Introduction
Iris ectropion syndrome (ectropion iridis or ectropion uveae) is characterized by ectropion or the presence of the pigmented epithelium of the iris on the anterior surface of the iris. Iris ectropion syndrome can be congenital or acquired.[1][2] Congenital ectropion uveae (CEU, or primary iris pigment epithelial hyperplasia) was first reported by Colsman in 1869.[3] However, he was actually describing iris flocculi. Iris flocculi are congenital smooth cyst-like benign structures arising from the pigmented epithelium at the pupillary margin.[4]
Boleslaw Wicherkiewicz, a Polish ophthalmologist in 1891 and Spiro in 1896, are credited with reporting iris ectropion syndrome. Congenital iris ectropion is usually unilateral and nonprogressive, though bilateral cases have been reported.[5][6][7][8] Progressive open-angle glaucoma may be associated in some cases due to the dysgenesis of the angle of the anterior chamber.[9][10][11]
The posterior pigment epithelium of the iris is found on the anterior stroma at birth. The iris surface is glassy smooth and devoid of crypts. The pupil is usually round and reactive. Other features are anterior insertion of the root of the iris and trabecular dysgenesis. The sphincter muscle of the iris and the stroma are not affected. Developmental glaucoma is a frequent association. Mild to moderate ptosis with good levator function may be seen.[12][13][12] Neurofibromatosis, Prader-Willi syndrome, facial hemihypertrophy, prominent corneal nerves, asthma, dental problems (late-onset), and Rieger anomaly may be associated with congenital iris ectropion.[14][15][16]
Acquired iris ectropion can occur in many conditions and is more common than congenital ectropion uveae. Fibrovascular membrane formation on the anterior surface of the iris causes tractional forces pulling the posterior pigment epithelium of the iris to the anterior surface. This is associated with neovascularization of the iris.[17] This membrane can form due to proliferative diabetic retinopathy, venous occlusions, and ischemic, inflammatory, and neoplastic etiologies. Unlike congenital ectropion uveae, acquired ectropion is progressive unless the underlying causes are treated.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Iris ectropion syndrome can be congenital or acquired. Anatomically, the term ectropion uveae is inaccurate as the pigment layer of the iris is separate from the uvea.[18] However, the term ectropion uveae is widely used in the clinical setting. Genetic predisposition has not been reported except by a few reports. Neonatal-onset congenital ectropion uveae (NO-CEU) may be a phenotypic marker for specific CYP1B1 genotypes causing severe bilateral congenital glaucoma. Bilateral presumed ectropion uveae in a patient with pathological myopia has been reported.[19]
Congenital iris ectropion or congenital ectropion uveae (primary iris pigment epithelial hyperplasia) is a developmental anomaly due to the proliferation of the pigment epithelium in the anterior surface of the iris. In a normal iris, the posterior pigmented epithelium of the iris comes anteriorly at the pupillary margin and creates the pupillary ruff. In CEU, there is an exaggeration of this process (an apparent spread of the pigmented epithelium beyond the pupillary ruff to the anterior surface of the iris), and the pigmented epithelium covers the anterior surface of the iris to a variable extent though typically, the pigmentation does not reach the angle. Congenital iris ectropion has been attributed to arrested development of the neural crest cells and may fall in the spectrum of neurocristopathy.[20]
Neural crest cell migration, proliferation, and differentiation are essential in developing several ocular structures such as the corneal endothelium, trabecular meshwork, and the iris. Disorders in these aspects of neural crest cell proliferation, migration, or differentiation form the basis of the etiology of anterior segment dysgenesis syndromes such as Axenfeld Rieger syndrome, Peters anomaly, and posterior embryotoxon. During embryological development, after forming the optic cup, neural crest cells invade the primary mesenchyme surrounding it to form the secondary mesenchyme.
The neural crest cells in the secondary mesenchyme are responsible for the corneal stroma, the endothelium, the iris stroma, and the structures at the angle of the anterior chamber. The migration of the neural crest cells between the surface ectoderm and the lens vesicle occurs in three waves. Primordial corneal endothelium (and later corneal endothelium and Descemet membrane) and the trabecular meshwork form in the first wave. The keratocytes in the corneal stroma result from the second wave, and the iris (pupillary membrane and the iris stroma) forms in the third wave. The epithelial layers of the iris and ciliary develop from the neural ectoderm, and hence these are not considered a part of the uvea. The posterior non-pigmented epithelium (PNPE) of the iris and ciliary body is continuous.
