Introduction
An osmometer is a device used in clinical laboratories for measuring the concentration of particles in a solution, known as the osmolar concentration. This quantity can be expressed as osmolality (in mmol/kg) or osmolarity (in mmol/L). Clinical laboratories typically measure osmolality, which is considered more precise as weight is temperature-independent.[1] In laboratory analysis, a dissolved substance is referred to as a solute, and the substance in which the solute is dissolved is referred to as a solvent. A solute dissolved into a solvent creates a solution. The unit for osmolar concentration or osmolality is milliosmole (abbreviated mOsm or mOsmol). For nonelectrolytes, 1 mmol equals 1 mOsm, whereas for electrolytes, the number of particles in a solution depends on the electrolyte's dissociation.[2][3]
When a solute is dissolved into a solvent, 4 colligative properties of the solvent change. These properties include:
- Osmotic pressure
- Vapor pressure
- Boiling point
- Freezing point
These properties are tied to osmolality and depend on the solution's number of solute particles. Dissolving a solute into a solvent generally increases the osmotic pressure and boiling point and decreases a solution's vapor pressure and freezing point. Although any of the 4 colligative properties could be used to determine the osmolality of a solution, technical limitations restrict the commercial measurement of osmolality to freezing point, vapor pressure, and membrane osmometers. The freezing point depression method is commonly employed in clinical laboratories due to its high accuracy and ease of execution.[4]
The general principle of freezing point depression osmometers involves the relationship between the number of moles of dissolved solute in a solution and the change in freezing point. For example, 1 mole of a dissolved solute reduces the freezing point of water by approximately 1.86 °C (~35.35 °F).[5] Therefore, freezing and thawing a solution and comparing the relative change in freezing point to that of a pure solvent allows for determining the approximate number of moles of dissolved solute in a sample. In clinical laboratory analysis, most samples are in water-based aqueous solutions, and the reference for solutions is generally pure water.
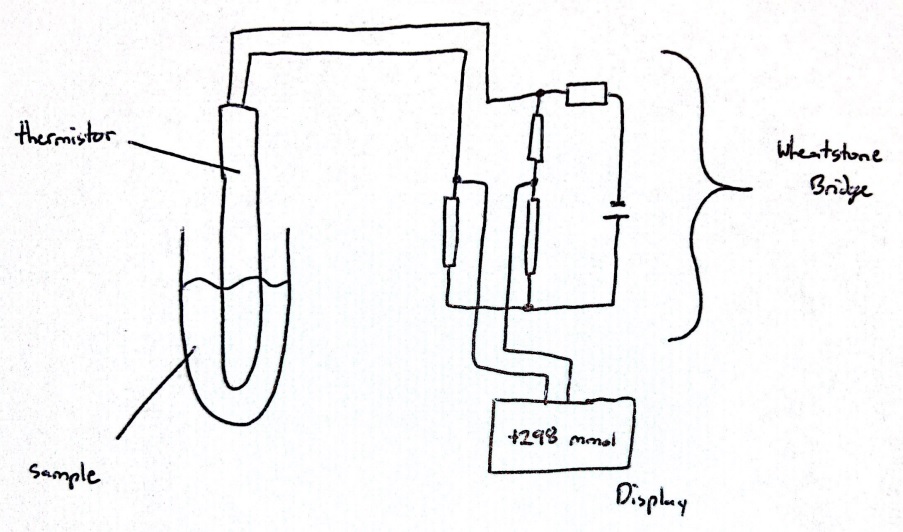
The setup of a freezing point depression osmometer includes a temperature-controlled bath to allow for sub-freezing temperatures, a thermistor probe connected to a Wheatstone bridge circuit to measure the temperature of a clinical sample, and a thermistor readout circuit, which represents a combination of a galvanometer and a potentiometer (see Image. Circuit Diagram of an Osmometer).[5][6] Although many osmometers are standalone tabletop devices, there are computerized, automatic, and handheld osmometers available (see Image. Computerized Osmometer).[7] Vapor pressure osmometers determine the osmolality of a solution by measuring the voltage difference between 2 thermistors. One thermistor is exposed to a sample solution, whereas the other is exposed to only the pure solvent corresponding to the sample solution. This method establishes a correlation between voltage and solute concentration, enabling the determination of the sample's osmolality.[8][9]
On the other hand, membrane osmometers measure the flow of solvent, often water, from a pure solvent container across a semipermeable membrane into a solution containing a solute and the same solvent. The flow across the semipermeable membrane can be measured as the osmotic pressure of a sample and is related to the concentration of solute in the sample solution.[1][10] The semipermeable membrane in this device only allows the flow of solvent and blocks the flow of dissolved particles. These osmometers are limited in their application by the range of osmolality they can measure and the membrane material with which the semipermeable membrane can be made.
