Introduction
Iridocorneal endothelial syndrome (ICE) is a rare disorder characterized by the increased proliferation and migration of corneal endothelial cells to the iris and iridocorneal angle, leading to secondary angle-closure glaucoma, corneal edema, and atrophy of the iris.[1] This condition is often associated with secondary glaucoma due to obstruction of the trabecular meshwork in the iridocorneal angle, the formation of peripheral anterior synechiae of the iris, or both.[1] Patients typically present with changes in the shape of the iris, a decrease in visual acuity, blurred vision, or a combination thereof.[2] Vision loss typically occurs due to the progression of secondary glaucoma and corneal decompensation.[3] If left untreated, ICE can progress to blindness.[4]
ICE is typically a unilateral disorder and more frequently affects young adults and middle-aged females.[5][6] The 3 clinical variants of ICE are essential iris atrophy (EIA), Chandler syndrome (CS), and Cogan-Reese syndrome (CRS).[1][2] The clinical features of EIA include iridic changes such as full-thickness holes and endothelial dystrophy (see Image. Iridocorneal Endothelial Syndrome: Essential Endothelial Syndrome).[1] The most common variant of ICE is CS, characterized by less iridic involvement, unilateral visual impairment, iridic atrophy, corectopia, and significantly more corneal edema, epithelial bullae, and endothelial dystrophy.[1] The CRS typically presents with nodules on the anterior surface of the iris and perhaps endothelial disease and corneal edema.[1][2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The pathogenesis of ICE is not fully understood; several working hypotheses exist. Corneal endothelial cells are postmitotic and typically do not proliferate in vivo; the quantity of these cells decreases with age.[7][8] ICE can develop when corneal endothelial cells regain proliferative capacity.[3] Corneal endothelial cells play an important role in regulating corneal hydration and transparency, and endothelial dysfunction leads to corneal edema and visual defects.[9]
The neural crest theory is one proposed pathogenic mechanism for ICE development. This theory stratifies different ocular endothelial disorders by neural crest origin. ICE was thought to be related to abnormal neural crest cell (NCC) proliferation, compared to NCC formation in cyclopia, NCC migration in Peters anomaly, or NCC differentiation in Fuchs dystrophy.[10]
In Peters anomaly, an abnormality in the development of the first wave of neural crest cells leads to a failure of separation between lens vesicles and surface ectoderm, causing corneal opacification.[11][12][13] Type 1 Peters anomaly is characterized by iridocorneal adhesions, the development of corneal opacity, and a striking similarity to ICE.[14][15][16] However, compared with ICE, Peters anomaly presents at a younger age and occurs bilaterally in 80% of cases.[17] Fuchs dystrophy is also a bilateral endothelial disorder, but unlike Peters anomaly and ICE, it does not present with iridocorneal adhesions.[1] Similar to ICE, Fuchs dystrophy may present with corneal edema.[18]
Cambell, Shields, and Smith proposed their membrane theory in 1978 to better explain the corneal endothelial structural changes and abnormalities associated with ICE.[19] The membrane theory characterizes ICE as primary proliferative endothelial degeneration and emphasizes that this disease is primarily corneal in origin with secondary impacts on the iris. According to the membrane theory, the initial pathologic insult involves corneal endothelial degeneration that progresses to abnormal endothelial membrane overgrowth around the iridocorneal angle. Obstruction of the trabecular meshwork and contraction of the membrane leads to structural changes in the iris, secondary glaucoma, and ectropion uveae.[20] Further histological studies of ICE cell characteristics have supported the membrane theory.[19][21]
Although these hypotheses discuss the progression of ICE, the causal factor of ICE remains undetermined. Various studies have described an association between ICE and inflammation or uveitis.[1] Another proposed pathogenic mechanism points to a viral trigger. In 1994, Alvarado et al investigated ICE's potential viral etiology using polymerase chain reaction and identified Herpes simplex viral (HSV) DNA in >60% of tested ICE specimens.[22] In this study, corneal control specimens consisted of normal corneas and corneas with keratoconus, interstitial keratitis, or aphakic bullous keratopathy. None of the control corneas tested positive for HSV DNA. Interestingly, when the corneal endothelial layer was removed from ICE specimens, HSV DNA was no longer detected. This finding provided direct evidence to support the idea that viral DNA was confined to the endothelial cell layer.[1][22] Other studies report an association between Epstein-Barr virus (EBV) and ICE.[23]
Epidemiology
ICE is an acquired syndrome that typically presents unilaterally and predominantly affects females between the age of 20 and 50.[5] However, there are case reports of bilateral presentation, and ICE has been diagnosed in male patients.[24] Despite reports of concurrent ICE and sensorineural hearing loss, no definitive association has been established.