PNPE of the iris later acquired pigments during the iris development. The pigmentation starts at the pupillary margin and progresses radially to the iris root. The anterior epithelial layer of the iris remains non-pigmented. The dilator muscles of the iris (dilator pupillae) develop directly from the anterior iris epithelial layer. The sphincter pupillae develops from neural ectoderm near the anterior epithelial layer of the iris adjacent to the pupillary border. The occurrence of CEU has been proposed due to the failure of regression of the primordial endothelium in the anterior segment.[21]
The neural crest cell origin disorders have been classified by Bahn et al. into 1) deficient neural crest formation and 2) disorders of neural crest cells migration, proliferation, and differentiation. Abnormal migration may lead to sclerocornea, Axenfeld anomaly and syndrome, congenital glaucoma, and posterior embryotoxon.[5][18][22]
In addition, abnormal proliferation may lead to Chandler syndrome, Essential iris atrophy, Iris nevus (Cogan Reese) syndrome, and abnormal differentiation results in the formation of either congenital hereditary endothelial dystrophy (CHED), posterior polymorphous dystrophy (PPMD), or Fuchs endothelial corneal dystrophy (FECD).[22] This classification has seen several changes. Chandler syndrome, essential iris atrophy, and iris nevus syndrome are now classified under ICE (iridocorneal endothelial) syndrome. It is distinguished by later onset, unilaterality, female preponderance, and is non-hereditary.[18] ICE syndrome also shows iris atrophy and acquired ectropion uveae.
Edward Wilson, in 1990 proposed an alternative classification wherein CEU was accepted as an addition to the anterior segment dysgenesis. This is based on the concept of developmental arrest. Examples of late arrest with retained primordial endothelium are posterior embryotoxon alone, Axenfeld- Rieger syndrome, and congenital iris ectropion syndrome. The second category in this classification is late partial arrest in the posterior iris migration. Classic congenital glaucoma falls in this category.
The third category is the late arrest of final differentiation, wherein the central cornea is also involved. ICE syndrome, CHED, and PPMD fall in this group. Shields proposed congenital iris ectropion to occur late in gestation due to a developmental arrest leading to retention of primordial tissue and anterior insertion of the iris. Anterior migration of pigment epithelium may be due to contact with the primordial tissue.[23] The activity or static state of the primordial tissue determines the quiescence or progression of the iris ectropion.
Acquired iris ectropion is seen in cases of rubeosis iridis and is frequently associated with neovascular glaucoma (NVG). Acquired iris ectropion is due to a fibrovascular membrane on the iris that contracts and pulls the posterior pigment epithelium anteriorly. The various etiologies of neovascularization of the iris and subsequently the development of acquired ectropion uveae include:
- Central retinal venous occlusion (CRVO): This is one of the most common causes. Up to 60% of eyes with ischemic CRVO may be associated with new vessels in the anterior segment (iris or the angle of the anterior chamber). The new vessels formed cause neovascular glaucoma, which typically occurs after 90 days (90-day glaucoma). The most important risk factors (according to the central retinal vein occlusion study) for the development of iris neovascularization include poor initial visual acuity, intraretinal hemorrhages, and a large area of capillary nonperfusion.[24]
- Proliferative diabetic retinopathy[25][26]
- Central retinal arterial occlusion (CRAO): The NVI usually forms in 18% of the eyes around 1 week to 3 months after CRAO (mean time around 1 month).
- Ocular ischemic syndrome (carotid artery occlusive disease): Around two-thirds of eyes have new vessels in the iris (NVI).