Alternative methods to osmometers can be used to measure osmolality, including electrical conductivity and specific gravity.[11] Specific gravity was utilized before osmometers were practical to measure the osmolality of urine, utilizing various instruments such as refractometers, which measure the refraction of light through a fluid, or hydrometers. The applicability and accuracy of these methods besides osmometry for determining a sample's osmolality depend on the specific method and the sample.[8][9][10][11]
Specimen Requirements and Procedure
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Specimen Requirements and Procedure
Specimen requirements vary between the various commercially available osmometers and are included in the respective machine's instruction manual and laboratory protocols for specimen preparation. Acceptable specimens, in general, include serum, heparinized plasma, tears, sweat, and urine.[1][12] Some osmometers allow for the use of gel tubes to hold samples. In general, urine does not require the addition of preservatives during collection. Specimens should undergo centrifugation before analysis to eliminate any particulate matter. While dilution is unnecessary for many machines, it may be required for certain types of samples.[13][14]
The stability of different samples varies, with some general guidelines as follows:
- Unspun serum/plasma (room temperature or refrigerated at 2 to 8 °C): 3 hours
- Separated serum/plasma (room temperature or refrigerated at 2 to 8 °C): 48 hours
- Urine (room temperature): 24 hours
- Urine (refrigerated or frozen): 7 days
The general procedure for osmometry begins by employing a properly calibrated osmometer. A full calibration of osmometers is required at least every 6 months. Calibration may also be necessary following a part replacement or service of the instrument or if the quality control (QC) reading is repeatedly out of range. For QC purposes, laboratories are typically mandated to conduct 2 controls at 2 different concentrations daily or with each batch of samples. Acceptable controls are generally within ±2 SD from the mean. The institution's QC policy, protocol, and instrument user manual should be followed to ensure accurate sample analysis.
Testing Procedures
General Osmometry Procedure
The testing procedure for specific osmometers varies and is detailed in the user manual for each device. However, a general procedure outline is as follows:
- Enter or scan the sample ID.
- Load the sample to be tested into the osmometer. For freezing point depression osmometers, a small sample amount is drawn in a sampler with a plunger. The sampler tip is inserted into the instrument's sample port, and the analysis begins. Loading the sample into the osmometer may start the analysis automatically. Other systems may require the lab technologist to initiate the process by pressing a button.
- Record the osmolality of the sample.
- Once the test is completed, remove the sampler from the osmometer and discard the sampler tip. Ensure that both the sampler and sample chamber are thoroughly cleaned.
Interfering Factors
Freezing point depression osmometers have a few limitations. Particulate matter can cause premature crystallization of samples, which can be avoided by centrifuging blood and urine samples or filtering urine samples. The presence of air bubbles in a sample may also result in the inadequate freezing of samples. Test samples allowed to evaporate, non-aqueous, or highly viscous may not freeze well and yield an inaccurate result. High concentrations of substances, including ethanol, methanol, acetone, paraldehyde, trichloroethane, or propylene glycol, may not freeze reliably, leading to inaccurate results.