Observational studies indicate varying incidences of ICE subtypes among racial and ethnic groups. For instance, CS is the most common variant of ICE diagnosed in White patients.[1] In contrast, CRS is the least frequently reported subtype in India at 14.29%, and EIA has the highest incidence at 66.67%.[25] This study also documented the incidence of ICE in Indian male patients at 62%, compared with studies from North America and Europe, which reported the incidence in males as 17%.[26]
Pathophysiology
The pathophysiology of ICE results from the increased proliferation and migration of corneal endothelial cells to the iridocorneal angle and iris, leading to secondary angle-closure glaucoma (see Image. Iridocorneal Endothelial Syndrome: Secondary Angle-Closure Glaucoma), corneal edema, and iridic atrophy.[1]
Histopathology
The cornea comprises multiple highly organized layers that maintain corneal transparency and barrier function. The nonproliferative layer of corneal endothelial cells is a barrier and a pump, helping to maintain the clarity and transparency of the cornea.[27] The corneal epithelial cells in patients with ICE are typically taller, and the multilayer organization is abnormal. These abnormal endothelial cells retain the microvilli or their normal counterparts.[1]
Corneal decompensation occurs when persistent corneal insults lead to increasing corneal opacity.[28] The development of secondary glaucoma is due to the formation of peripheral anterior synechiae (PAS) or the growth of abnormal endothelial cells over the trabecular meshwork.[1] These histological changes lead to elevated intraocular pressure (IOP), the characteristic finding of glaucoma.[1]
History and Physical
The typical presenting clinical symptoms of patients with ICE include changes in the shape of their pupil due to atrophy of the iris and visual changes such as decreased vision upon waking or blurred halo of lights due to corneal edema and subsequent corneal decompensation.[1] Patients may also develop ocular pain or headache if they have a severe increase in IOP due to secondary glaucoma.[29]
The external appearance of the affected eye is abnormal in patients with ICE but will vary with the stage of the disease and clinical variant.[1] The atrophic iridic changes characteristic of EIA may not be seen without careful slit-lamp examination.[1] Due to extensive endothelial dystrophy, patients with CS or CRS may develop significantly increased IOP, severe glaucoma, and corneal edema, which grossly appears as corneal opacification.[1]
Ophthalmic examination of the corneal endothelium may reveal “hammered silver” or “beaten bronze” irregularities during slit-lamp examination, a reversal light pattern during specular microscopy, or changes in the iridocorneal angle on gonioscopy. (see Image. Iridocorneal Endothelial Syndrome: Corneal Endothelium Irregularities). Cupping of the optic nerve may be seen in patients with secondary glaucoma).[30]
A thorough physical examination includes a slit-lamp examination, a careful exam of the angle with gonioscopy, and tonometry or applanation. Techniques such as specular microscopy and in vivo corneal confocal microscopy (IVCM) can reveal pathognomonic findings.[1][2]
Evaluation
Early diagnosis of ICE is essential to prevent the complications of corneal edema and secondary glaucoma.[2] If significant corneal edema prevents clear visualization of the anterior chamber structures, IVCM can be employed.
IVCM is a noninvasive imaging technique that allows visualization of corneal layers at the cellular level. The abnormal corneal epithelial cells of patients with ICE appear as epithelial-like endothelial cells varying in size and shape, with bright cell borders and hyperreflective nuclei.[26] Specular microscopy is another imaging tool to evaluate the corneal epithelium; epithelial cells in patients with ICE are characterized by an abnormally large and round shape and display a pattern of "light-dark reversal." These abnormally dark cells have a light central spot with light borders, which is the opposite of normal endothelial cell light patterns. In addition to IVCM and specular microscopy, gonioscopy is required to visualize the iridocorneal angle. In ICE, there are often progressive broad-based synechiae located on the iris and around the trabecular meshwork.