- Hemiretinal vein occlusion
- Branch retinal vein occlusion
- Chronic or very severe ocular inflammation
- Trauma
- Chronic retinal detachment[27]
- Coats disease[28]
- Intraocular tumor[29]
- Retinopathy of prematurity
- Radiation retinopathy
- Sickle cell disease
- Retinal vasculitis and Eales disease[30]
- Uveitis glaucoma hyphema syndrome
- Carotid-cavernous fistula
- Giant cell arteritis
- Takayasu disease
- Anterior segment ischemia due to various causing including squint surgery involving multiple extraocular muscles and scleral buckling)
- Iris melanoma or metastasis can cause vascularization of the iris and the angle of the anterior chamber followed by ectropion uveae. In a series of 25 cases with diffuse iris melanoma, 21 patients (84%) had ectropion uveae.[31] Breast cancer metastasizing to the iris may show ectropion uveae.[32] A rare association of Fuchs heterochromatic cyclitis, Usher syndrome, and ectropion uveae has been reported.[33]
Epidemiology
Acquired ectropion uveae is more common than congenital ectropion uveae (CEU). Till 1914, twenty-four patients with CEU were reported in the literature.[34] Ritch et al., in 1984, presented a series of eight cases and, in their review of the literature, found references of 30 patients. In the series by Ritch and colleagues, 7 out of 8 cases developed glaucoma. Dowling et al. reported nine patients with unilateral CEU ('iris pigment border hyperplasia') with glaucoma, iridotrabecular dysgenesis, stromal hypoplasia of the iris, anterior insertion of the root of the iris, and mild ptosis.[18] Subsequently, there have been isolated case reports of unilateral and bilateral CEU. In 2022, Kaushik et al. reported 13 neonates with bilateral ectropion uveae with severe glaucoma.
The epidemiology of acquired ectropion uveae varies according to the underlying cause. Ectropion uveae was found in twenty-four patients out of the 317 patients with iris melanoma by Shields et al. in a retrospective study over 40 years.[35] Acquired ectropion uveae are not uncommon in neovascular glaucoma and absolute glaucoma patients.
Pathophysiology
Congenital iris ectropion is attributed to a developmental arrest late in gestation, which leads to retention of the primordial endothelium over parts of the iris. There is a high insertion of the iris over the posterior trabecular meshwork due to incomplete regression of uveal tissue. The retention of the primordial tissue leads to a smooth cryptless anterior iris surface.[23]
An animal (cat) model of iris ectropion demonstrated the role of ischemia of the posterior segment. Lensectomy, vitrectomy, transection, and cautery of branch retinal vein consistently produced ectropion uveae and iris neovascularization. A cellular membrane with vessels developed on the anterior surface of the iris. Sphincter muscle and pigment epithelium of the iris was also pulled to the anterior surface. The contractile nature of the membrane was supported by this finding.[36]
Histopathology
Dowling et al. provided a detailed description of the histopathology of iris ectropion syndrome (congenital ectropion uveae or CEU) in the enucleated specimen from a patient with congenital ectropion uveae with iridotrabecular dysgenesis and loss of vision from secondary glaucoma.[18]
The surface of the iris was endothelialized at the termination of the iris pigment epithelial ectropion but not covering the ectropion. There was hyperplasia of the pigmented epithelium of the iris.[18] Wilson proposed that this tissue may induce or be a tractional force. Certain similarities are noted between Axenfeld Rieger syndrome and iris ectropion syndrome.[23]
Trabecular meshwork and Schelmm's canal are rudimentary in both syndromes. Arrested posterior migration of iris insertion and glassy smooth iris surface may also be seen in both. However, iris ectropion is usually nonprogressive, unlike Axenfeld Rieger syndrome, where a large amount of primordial endothelium, if present, may lead to continued contracture. This will result in corectopia and progressive iris holes. Ectropion uveae and smooth iris surface are usually not seen in congenital glaucoma.[37]
Goniotomy may be curative in congenital glaucoma, unlike Axenfeld Rieger and iris ectropion syndrome. The iris may show new vessels and intrastromal hemorrhage on histopathological examination of eyes with congenital iris ectropion. It has been suggested that the primary cause of CEU may be related to a vascular insult resulting in an abnormality of the migration of neural crest cells. A similar defect in neural crest cell migration has been noted in mice embryos deficient in hypoxia-inducible factor-1alpha leading to anomalous cardiac development and cardia bifida.[38]
History and Physical
Patients with congenital iris ectropion may present in infancy, childhood, or adolescence. Usually, there is no genetic predisposition. It is typically unilateral.[18] Glaucoma usually develops at mid-teen years. However, bilateral congenital ectropion in neonates with severe glaucoma (NO-CEU) has been reported. Patients may present for routine examination with no loss of visual acuity. Patients may also have decreased visual acuity, headache, redness, and watering.[39] Usually, the features of congenital glaucoma, including photophobia, epiphora, and blepharospasm, are not seen in CEU as congenital glaucoma usually does not occur.