Finally, failure to clean the sample container after sample analysis can lead to the subsequent osmometer measurement being skewed by the previous sample. Vapor pressure osmometers are limited by a lack of precision compared to freezing point depression osmometers. Vapor pressure osmometers cannot be used to measure the osmolality of solutions containing volatile solutes.[1]
Results, Reporting, and Critical Findings
Certain sample types are more prone to inter-sample variability and may require 2 or more replicates. For instance, volatile samples may need to be analyzed in duplicate and the results should agree within a certain range of mOsm. The osmometer's specific protocol and user manual should be followed. The measurement range of osmometers varies depending on the instrument and sample being used. However, a common reportable range for freezing point depression osmometers is 0 to 2000 mOsm/kg. The normal reference range for serum is 275 to 295 mOsm/kg, and urine is 300 to 750 mOsm/kg, although these ranges may vary.[15][16]
Clinical Significance
Serum Osmolality
Changes in serum osmolality can be useful in differentiating the underlying causes of various electrolyte and acid-base balance disorders.[1][3] Serum osmolality is an important initial test employed in investigating hyponatremia. Hyponatremia in the context of normal serum osmolality is termed pseudohyponatremia. This condition is caused by the electrolyte exclusion effect when sodium is measured by the indirect ion-selective electrode method in patients with increased levels of lipids (hypertriglyceridemia) or total protein. Hyponatremia in the context of elevated serum osmolality (>295 mOsm/kg) is most commonly caused by elevated levels of serum glucose, or it may also occur when osmotically active substances such as mannitol, radiographic contrast agents, and glycine (surgical irrigant solutions) are administered. Serum osmolality can be directly measured using osmometers, and it can also be calculated by the measurements of osmotically active substances in the serum. Many formulas for calculating osmolality exist, among which the Smithline-Gardner formula stands out as one of the most commonly employed and straightforward.
Osmolality (mOsm/kg) = 2×Na (mmol/L) + glucose (mg/dL)/18 + urea (mg/dL)/2.8 [3][17]
The difference between the calculated and measured osmolality is known as the osmol gap, also known as the osmolal or osmolar gap. A normal osmol gap is 2 or less, although according to some sources, it is greater than 10 mOsm/kg in the clinical environment.[1][18]
An elevated serum osmol gap can be found in the following clinical scenarios or disease states:
- Pseudohyponatremia: This condition occurs when a low reported serum sodium concentration (<135 mEq/L) is observed in the presence of normally measured serum osmolality. This discrepancy can be caused by hyperlipidemia or hyperproteinemia, where these molecules comprise an increased portion of the serum sample, resulting in a falsely decreased sodium reading. Based on the aforementioned Smithline-Gardner formula, the falsely decreased sodium concentration decreases the calculated serum osmolality, resulting in an elevated osmol gap compared to the normal range measured osmolality.[1][19]
- Exogenous substances: Ingestion of toxic alcohols such as ethanol, methanol, propylene glycol, and acetone can cause elevated osmol gap. The presence of an elevated osmol gap and suspicion of volatile alcohol poisoning often prompt further investigation by a volatile screen.[20] In addition, osmotically active medications such as mannitol or glycerol are sometimes used to manipulate the osmol gap to drive fluid shifts within the body. For instance, mannitol shifts fluid from the brain into the blood to relieve increased intracranial pressure. The increased solute in the blood after mannitol ingestion results in an osmotic gradient moving fluid across the blood-brain barrier.[21] An important but often overlooked cause of exogenous substances elevating a patient's osmol gap is ingesting propylene glycol from medications using this molecule as a vehicle, such as nitroglycerin, lorazepam, or etomidate.[18]
- Endogenous substances: Metabolic states such as diabetic ketoacidosis or alcoholic ketoacidosis increase the concentration of ketones in the bloodstream, increasing the osmolality within the blood and creating an osmol gap. Lactic acidosis can result in moderate lactic acidosis due to the accumulation of products from the breakdown of glycogen.[18] A hyperglycemic hyperosmolar state can also drive an increased osmol gap secondary to hyperglycemia.[22] In addition, diseases causing the retention of small molecules, such as renal failure, can also produce a similar effect.[15] Finally, shock can result in the accumulation of cationic or neutral amino acids, increasing the osmol gap.[18]
The value of change in the osmol gap can also give some insight into the cause of the elevation. For instance, renal failure and ketoacidosis often present with an osmol gap equal to or less than 15 to 20 mOsm/kg, whereas an osmol gap greater than 20 mOsm/kg indicates alcohol or acetone accumulation. Lactic acidosis often produces an average osmol gap of approximately 11 mOsm/kg.[18]
