and the needle is advanced downwards until the desired depth is achieved. The needle is then advanced forward without applying any downward pressure, making sure that it is visible throughout its course. After an adequate intrascleral length is achieved, the needle is taken out by applying slight upward pressure. The tip of the needle can then be grasped to remove the needle along its curve. The needle should be removed gently, as applying too much downward pressure on the heel of the needle can lead to scleral perforation. The entrance and exit wounds should not be too shallow as they can partially tear out under tension.
While placing sutures around a circumferential explant, the posterior bite of the suture is taken first. Similarly, while placing sutures around a radial explant, the anteroposterior bite is taken first. While placing sutures in the inferior half, the posterior bite is taken in a forward direction, and the anterior bite is taken in a reverse manner. While placing sutures in the superior half, the surgeon has to fold their arm such that his hand faces up toward them and their elbow faces down toward the patient’s feet. Both the bites are then taken in a reverse manner.
The distance between the suture bites should be greater than the width of the explant so that the sclera partially envelops the explant, thus creating an indent. The suture should never lie between the explant and sclera as it increases the chances of scleral erosions.
i) Radial explant: The width of the sutures should be at least 1.5 times that of the explant. For example, sutures for placing a 5mm sponge should be placed at least 8mm apart. The assistant needs to tightly pull both the ends of the sponge during suture placement.
ii) Circumferential explant: The anterior bite is placed at least 1mm posterior to the muscle insertion. The suture bites are placed 1 to 2 mm wider than the buckle width on either side. For example, the posterior bite for placing tire number 276 or 277 is placed around 11 mm (1+1.5+7+1.5) posterior to the muscle insertion.
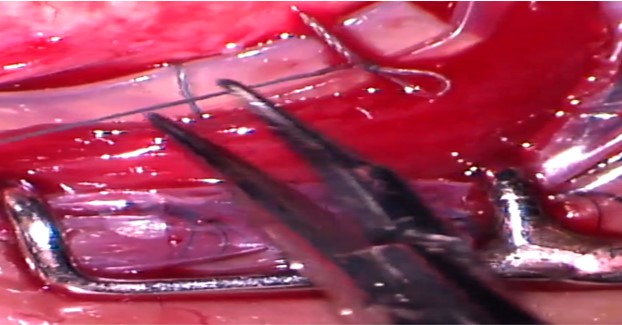
A Watzke sleeve or a clove hitch knot (CHK) is used to suture the encircling band end-to-end. Segmental buckles (without 360 degrees encirclage) have been found to be effective in eyes with localized shallow RD caused by a single break or retinal dialysis or multiple breaks localized within 1 or 2 clock hours.[18]
8. SRF drainage brings the retinal break(s) closer to the RPE. This reverses the fluid flow and provides volume for the buckling element. The various indications for SRF drainage include bullous and chronic RD, high myopia, aphakia, inferior retinal break(s), old patients with poor RPE function, inability to localize any retinal break, and intolerance to high IOP (glaucoma).
The following points should be kept in mind while selecting the site for SRF drainage:
- Presence of sufficient SRF
- Avoid vertical meridian to avoid damaging the vortex veins
- Prefer location at or slightly anterior to the equator to reduce the chances of choroidal bleed as the choroid is less vascular in this region
- Prefer the nasal quadrant as any accidental subretinal bleed is less likely to track under the macula
- Prefer under the explant as any inadvertent retinal break gets supported
- Avoid area treated with cryotherapy to prevent choroidal hemorrhage secondary to the choroidal congestion
- Avoid areas with a large break to prevent vitreous loss
There are two major techniques for SRFD.
i) “Cut down technique”: The proposed site is treated with moderate cautery. A 3 mm scleral incision is then made deep enough to make a small knuckle of the dark choroid bulge into the incision. This knuckle of the choroid is treated with diathermy to coagulate the choroidal vessels. Pressure from the globe is completely relieved, and the choroidal knuckle is slowly penetrated with a needle for about 2 mm until SRF starts draining.