If gonioscopy is challenging due to corneal edema, ultrasound biomicroscopy can be used to visualize the anterior chamber angle and detect abnormalities.[31] Gonioscopy, in combination with ultrasound biomicroscopy, can be used to assess the size and extent of PAS fully.[2]
Patients with ICE and subsequent secondary glaucoma should be evaluated with Humphrey visual field testing, central corneal thickness measurements, intraocular pressure evaluation with Goldmann applanation tonometry, evaluation of the iridocorneal angle and trabecular meshwork with gonioscopy, optic nerve evaluation on physical examination, as well as optical coherence tomography of the optic nerve head and retinal nerve fiber layer.[32][33]
Treatment / Management
The treatment of ICE is typically centered around managing corneal decompensation and preventing irreversible glaucomatous vision loss. Surgical procedures may address iridic changes to improve cosmesis or vision-impacting defects.[1] (B3)
Endothelial keratoplasty techniques such as Descemet stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK) are preferred over penetrating keratoplasty (PK) for the treatment of ICE-related corneal edema.[1] DMEK improves outcomes and shortens recovery time when compared to DSAEK.[34] A recent study evaluating the effectiveness of DMEK in patients with ICE showed an 85.7% cumulative graft success rate after 1 year.[34] However, DSAEK is the preferred procedure for patients with significant iris changes or copious synechiae. If patients have failed multiple keratoplasty procedures, keratoprosthesis may be considered.[35] (B3)
ICE damages the functionality of the trabecular meshwork in the iridocorneal angle, blocking aqueous outflow and causing secondary glaucoma. Therefore, topical eyedrops that decrease aqueous humor production are preferred over those that increase trabecular aqueous outflow.[1] The primary treatment regimen for secondary glaucoma includes topical beta blockers, carbonic anhydrase inhibitors, and alpha agonists. Topical prostaglandins have been associated with intraocular reactivation of HSV and should be avoided in patients with ICE.[1][36] (B3)
ICE-induced secondary glaucoma may be very challenging to control. If IOP is not adequately controlled with topical ophthalmic medications, surgical interventions such as goniotomy, trabeculectomy with antifibrotic agents such as mitomycin-C or 5-fluorouracil, glaucoma drainage implants, or cyclodestructive procedures must be considered.
Goniotomy is an excellent surgical option to relieve the obstruction to aqueous outflow caused by the ICE membrane over the angle.[37] However, a new membrane can subsequently form over the goniotomy site resulting in recurrent obstruction. Trabeculectomy with antimetabolites can be performed to manage IOP but usually fails over time due to the proliferation of the endothelial membrane in the trabecular meshwork, ostium, and fistula, and the deposition of abnormal basement membrane within the bleb.[38] (B2)
Trabeculectomy outcomes in patients with ICE are typically worse than in other disease processes, such as primary open-angle glaucoma, pigmentary glaucoma, and juvenile open-angle glaucoma.[39][40] Success rates for patients with ICE are higher when trabeculectomy is performed early in the disease process.[41] In general, the success rate of trabeculectomy is approximately 60% at 1 year and 40% at 2 years.[42] (B2)
Trabeculectomy with antimetabolites and glaucoma drainage implants offer postoperative IOP reduction for patients with ICE syndrome. Still, the long-term failure rate for trabeculectomy may be higher than for glaucoma drainage devices.[43] Glaucoma drainage devices may fail if the continued proliferation of the endothelial membrane blocks the drainage tube. Inserting the tube behind the iris at the ciliary sulcus, especially in pseudophakic eyes, may avoid this phenomenon.[1] Nd:YAG laser may be employed to reopen blocked drainage tubes due to endothelial proliferation.(B3)
Cyclodestructive procedures may be employed when surgical interventions fail to control the IOP, resulting in a painful blind eye. The options for cyclodestructive procedures include diode laser cyclophotocoagulation, cyclocryotherapy, and endocyclophotocoagulation.
Femtosecond-assisted keratopigmentation (KTP) has shown promising results in eliminating the diplopia and photophobia common in patients with ICE.[44] Alternatively, multiple endocapsular iris prosthesis devices can be implanted inside the capsular bag in front of the intraocular lens to address iris atrophy and photophobia and improve cosmesis.[1][45](B3)
Differential Diagnosis
ICE should be considered a potential diagnosis in any young female with unilateral corneal edema, visual disturbances, iris abnormalities, or glaucoma.[2] The variants of ICE may present similarly to other disease processes, and it is essential to obtain a comprehensive medical history and perform a thorough physical examination to identify the correct underlying disease.
The differential diagnosis for CS includes posterior polymorphous corneal dystrophy (PPCD) and Fuchs endothelial dystrophy. PPCD is a corneal disorder that is typically bilateral and inherited in an autosomal dominant fashion. Similar to the ICE variants, patients with PPCD have areas of PAS that increase their risk for secondary glaucoma. However, the Descemet membrane of patients with PPCD is thickened and may reveal vesicular, band-like, or diffuse opacities; the Descemet membrane in CS is normal. Fuchs endothelial dystrophy is a bilateral disorder and does not present with iridocorneal abnormalities, and IVCM reveals hyporeflective nuclei compared to the hyperreflective nuclei seen in ICE. Fuchs endothelial dystrophy often presents with endothelial guttae, or the deposition of an extracellular collagen-like matrix, in addition to corneal edema.[1][18]
The differential diagnosis for EIA includes Axenfeld-Rieger syndrome (ARS), aniridia, and iridoschisis.[46][47][13] ARS is a congenital disorder usually inherited in an autosomal dominant fashion but can occur sporadically. Patients with ARS display changes at birth and may have associated anomalies such as abnormal teeth. ARS is typically a bilateral disease, whereas ICE is usually unilateral. ICE and ARS display a similar pattern of an endothelial cell monolayer extending over the cornea, iridocorneal angle, and iris. However, since ARS is a congenital disorder, it is hypothesized that the iridocorneal membrane is due to the retention of primordial endothelium. A distinct differentiating factor between ARS and ICE is the posterior embryotoxon with iris strands of ARS.