Urine Osmolality
Urine osmolality is useful for investigating electrolyte disturbances, particularly in hypo- or hypernatremia.[23][24] Hypernatremia and hyperosmolar plasma in the context of hypoosmolar urine (<300 mOsm/kg) are suggestive of diabetes insipidus. In this disease, a large volume of urine is excreted due to the failure to produce antidiuretic hormone by the hypothalamus (central diabetes insipidus) or lack of response to antidiuretic hormone by the kidney (nephrogenic diabetes insipidus). Hypernatremia in the presence of hyperosmolar urine (>600 mOsm/kg) is suggestive of extrarenal water loss, such as diarrhea, burn, or fever, or sodium overload resulting from the administration of hypertonic saline or salt poisoning.[24]
In patients with low serum osmolality and a clinically normal extracellular fluid volume, urine osmolality can aid in distinguishing between various etiologies. In these cases, a urine osmolality >100 mOsm/kg is indicative of the syndrome of inappropriate antidiuretic hormone, hypothyroidism, or glucocorticoid deficiency. On the other hand, a urine osmolality <100 mOsm/kg is observed in patients with primary polydipsia or beer potomania.[23]
Stool Osmolality
Measuring stool osmolality can help evaluate patients with diarrhea to differentiate osmotic diarrhea from secretory diarrhea. The stool osmotic gap is defined as the difference between the measured osmolality and a calculated osmolality (2*([fecal Na+] + [fecal K+]).[25] A stool osmotic gap <50 mOsm/kg implies that the diarrhea is secretory. In patients with osmotic diarrhea, the stool osmotic gap is typically >75 mOsm/kg. The osmolality should be measured within 30 minutes of stool sample collection, or the sample should be refrigerated until analysis because bacterial metabolism produces osmotically active substances that can alter the reading.[26] A stool osmolality <290 mOsm/kg may result from the intentional addition of water to a stool sample. In contrast, a stool osmolality >330 mOsm/kg without an elevated serum osmolality can suggest improper storage of the sample or contamination with concentrated urine.[27]
Quality Control and Lab Safety
Accurate osmolality testing is crucial for patient care. Daily analysis of commercially prepared control solutions with known low, normal, and high osmolality values is vital. Measured control values must fall within pre-defined limits, with deviations triggering corrective and preventive action.[17]
Maintaining instrument accuracy extends beyond regular control checks. Regular calibration employing manufacturer-supplied solutions with known osmolality values is vital. The calibration frequency may vary based on the specific instrument model, laboratory workload, and manufacturer recommendations. Furthermore, adhering to the manufacturer's guidelines for preventive maintenance, which encompasses cleaning, part replacement, and verification of proper component function, is essential.[28]
Participation in external proficiency testing programs offered by external organizations is highly recommended to ensure consistent quality and minimize inter-laboratory variability. These programs involve analyzing blinded samples with known osmolality and comparing the results to those of other laboratories. This collaborative approach facilitates identifying and rectifying any systematic biases that might be present within the testing procedures.[29] The documentation of all QC procedures is paramount for establishing a robust and reliable osmolality testing process within the laboratory. This documentation should include control results, calibration records, and any corrective actions taken. In addition, it is crucial to provide comprehensive training for personnel on instrument operation, QC procedures, and troubleshooting protocols.[30]
A robust safety program safeguarding patients and staff is paramount for any clinical laboratory. This program proactively identifies and manages potential hazards inherent to laboratory operations. Stringent protocols, including consistent use of barrier protection and frequent hand washing, minimize exposure risks. All specimens are treated as potentially infectious, and proper disposal procedures for sharps and infected materials are followed.[31] The Occupational Safety and Health Administration mandates hepatitis B vaccination for at-risk personnel, whereas the International Organization for Standardization 15190 standard ensures safety excellence through defined roles, fire and radiation safety measures, and continuous improvement initiatives.[31][32]
Enhancing Healthcare Team Outcomes
Determining serum and urine osmolality and assessing the difference between the calculated and measured osmolality (measurement of osmolar gap) are crucial for diagnosing and treating patients in various clinical scenarios. Modern osmometers in clinical laboratories are simple to operate and produce rapid and accurate results using a small specimen volume. Performing osmolality measurements is recommended for better accuracy and minimum errors.