ii) Trans-scleral needle drainage: A 26G needle is used to perforate the sclera. The needle is slowly advanced until the resistance gives away, and SRF starts draining. This is achieved roughly when the hub of the needle penetrates.[19] Using a 26G needle bent like a cystitome used for capsulorhexis has also been described. This arrangement adds a safety feature as the shaft acts as a guard to prevent the tip from entering beyond 2 mm.[20]
Once SRF drainage starts, further drainage is facilitated by pulling the traction sutures to increase the intraocular pressure and pressing the sclera anterior to the drainage site. The endpoint of drainage is indicated by the appearance of pigment particles. If drainage stops pre-maturely, the drainage site should be inspected with indirect ophthalmoscopy to look for any retinal incarceration, which appears like star-shaped folds radiating from the drainage site. No attempt should be made to reposition the incarcerated retina as this can cause greater damage. Instead, another retinotomy should be made.
The eye usually becomes soft after SRF drainage and needs to be reformed with an intravitreal injection of saline or air. The assistant should not release the traction sutures until the lost volume is replaced, as hypotony can cause suprachoroidal effusion or hemorrhages, hyphaema, and pupillary constriction.
9. Adjustment of the buckle height: The loose scleral sutures are tightened to achieve an adequate buckle height. The knots can be rotated posteriorly to prevent conjunctival erosions. Excessive tightening of the encircling band should be avoided as this can lead to “fish-mouthing.”
10. Final examination: Retina should be examined to check the optic disc perfusion, the buckle height, the retinal breaks, and the site of SRF drainage. A pale disc or arterial pulsations warrant paracentesis to reduce the intraocular pressure
11. Closure of tenon and conjunctiva: The tenon’s capsule and conjunctiva are irrigated with a balanced salt solution to remove the blood clots and other debris as well as broad-spectrum antibiotics (gentamycin, 40 mg/mL). It is better to suture them in two different layers. A complete closure prevents complications like buckle exposure and buckle infection.
12. Documentation: All details regarding the break(s), site of SRF drainage, the type of explant used, the position of scleral sutures and Watzke sleeve or CHK, and any intraoperative complications should be correctly documented for future reference or re-surgery.
Complications
Several intra- and postoperative complications are associated with SB surgery.
1. The various steps-wise intra-operative complications include:
i) During anesthesia: Peribulbar anesthesia can cause inadvertent globe perforation in around 0.06 to 0.13% of cases.[21] Other rare complications include retrobulbar hemorrhage, optic neuropathy, central retinal artery occlusion, diplopia secondary to muscle injury, and respiratory arrest due to accidental intradural injection leading to brainstem anesthesia. Severe retrobulbar hemorrhage may warrant a lateral canthotomy.
ii) During muscle bridling: The complications associated with this step include ocular cardiac reflex and resultant bradycardia, muscle rupture, lost muscle, damage to the vortex veins, and inadvertent scleral perforation. The prevention and management of these complications have been discussed in the technique section.
iii) During break localization: The complications associated with this step include damage to the vortex vein and scleral perforation. A torn vortex vein has to be cauterized immediately.
iv) Placement of scleral sutures can be complicated by scleral perforation and iatrogenic retinal breaks. An inadvertent scleral perforation can lead to SRF drainage, retinal break, vitreous loss, subretinal or sub-choroidal hemorrhage, and hypotony. An inadvertent perforation is suspected if SRF, blood, or pigments appear on the scleral surface while placing the sutures. It is crucial for the surgeon not to panic. The suture should be removed, and the intraocular pressure (IOP) should be checked. An intravitreal saline injection may be needed if the eyeball is becoming hypotonus. The retina is then examined with indirect ophthalmoscopy at the site of perforation. Any iatrogenic break warrants cryo-treatment and extension of the explant to support the break. Subretinal or sub-choroidal hemorrhage can be controlled by increasing the IOP by pulling the traction sutures. The eyeball is then placed in a position to prevent subfoveal migration of blood. Suture placement can be continued after managing the complications.
v) SRF drainage can be complicated by damage to vortex vein, choroidal hemorrhage, retinal incarceration, retinal perforation, vitreous gel incarceration, vitreous hemorrhage, hypotony, and serous and hemorrhagic choroidal detachment. The appearance of blood at the drainage site indicates choroidal hemorrhage and can be managed by increasing the IOP by pulling the traction sutures.