Aniridia is another congenital disorder that is typically bilateral and often associated with other conditions, such as optic nerve hypoplasia. The age of onset can be an important factor in helping differentiate aniridia from late-stage EIA. Most cases of aniridia present within the first 6 weeks of life; other associated sensory deficits, such as hearing loss and decreased sense of smell, are common.[48] Iridoschisis is the progressive separation of the layers of the iris, is frequently bilateral, and most often occurs in older patients.[1]
The differential diagnosis of CRS is broad and includes Tapioca melanoma and diffuse malignant melanoma of the iris. Tapioca melanoma presents with nodules on the iris similar to those in CRS. However, these nodules are typically hypopigmented compared to the hyperpigmented nodules in CRS. In addition, typical features of CRS, including PAS and iris atrophy, are not present in Tapioca melanoma. Diffuse malignant melanoma of the iris typically does not present with distortion of the pupillary margin and PAS, which are more suggestive of CRS.[1]
Prognosis
The prognosis of ICE is dependent on how advanced the disease is at the time of diagnosis and the presence of secondary complications. Corneal surgeries may not be able to excise the abnormal endothelium entirely and, therefore, may not prevent the progression of PAS and the development of secondary glaucoma.[2]
A small case series of 8 eyes in 7 patients studied the outcome of DMEK in patients with both ICE and PPCD. This 2018 case series reported a statistically significant increase in best-corrected visual acuity in all eyes at 6 and 24 months following the procedures.[49] A more extensive study in 2020 analyzed 86 patients undergoing their first corneal transplants for ICE syndrome and the 5-year graft survival rates in patients who underwent penetrating keratoplasty (PKP) or endothelial keratoplasty (EK). There was no significant difference between corneal graft survival rates for PKP or EK procedures, with 64.3% and 66.8% survival rates, respectively.[50]
Complications
The complications of ICE include iris abnormalities, corneal edema and decompensation, and secondary glaucoma.[2] Glaucoma occurs in approximately 50% of patients with ICE and is more common in the EIA variant.[29][37] Corneal edema and decompensation, however, are higher in the CS subtype. Depending on the severity of endothelial disease in any subtype of ICE, patients may be approached differently during intraocular surgeries such as cataract extraction; patients may undergo combined DSAEK and cataract extraction to address their corneal endothelial disease.[51]
Deterrence and Patient Education
Although ICE is a rare disorder, patients with unilateral corneal edema, iridic changes, vision changes, or the development of glaucoma need to be carefully evaluated for this condition, particularly if the changes occur in a woman between 20 and 50 years of age.[1][6] A thorough evaluation and prompt diagnosis can prevent the development or worsening of corneal decompensation due to corneal edema or secondary glaucoma due to PAS.[1] Education about ICE and its variants and early diagnosis can help prevent the progression of this vision-threatening disease.
Enhancing Healthcare Team Outcomes