Effective communication among all members of a patient's interprofessional care team, including laboratory technologists, clinicians, pharmacists, and other healthcare professionals, is essential for ensuring patients receive appropriate and timely medical care. A systematic process for immediate osmolality measurement is beneficial, as is a properly equipped and calibrated osmometer and fully qualified and trained laboratory technologists to run this instrument.[33][34]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Lord RC. Osmosis, osmometry, and osmoregulation. Postgraduate medical journal. 1999 Feb:75(880):67-73 [PubMed PMID: 10448464]
Tuchman S, Khademian ZP, Mistry K. Dialysis disequilibrium syndrome occurring during continuous renal replacement therapy. Clinical kidney journal. 2013 Oct:6(5):526-9. doi: 10.1093/ckj/sft087. Epub 2013 Aug 13 [PubMed PMID: 26120445]
Rasouli M. Basic concepts and practical equations on osmolality: Biochemical approach. Clinical biochemistry. 2016 Aug:49(12):936-41. doi: 10.1016/j.clinbiochem.2016.06.001. Epub 2016 Jun 22 [PubMed PMID: 27343561]
Pena-Verdeal H, García-Resúa C, Vazquez-Sanchez C, Garcia-Queiruga J, Yebra-Pimentel E, Giráldez MJ. Reproducibility in measuring tear samples using a freezing point depression osmometer. Clinical & experimental optometry. 2019 Nov:102(6):571-575. doi: 10.1111/cxo.12886. Epub 2019 Feb 28 [PubMed PMID: 30818419]
Lamas CP, Vega C, Noya EG. Freezing point depression of salt aqueous solutions using the Madrid-2019 model. The Journal of chemical physics. 2022 Apr 7:156(13):134503. doi: 10.1063/5.0085051. Epub [PubMed PMID: 35395902]
Jeon JG, Hong GW, Park HG, Lee SK, Kim JH, Kang TJ. Resistance Temperature Detectors Fabricated via Dual Fused Deposition Modeling of Polylactic Acid and Polylactic Acid/Carbon Black Composites. Sensors (Basel, Switzerland). 2021 Feb 24:21(5):. doi: 10.3390/s21051560. Epub 2021 Feb 24 [PubMed PMID: 33668114]
Braslavsky I, Drori R. LabVIEW-operated novel nanoliter osmometer for ice binding protein investigations. Journal of visualized experiments : JoVE. 2013 Feb 4:(72):e4189. doi: 10.3791/4189. Epub 2013 Feb 4 [PubMed PMID: 23407403]
Gray MF, Nilsson M. Accuracy, Repeatability, and Limitations for Determination of Chemical Activities from Vapor Pressure Osmometry. Analytical chemistry. 2018 Nov 6:90(21):12761-12767. doi: 10.1021/acs.analchem.8b03129. Epub 2018 Oct 19 [PubMed PMID: 30298731]
Kolling WM, McPherson TB. Assessment of the accuracy of pharmacy students' compounded solutions using vapor pressure osmometry. American journal of pharmaceutical education. 2013 Apr 12:77(3):58. doi: 10.5688/ajpe77358. Epub [PubMed PMID: 23610476]