2. The various early postoperative complications include lid edema, conjunctival chemosis, corneal edema, impaired function of the recti, anterior segment ischemia, open- and closed-angle glaucoma, open retinal breaks, recurrent retinal detachment, serous and hemorrhagic choroidal detachment, and persistent SRF.
i) Anterior segment ischemia: The patient may present with extreme pain and decreased vision. The signs include corneal edema (epithelial and stromal), corneal endothelium precipitates, shallow anterior chamber, marked uveitis, lens opacification, and an excessive buckle height (vitreous cavity may appear like a dumb-bell shape). Mild cases may respond to topical steroids and cycloplegics. However, severe cases require immediate release of the encircling band. Untreated eyes may lead to phthisis bulbi. Patients with sickle cell disease are at increased risk of developing this complication. They may benefit from pre-operative exchange transfusion.
ii) Angle-closure glaucoma may occur secondary to ciliary body detachment. It can be treated with topical steroids and cycloplegics. However, choroidal drainage with anterior chamber reformation may be needed if the anterior chamber doesn’t form within seven days.
iii) Open-angle glaucoma: The most common cause is steroid response.
iv) Persistent SRF: In a few cases, SRF may persist for up to 3 months despite the closure of all the breaks. Such eyes can be observed safely.
v) Recurrent retinal detachment: Persistent SRF with an open break and increasing SRF need further evaluation and appropriate treatment. The various causes of recurrent retinal detachment include:
- Inadequate buckle: An unchanged pattern of RD may be due to low buckle height. This may need revision surgery and the addition of extra sutures.
- Misplaced buckle: Sometimes recurrent RD may be caused by an untreated tear lying posterior to the buckle indent. This can be managed by adding a radial explant to support the break.
- Missed retinal break: A changed pattern of RD may indicate an undetected break. Such eyes may benefit from revision surgery to treat that break.
- Fishmouthing: Persistent SRF with retinal folds on the buckle is suggestive of fish mouthing of the retinal break. This warrants intravitreal injection of expansile gas.
3. The various late postoperative complications include diplopia, refractive change, buckle extrusion, buckle infection, transscleral erosion, cystoid macular edema (CME), epiretinal membrane (ERM), and PVR changes.
i) Diplopia may occur due to mechanical muscle restriction or ischemia. Such patients may initially be managed with prisms. However, muscle surgery with or without buckle removal may be required if symptoms persist.
ii) The refractive changes occur secondary to the surgery-induced changes in the shape of the eyeball. The main risk factor for postoperative astigmatism is the use of radial elements, while a myopic shift may occur secondary to an increase in the axial length due to circumferential elements or anterior displacement of the lens.[22][23]
iii) Patients with buckle extrusion present with pain, redness, and discharge. Such patients need buckle explantation.
iv) Buckle intrusion was a common complication in the era of intrascleral implants. It can lead to recurrent RD, hypotony, vitreous hemorrhage, and endophthalmitis. While the asymptomatic patients can be safely observed, the presence of symptoms warrants buckle removal with or without vitrectomy. The scleral defect can be treated with cyanoacrylate glue, scleral imbrication, or other scleral patching techniques.
v) ERM is the most common cause of visual loss after a successful SB surgery. The reported incidence of CME and ERM is 5.6 to 43.0% and 7.7 to 18.0%, respectively.[24] They occur due to the inflammation-induced secondary to cryopexy.
Clinical Significance
SB is an elegant and safe extraocular surgery, especially because it doesn’t disturb the vitreous. It doesn’t require the use of sophisticated machines and is significantly less expensive than a vitrectomy.[25] Although vitrectomy is a better option for eyes with advanced PVR changes, anatomical and functional results produced by SB in primary uncomplicated RRD are comparable. The need to master the surgery becomes even more important as the incidence of myopia is rising sharply, and the incidence of young patients developing RRD is expected to follow the same trend.[26]
Enhancing Healthcare Team Outcomes
SB is an effective option for the management of patients with fresh RRD. Several patients initially seek the opinion of an optometrist for decreased vision. An early referral can ensure a good outcome.