Enhancing outcomes for patients with ICE involves a comprehensive approach that addresses both the ocular manifestations and associated complications. ICE is a rare but serious condition often overlooked or misdiagnosed in a clinical setting. Prompt diagnosis is crucial for patients with ICE, and regular eye examinations conducted by eye care professionals or primary care providers can help to identify the disease in its early stages. ICE should be considered in patients presenting with changes in the shape of the iris or visual acuity, corneal edema, or blurred vision, particularly if the patient is a woman between the ages of 20 and 50.[1][5]
Patients exhibiting symptoms suggestive of ICE should be referred to healthcare providers capable of performing comprehensive ophthalmic examinations, which may include slit-lamp examination, specular microscopy, and gonioscopy.[2] Timely intervention with medical or surgical treatment can help prevent or mitigate potential complications. Collaboration among ophthalmologists, glaucoma specialists, corneal specialists, and other relevant healthcare professionals can provide comprehensive care for patients with ICES. This multidisciplinary approach ensures that all aspects of the condition are addressed effectively.[1][5]
Ensuring patients are educated about ICE, including its symptoms, available treatment options, and the significance of medication adherence and follow-up visits, is paramount. Providing support and addressing patient concerns can enhance their understanding and cooperation with the treatment plan, leading to better outcomes. The specific management plan should be tailored to each individual based on their unique circumstances and needs.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
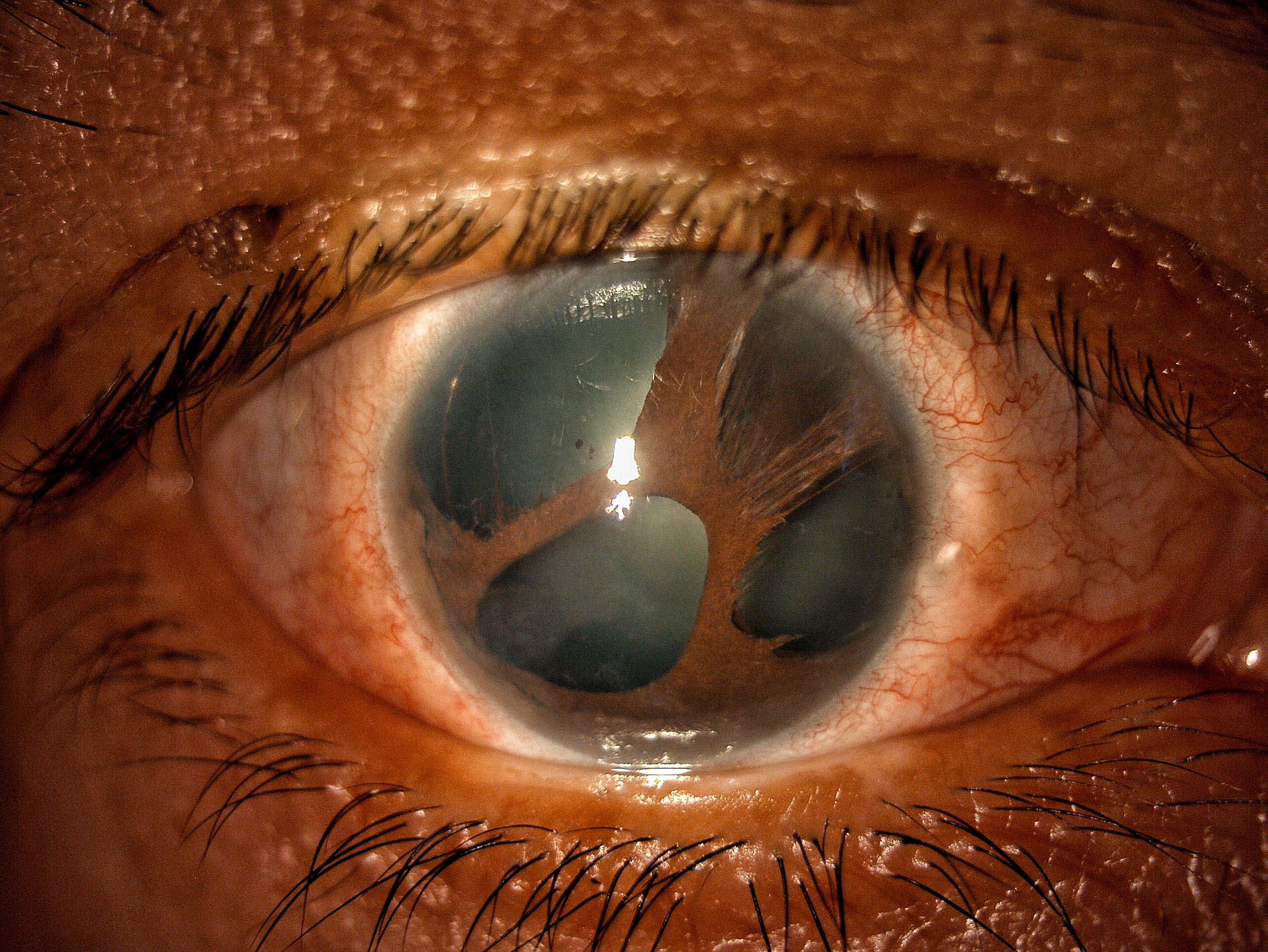
Iridocorneal Endothelial Syndrome: Essential Endothelial Syndrome. This image shows essential iris atrophy (or progressive essential iris atrophy), one of the 3 variants of iridocorneal endothelial syndrome. This variant has more iris changes like atrophic patches, heterochromia, corectopia, polycoria, and a peripheral hole compared to the other 2 variants. Contributed by Gustavo Espinoza, MD
(Click Image to Enlarge)
References
Silva L, Najafi A, Suwan Y, Teekhasaenee C, Ritch R. The iridocorneal endothelial syndrome. Survey of ophthalmology. 2018 Sep-Oct:63(5):665-676. doi: 10.1016/j.survophthal.2018.01.001. Epub 2018 Jan 11 [PubMed PMID: 29331589]
Level 3 (low-level) evidenceSacchetti M, Mantelli F, Marenco M, Macchi I, Ambrosio O, Rama P. Diagnosis and Management of Iridocorneal Endothelial Syndrome. BioMed research international. 2015:2015():763093. doi: 10.1155/2015/763093. Epub 2015 Sep 16 [PubMed PMID: 26451377]
Li F, Liu Y, Sun Y, Zhang X. Etiological mechanism of iridocorneal endothelial (ICE) syndrome may involve infection of herpes simplex virus (HSV) and integration of viral genes into human genome. Medical hypotheses. 2018 Jan:110():50-52. doi: 10.1016/j.mehy.2017.10.023. Epub 2017 Oct 26 [PubMed PMID: 29317068]
Dubey S, Jain K, Singh S, Mukhejee S. Iridocorneal Endothelial Syndrome with Coexisting Macular Edema and Neurosensory Detachment: An Unusual Case Report. Journal of current glaucoma practice. 2021 Sep-Dec:15(3):149-152. doi: 10.5005/jp-journals-10078-1315. Epub [PubMed PMID: 35173398]
Level 3 (low-level) evidenceWalkden A, Au L. Iridocorneal endothelial syndrome: clinical perspectives. Clinical ophthalmology (Auckland, N.Z.). 2018:12():657-664. doi: 10.2147/OPTH.S143132. Epub 2018 Apr 9 [PubMed PMID: 29670326]