Hale CS, McBride DW, Batarseh R, Hughey J, Vang K, Rodgers VGJ. Development and applications of a concentrating membrane osmometer for colloid solutions. The Review of scientific instruments. 2019 Mar:90(3):034102. doi: 10.1063/1.5065512. Epub [PubMed PMID: 30927796]
Yoo DW, Lee SM, Moon SY, Kim IS, Chang CL. Evaluation of conductivity-based osmolality measurement in urine using the Sysmex UF5000. Journal of clinical laboratory analysis. 2021 Jan:35(1):e23586. doi: 10.1002/jcla.23586. Epub 2020 Sep 24 [PubMed PMID: 32969530]
Barley OR, Chapman DW, Abbiss CR. Reviewing the current methods of assessing hydration in athletes. Journal of the International Society of Sports Nutrition. 2020 Oct 30:17(1):52. doi: 10.1186/s12970-020-00381-6. Epub 2020 Oct 30 [PubMed PMID: 33126891]
Chan CC, Borovik A, Hofmann I, Gulliver E, Rocha G. Validity and Reliability of a Novel Handheld Osmolarity System for Measurement of a National Institute of Standards Traceable Solution. Cornea. 2018 Sep:37(9):1169-1174. doi: 10.1097/ICO.0000000000001653. Epub [PubMed PMID: 29877926]
Gokhale M, Stahl U, Jalbert I. In situ osmometry: validation and effect of sample collection technique. Optometry and vision science : official publication of the American Academy of Optometry. 2013 Apr:90(4):359-65. doi: 10.1097/OPX.0b013e31828aaf10. Epub [PubMed PMID: 23518677]
Level 1 (high-level) evidenceBüyükkaragöz B, Bakkaloğlu SA. Serum osmolality and hyperosmolar states. Pediatric nephrology (Berlin, Germany). 2023 Apr:38(4):1013-1025. doi: 10.1007/s00467-022-05668-1. Epub 2022 Jul 2 [PubMed PMID: 35779183]
Anyabolu EN, Chukwuonye II, Ezeani I. Urine osmolality in treatment-naïve HIV-positive subjects in Southeast Nigeria. Nigerian journal of clinical practice. 2017 Aug:20(8):936-942. doi: 10.4103/njcp.njcp_253_16. Epub [PubMed PMID: 28891536]
Najem O, Shah MM, Zubair M, De Jesus O. Serum Osmolality. StatPearls. 2024 Jan:(): [PubMed PMID: 33620841]
Liamis G, Filippatos TD, Liontos A, Elisaf MS. Serum osmolal gap in clinical practice: usefulness and limitations. Postgraduate medicine. 2017 May:129(4):456-459. doi: 10.1080/00325481.2017.1308210. Epub 2017 Mar 23 [PubMed PMID: 28306366]
Theis SR, Khandhar PB. Pseudohyponatremia. StatPearls. 2024 Jan:(): [PubMed PMID: 31985988]
Althwanay A, Alharthi MM, Aljumaan M, Almubarak Y, Alamri A. Methanol, Paracetamol Toxicities and Acute Blindness. Cureus. 2020 May 18:12(5):e8179. doi: 10.7759/cureus.8179. Epub 2020 May 18 [PubMed PMID: 32566420]
Mangat HS, Wu X, Gerber LM, Schwarz JT, Fakhar M, Murthy SB, Stieg PE, Ghajar J, Härtl R. Hypertonic Saline is Superior to Mannitol for the Combined Effect on Intracranial Pressure and Cerebral Perfusion Pressure Burdens in Patients With Severe Traumatic Brain Injury. Neurosurgery. 2020 Feb 1:86(2):221-230. doi: 10.1093/neuros/nyz046. Epub [PubMed PMID: 30877299]