Nursing, Allied Health, and Interprofessional Team Interventions
Unlike vitrectomy, the success of SB surgery is highly dependent on the skill of the assistants. The operating room staff should be well versed in identifying the various buckle elements. The role of assistants in rotating and fixing the eyeball to provide adequate scleral exposure for placing scleral sutures and SRF drainage is immense and has been discussed in detail in the manuscript.
Nursing, Allied Health, and Interprofessional Team Monitoring
The assistants must be aware of the potential complications that can occur during the procedure. They should be able to identify the complication and get ready with the instrumentation necessary for further management. In particular, special attention should be paid to monitoring vitals during muscle handling and signs of scleral perforation while placing scleral sutures.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Mitry D, Charteris DG, Fleck BW, Campbell H, Singh J. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. The British journal of ophthalmology. 2010 Jun:94(6):678-84. doi: 10.1136/bjo.2009.157727. Epub 2009 Jun 9 [PubMed PMID: 19515646]
Level 1 (high-level) evidenceWolfensberger TJ. Jules Gonin. Pioneer of retinal detachment surgery. Indian journal of ophthalmology. 2003 Dec:51(4):303-8 [PubMed PMID: 14750617]
Albert DM, Gloor BP, McPherson AR. Why Jules Gonin Achieved His "Audacious Goal Initiative"—and Why He Is a Model for the Present Day. Ophthalmology. 2015 Oct:122(10):1955-7. doi: 10.1016/j.ophtha.2015.05.037. Epub [PubMed PMID: 26398047]
LINCOFF HA, BARAS I, MCLEAN J. MODIFICATIONS TO THE CUSTODIS PROCEDURE FOR RETINAL DETACHMENT. Archives of ophthalmology (Chicago, Ill. : 1960). 1965 Feb:73():160-3 [PubMed PMID: 14237781]
Sodhi A, Leung LS, Do DV, Gower EW, Schein OD, Handa JT. Recent trends in the management of rhegmatogenous retinal detachment. Survey of ophthalmology. 2008 Jan-Feb:53(1):50-67. doi: 10.1016/j.survophthal.2007.10.007. Epub [PubMed PMID: 18191657]
Level 3 (low-level) evidenceHeimann H, Bartz-Schmidt KU, Bornfeld N, Weiss C, Hilgers RD, Foerster MH, Scleral Buckling versus Primary Vitrectomy in Rhegmatogenous Retinal Detachment Study Group. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: a prospective randomized multicenter clinical study. Ophthalmology. 2007 Dec:114(12):2142-54 [PubMed PMID: 18054633]
Level 1 (high-level) evidenceHeimann H, Hellmich M, Bornfeld N, Bartz-Schmidt KU, Hilgers RD, Foerster MH. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment (SPR Study): design issues and implications. SPR Study report no. 1. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2001 Aug:239(8):567-74 [PubMed PMID: 11585312]
Level 1 (high-level) evidenceSaw SM, Gazzard G, Wagle AM, Lim J, Au Eong KG. An evidence-based analysis of surgical interventions for uncomplicated rhegmatogenous retinal detachment. Acta ophthalmologica Scandinavica. 2006 Oct:84(5):606-12 [PubMed PMID: 16965489]
Park SW, Lee JJ, Lee JE. Scleral buckling in the management of rhegmatogenous retinal detachment: patient selection and perspectives. Clinical ophthalmology (Auckland, N.Z.). 2018:12():1605-1615. doi: 10.2147/OPTH.S153717. Epub 2018 Aug 30 [PubMed PMID: 30214145]
Level 3 (low-level) evidenceSagong M, Chang W. Learning curve of the scleral buckling operation: lessons from the first 97 cases. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2010:224(1):22-9. doi: 10.1159/000233232. Epub 2009 Aug 13 [PubMed PMID: 19684425]