Level 3 (low-level) evidenceGupta V, Kumar R, Gupta R, Srinivasan G, Sihota R. Bilateral iridocorneal endothelial syndrome in a young girl with Down's syndrome. Indian journal of ophthalmology. 2009 Jan-Feb:57(1):61-3 [PubMed PMID: 19075416]
Level 3 (low-level) evidenceJoyce NC. Proliferative capacity of corneal endothelial cells. Experimental eye research. 2012 Feb:95(1):16-23. doi: 10.1016/j.exer.2011.08.014. Epub 2011 Aug 30 [PubMed PMID: 21906590]
Level 3 (low-level) evidenceWang Q, Dou S, Zhang B, Jiang H, Qi X, Duan H, Wang X, Dong C, Zhang BN, Xie L, Cao Y, Zhou Q, Shi W. Heterogeneity of human corneal endothelium implicates lncRNA NEAT1 in Fuchs endothelial corneal dystrophy. Molecular therapy. Nucleic acids. 2022 Mar 8:27():880-893. doi: 10.1016/j.omtn.2022.01.005. Epub 2022 Jan 10 [PubMed PMID: 35141048]
Level 2 (mid-level) evidencePrice MO, Mehta JS, Jurkunas UV, Price FW Jr. Corneal endothelial dysfunction: Evolving understanding and treatment options. Progress in retinal and eye research. 2021 May:82():100904. doi: 10.1016/j.preteyeres.2020.100904. Epub 2020 Sep 22 [PubMed PMID: 32977001]
Level 3 (low-level) evidenceBahn CF, Falls HF, Varley GA, Meyer RF, Edelhauser HF, Bourne WM. Classification of corneal endothelial disorders based on neural crest origin. Ophthalmology. 1984 Jun:91(6):558-63 [PubMed PMID: 6462621]
Williams AL, Bohnsack BL. The Ocular Neural Crest: Specification, Migration, and Then What? Frontiers in cell and developmental biology. 2020:8():595896. doi: 10.3389/fcell.2020.595896. Epub 2020 Dec 23 [PubMed PMID: 33425902]
Jat NS, Tripathy K. Peters Anomaly. StatPearls. 2023 Jan:(): [PubMed PMID: 35593847]
Singh P, Gupta A, Tripathy K. Iridocorneal Dysgenesis. StatPearls. 2024 Jan:(): [PubMed PMID: 36251848]
Alkatan HM, Al Dhaheri H, Al Harby M. Terminology of Peters' anomaly variants: Summary of histopathological findings in 6 corneas and detailed clinicopathological correlation in 2 cases. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2019 Jul-Sep:33(3):277-282. doi: 10.1016/j.sjopt.2018.02.015. Epub 2018 Mar 8 [PubMed PMID: 31686970]
Level 3 (low-level) evidenceAlmarzouki HS, Tayyib AA, Khayat HA, Alsulami RE, Alzahrani SM, Alkahtani AS, Alghifees LS. Peters Anomaly in Twins: A Case Report of a Rare Incident with Novel Comorbidities. Case reports in ophthalmology. 2016 Sep-Dec:7(3):186-192 [PubMed PMID: 27843434]
Level 3 (low-level) evidenceNi W, Wang W, Hong J, Zhang P, Liu C. A novel histopathologic finding in the Descemet's membrane of a patient with Peters Anomaly: a case-report and literature review. BMC ophthalmology. 2015 Oct 23:15():139. doi: 10.1186/s12886-015-0131-y. Epub 2015 Oct 23 [PubMed PMID: 26496717]
Level 3 (low-level) evidenceSault RW, Sheridan J. Peters' anomaly. Ophthalmology and eye diseases. 2013:5():1-3. doi: 10.4137/OED.S11142. Epub 2013 Feb 13 [PubMed PMID: 23650461]
Elhalis H, Azizi B, Jurkunas UV. Fuchs endothelial corneal dystrophy. The ocular surface. 2010 Oct:8(4):173-84 [PubMed PMID: 20964980]
Level 3 (low-level) evidenceCampbell DG, Shields MB, Smith TR. The corneal endothelium and the spectrum of essential iris atrophy. American journal of ophthalmology. 1978 Sep:86(3):317-24 [PubMed PMID: 717494]
Level 3 (low-level) evidenceSridhar U, Tripathy K. Iris Ectropion Syndrome. StatPearls. 2024 Jan:(): [PubMed PMID: 35593818]