Nishikawa T, Kinoshita H, Ono K, Kodama-Hashimoto S, Kobayashi Y, Nakamura T, Yoshinaga T, Ohkubo Y, Harada M, Toyonaga T, Takahashi T, Araki E. Clinical profiles of hyperglycemic crises: A single-center retrospective study from Japan. Journal of diabetes investigation. 2021 Aug:12(8):1359-1366. doi: 10.1111/jdi.13475. Epub 2021 Jan 8 [PubMed PMID: 33277786]
Level 2 (mid-level) evidenceRondon H, Badireddy M. Hyponatremia. StatPearls. 2024 Jan:(): [PubMed PMID: 29262111]
Sonani B, Naganathan S, Al-Dhahir MA. Hypernatremia. StatPearls. 2024 Jan:(): [PubMed PMID: 28722989]
Parakh R, Greene DN, Mathias PC, Block DR, Ranjitkar P. Laboratory Utilization and Analytical Validation of Fecal Electrolyte Tests. The journal of applied laboratory medicine. 2017 May 1:1(6):668-677. doi: 10.1373/jalm.2016.022590. Epub [PubMed PMID: 33379818]
Level 1 (high-level) evidenceShiau YF, Feldman GM, Resnick MA, Coff PM. Stool electrolyte and osmolality measurements in the evaluation of diarrheal disorders. Annals of internal medicine. 1985 Jun:102(6):773-5 [PubMed PMID: 3994188]
Eherer AJ, Fordtran JS. Fecal osmotic gap and pH in experimental diarrhea of various causes. Gastroenterology. 1992 Aug:103(2):545-51 [PubMed PMID: 1634072]
Barth JH. Selecting clinical quality indicators for laboratory medicine. Annals of clinical biochemistry. 2012 May:49(Pt 3):257-61. doi: 10.1258/acb.2011.011159. Epub 2012 Mar 15 [PubMed PMID: 22422153]
Level 2 (mid-level) evidenceJones GR. The role of EQA in harmonization in laboratory medicine - a global effort. Biochemia medica. 2017 Feb 15:27(1):23-29. doi: 10.11613/BM.2017.004. Epub [PubMed PMID: 28392723]
Nemenqani DM, Tekian A, Park YS. Competency assessment in laboratory medicine: Standardization and utility for technical staff assessment and recertification in Saudi Arabia. Medical teacher. 2017 Apr:39(sup1):S63-S74. doi: 10.1080/0142159X.2016.1254751. Epub 2017 Feb 5 [PubMed PMID: 28162028]
Meisenhelder J, Bursik S, Lunn G, Strober W. Laboratory safety. Current protocols in human genetics. 2008 Apr:Appendix 2():Appendix 2A. doi: 10.1002/0471142905.hga02as57. Epub [PubMed PMID: 18428418]
Denault D, Gardner H. OSHA Bloodborne Pathogen Standards. StatPearls. 2024 Jan:(): [PubMed PMID: 34033323]
Trepiccione F, Capasso G, Lippi G. [Serum and urine osmolality: clinical and laboratory features]. Giornale italiano di nefrologia : organo ufficiale della Societa italiana di nefrologia. 2014 Sep-Oct:31(5):. pii: gin/31.5.6. Epub [PubMed PMID: 25315724]
Kiles TM, Zhao AV, Wanat MA, Garey KW, Hatfield CL. Knowledge and self-perception comparisons between students with and without prior technician experience in community pharmacy lab courses. Currents in pharmacy teaching & learning. 2021 Mar:13(3):279-287. doi: 10.1016/j.cptl.2020.10.004. Epub 2020 Nov 27 [PubMed PMID: 33641739]