Level 2 (mid-level) evidencePujari A,Kumar S,Markan A,Chawla R,Damodaran S,Kumar A, Buckling surgery on a goat's eye: A simple technique to enhance residents' surgical skill. Indian journal of ophthalmology. 2019 Aug; [PubMed PMID: 31332123]
Lincoff H, Gieser R. Finding the retinal hole. Archives of ophthalmology (Chicago, Ill. : 1960). 1971 May:85(5):565-9 [PubMed PMID: 5087597]
Level 2 (mid-level) evidenceLincoff H, Kreissig I. Finding the retinal hole in the pseudophakic eye with detachment. American journal of ophthalmology. 1994 Apr 15:117(4):442-6 [PubMed PMID: 8154524]
Level 3 (low-level) evidenceBOSOMWORTH PP, ZIEGLER CH, JACOBY J. The oculo-cardiac reflex in eye muscle surgery. Anesthesiology. 1958 Jan-Feb:19(1):7-10 [PubMed PMID: 13498375]
Gilbert C, McLeod D. D-ACE surgical sequence for selected bullous retinal detachments. The British journal of ophthalmology. 1985 Oct:69(10):733-6 [PubMed PMID: 4052357]
Jaccoma EH, Conway BP, Campochiaro PA. Cryotherapy causes extensive breakdown of the blood-retinal barrier. A comparison with argon laser photocoagulation. Archives of ophthalmology (Chicago, Ill. : 1960). 1985 Nov:103(11):1728-30 [PubMed PMID: 4062641]
Level 3 (low-level) evidenceBonnet M, Fleury J, Guenoun S, Yaniali A, Dumas C, Hajjar C. Cryopexy in primary rhegmatogenous retinal detachment: a risk factor for postoperative proliferative vitreoretinopathy? Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1996 Dec:234(12):739-43 [PubMed PMID: 8986445]
Shanmugam PM, Ramanjulu R, Mishra KCD, Sagar P. Novel techniques in scleral buckling. Indian journal of ophthalmology. 2018 Jul:66(7):909-915. doi: 10.4103/ijo.IJO_136_18. Epub [PubMed PMID: 29941729]
Azad R, Kumar A, Sharma YR, Rajpal. Modified needle drainage. A safe and efficient technique of subretinal fluid drainage in scleral buckling procedure. Indian journal of ophthalmology. 2004 Sep:52(3):211-4 [PubMed PMID: 15510460]
Level 1 (high-level) evidenceAgarwal MK. Modified needle drainage. A safe and efficient technique of subretinal fluid drainage in scleral buckling procedure. Indian journal of ophthalmology. 2004 Dec:52(4):342-3; author reply 343 [PubMed PMID: 15693336]
Level 3 (low-level) evidenceBabu N, Kumar J, Kohli P, Ahuja A, Shah P, Ramasamy K. Clinical Presentation and Management of Eyes with Globe Perforation during Peribulbar and Retrobulbar Anesthesia: A Retrospective Case Series. Korean journal of ophthalmology : KJO. 2022 Feb:36(1):16-25. doi: 10.3341/kjo.2021.0090. Epub 2021 Nov 8 [PubMed PMID: 34743491]
Level 2 (mid-level) evidenceRubin ML. The induction of refractive errors by retinal detachment surgery. Transactions of the American Ophthalmological Society. 1975:73():452-90 [PubMed PMID: 1246813]
Thelen U, Amler S, Osada N, Gerding H. Success rates of retinal buckling surgery: relationship to refractive error and lens status: results from a large German case series. Ophthalmology. 2010 Apr:117(4):785-90. doi: 10.1016/j.ophtha.2009.12.016. Epub 2010 Mar 5 [PubMed PMID: 20207006]
Level 2 (mid-level) evidenceSabates NR, Sabates FN, Sabates R, Lee KY, Ziemianski MC. Macular changes after retinal detachment surgery. American journal of ophthalmology. 1989 Jul 15:108(1):22-9 [PubMed PMID: 2750831]
Level 1 (high-level) evidenceSeider MI, Naseri A, Stewart JM. Cost comparison of scleral buckle versus vitrectomy for rhegmatogenous retinal detachment repair. American journal of ophthalmology. 2013 Oct:156(4):661-6. doi: 10.1016/j.ajo.2013.05.019. Epub 2013 Jul 20 [PubMed PMID: 23876865]
Dolgin E. The myopia boom. Nature. 2015 Mar 19:519(7543):276-8. doi: 10.1038/519276a. Epub [PubMed PMID: 25788077]