Lee WR, Marshall GE, Kirkness CM. Corneal endothelial cell abnormalities in an early stage of the iridocorneal endothelial syndrome. The British journal of ophthalmology. 1994 Aug:78(8):624-31 [PubMed PMID: 7918291]
Level 3 (low-level) evidenceAlvarado JA, Underwood JL, Green WR, Wu S, Murphy CG, Hwang DG, Moore TE, O'Day D. Detection of herpes simplex viral DNA in the iridocorneal endothelial syndrome. Archives of ophthalmology (Chicago, Ill. : 1960). 1994 Dec:112(12):1601-9 [PubMed PMID: 7993217]
Herde J. [Iridocorneal endothelial syndrome (ICE-S): classification, clinical picture, diagnosis]. Klinische Monatsblatter fur Augenheilkunde. 2005 Oct:222(10):797-801 [PubMed PMID: 16240272]
Ye WQ, Deng YX, Zuo JJ, Zhang SD, Wang HO, Li JY, Zheng QX, Liang YB, Chen W. [Clinical characteristics of 114 patients with iridocorneal endothelial syndrome]. [Zhonghua yan ke za zhi] Chinese journal of ophthalmology. 2022 Jan 11:58(1):35-40. doi: 10.3760/cma.j.cn112142-20201231-00859. Epub [PubMed PMID: 34979791]
Malhotra C, Seth NG, Pandav SS, Jain AK, Kaushik S, Gupta A, Raj S, Dhingra D. Iridocorneal endothelial syndrome: Evaluation of patient demographics and endothelial morphology by in vivo confocal microscopy in an Indian cohort. Indian journal of ophthalmology. 2019 May:67(5):604-610. doi: 10.4103/ijo.IJO_1237_18. Epub [PubMed PMID: 31007217]
Le QH, Sun XH, Xu JJ. In-vivo confocal microscopy of iridocorneal endothelial syndrome. International ophthalmology. 2009 Feb:29(1):11-8. doi: 10.1007/s10792-007-9187-x. Epub 2008 Feb 23 [PubMed PMID: 18297250]
Feizi S. Corneal endothelial cell dysfunction: etiologies and management. Therapeutic advances in ophthalmology. 2018 Jan-Dec:10():2515841418815802. doi: 10.1177/2515841418815802. Epub 2018 Dec 7 [PubMed PMID: 30560230]
Level 3 (low-level) evidenceGupta K, Deng SX. Corneal Endothelial Decompensation. Klinische Monatsblatter fur Augenheilkunde. 2020 Jun:237(6):745-753. doi: 10.1055/a-1128-4445. Epub 2020 Jun 9 [PubMed PMID: 32516831]
Chandran P, Rao HL, Mandal AK, Choudhari NS, Garudadri CS, Senthil S. Glaucoma associated with iridocorneal endothelial syndrome in 203 Indian subjects. PloS one. 2017:12(3):e0171884. doi: 10.1371/journal.pone.0171884. Epub 2017 Mar 10 [PubMed PMID: 28282413]
Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet (London, England). 2017 Nov 11:390(10108):2183-2193. doi: 10.1016/S0140-6736(17)31469-1. Epub 2017 May 31 [PubMed PMID: 28577860]
Dada T, Gadia R, Sharma A, Ichhpujani P, Bali SJ, Bhartiya S, Panda A. Ultrasound biomicroscopy in glaucoma. Survey of ophthalmology. 2011 Sep-Oct:56(5):433-50. doi: 10.1016/j.survophthal.2011.04.004. Epub 2011 Jul 23 [PubMed PMID: 21783220]
Level 3 (low-level) evidenceRuia S, Tripathy K. Humphrey Visual Field. StatPearls. 2024 Jan:(): [PubMed PMID: 36256759]
Dietze J, Blair K, Havens SJ. Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 30855805]
Wu J, Dong X, Ouyang C, Ji J, Xie L, Hou C, Huang T. Comparison of Descemet Membrane Endothelial Keratoplasty for Iridocorneal Endothelial Syndrome and Fuchs Endothelial Dystrophy. American journal of ophthalmology. 2021 Jun:226():76-82. doi: 10.1016/j.ajo.2021.01.029. Epub 2021 Feb 5 [PubMed PMID: 33556383]
Phillips DL, Goins KM, Greiner MA, Alward WL, Kwon YH, Wagoner MD. Boston Type 1 Keratoprosthesis for Iridocorneal Endothelial Syndromes. Cornea. 2015 Nov:34(11):1383-6. doi: 10.1097/ICO.0000000000000616. Epub [PubMed PMID: 26398156]
Wand M, Gilbert CM, Liesegang TJ. Latanoprost and herpes simplex keratitis. American journal of ophthalmology. 1999 May:127(5):602-4 [PubMed PMID: 10334356]
Level 3 (low-level) evidenceLaganowski HC, Kerr Muir MG, Hitchings RA. Glaucoma and the iridocorneal endothelial syndrome. Archives of ophthalmology (Chicago, Ill. : 1960). 1992 Mar:110(3):346-50 [PubMed PMID: 1543451]
Level 2 (mid-level) evidenceIto YA, Walter MA. Genomics and anterior segment dysgenesis: a review. Clinical & experimental ophthalmology. 2014 Jan-Feb:42(1):13-24. doi: 10.1111/ceo.12152. Epub 2013 Jul 29 [PubMed PMID: 24433355]
Mahabadi N, Foris LA, Tripathy K. Open Angle Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 28722917]
Zeppieri M, Tripathy K. Pigment Dispersion Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 35593820]
Denis P. [Iridocorneal endothelial syndrome and glaucoma]. Journal francais d'ophtalmologie. 2007 Feb:30(2):189-95 [PubMed PMID: 17318107]
Kidd M, Hetherington J, Magee S. Surgical results in iridocorneal endothelial syndrome. Archives of ophthalmology (Chicago, Ill. : 1960). 1988 Feb:106(2):199-201 [PubMed PMID: 3341974]
Level 2 (mid-level) evidenceDoe EA, Budenz DL, Gedde SJ, Imami NR. Long-term surgical outcomes of patients with glaucoma secondary to the iridocorneal endothelial syndrome. Ophthalmology. 2001 Oct:108(10):1789-95 [PubMed PMID: 11581050]
Alió JL, Rodriguez AE, Toffaha BT, Piñero DP, Moreno LJ. Femtosecond-assisted keratopigmentation for functional and cosmetic restoration in essential iris atrophy. Journal of cataract and refractive surgery. 2011 Oct:37(10):1744-7. doi: 10.1016/j.jcrs.2011.08.003. Epub 2011 Aug 23 [PubMed PMID: 21865008]
Level 3 (low-level) evidenceKhng C, Snyder ME. Iris reconstruction with a multipiece endocapsular prosthesis in iridocorneal endothelial syndrome. Journal of cataract and refractive surgery. 2005 Nov:31(11):2051-4 [PubMed PMID: 16412914]
Level 3 (low-level) evidenceAgarwal R, Tripathy K, Jain M. Anterior segment optical coherence tomography of iridoschisis. BMJ case reports. 2021 Oct 5:14(10):. doi: 10.1136/bcr-2021-246020. Epub 2021 Oct 5 [PubMed PMID: 34610957]
Level 3 (low-level) evidenceTripathy K, Salini B. Aniridia. StatPearls. 2024 Jan:(): [PubMed PMID: 30844160]
Hingorani M, Hanson I, van Heyningen V. Aniridia. European journal of human genetics : EJHG. 2012 Oct:20(10):1011-7. doi: 10.1038/ejhg.2012.100. Epub 2012 Jun 13 [PubMed PMID: 22692063]
Sorkin N, Einan-Lifshitz A, Boutin T, Showail M, Borovik A, Chan CC, Rootman DS. Descemet membrane endothelial keratoplasty in iridocorneal endothelial syndrome and posterior polymorphous corneal dystrophy. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2019 Apr:54(2):190-195. doi: 10.1016/j.jcjo.2018.05.012. Epub 2018 Aug 23 [PubMed PMID: 30975342]
Rotenberg M, Downward L, Curnow E, Larkin DF, Tuft SJ, National Health Service Blood and Ocular Tissue Advisory Group and Contributing Ophthalmologists (OTAG Study 27). Graft Survival After Penetrating and Endothelial Keratoplasty in Iridocorneal Endothelial Syndrome. Cornea. 2020 Jan:39(1):18-22. doi: 10.1097/ICO.0000000000002039. Epub [PubMed PMID: 31335531]
García Caride S, Cuiña Sardiña R, Perucho González L. Combined cataract surgery and lamellar endothelial keratoplasty in iridocorneal endothelial syndrome. Archivos de la Sociedad Espanola de Oftalmologia. 2020 Sep:95(9):451-454. doi: 10.1016/j.oftal.2020.05.022. Epub 2020 Jun 25 [PubMed PMID: 32595005]