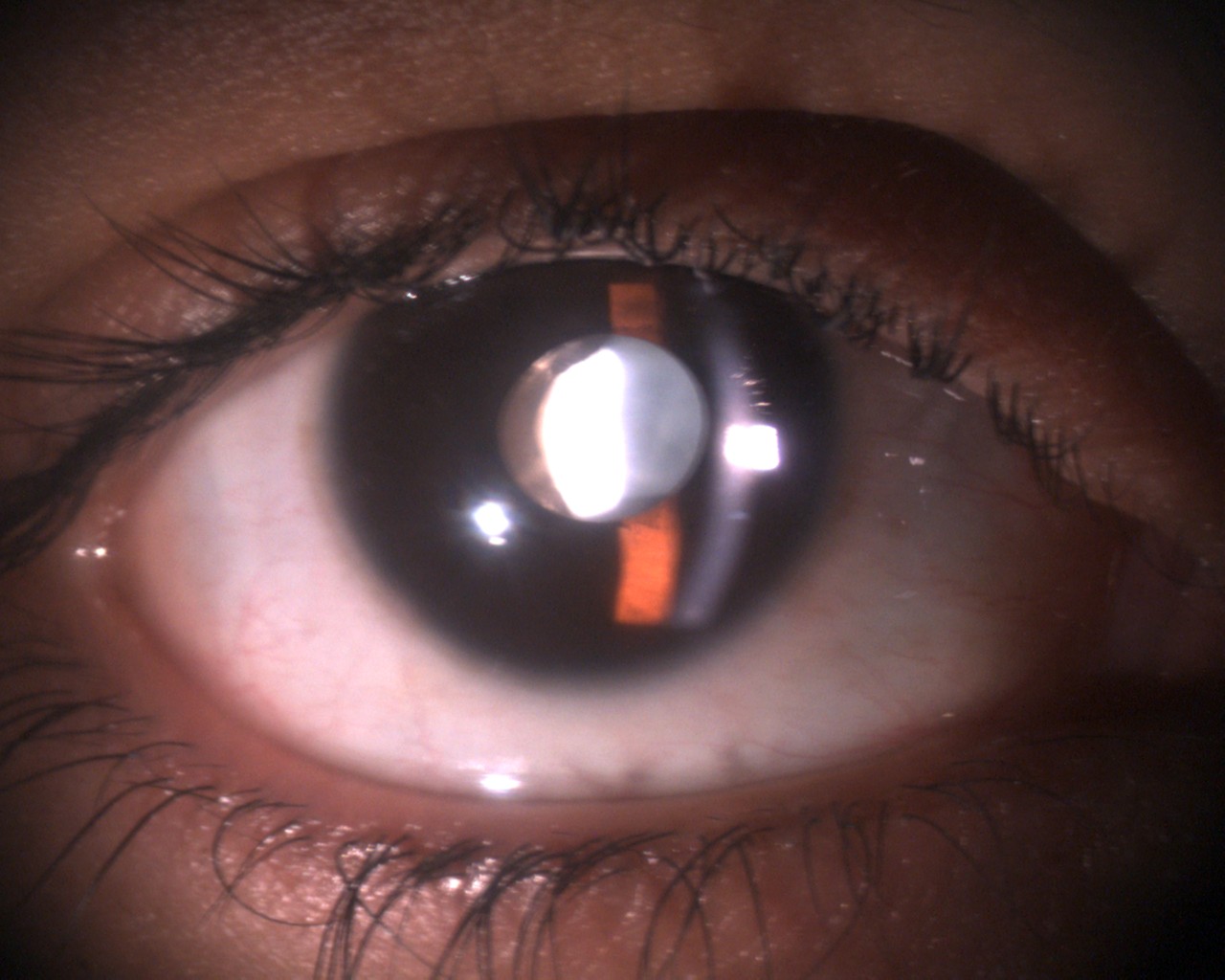
Slit-lamp biomicroscopy shows unilateral (rarely bilateral) pigmentation on the anterior iris surface. The extent of pigmentation varies. In CEU, the pigmentation nearly terminates with a scalloped border and does not reach the angle. The rest of the iris has a glassy and cryptless appearance without radial folds and concentric furrows. A variable degree of hypoplasia of the iris stroma is usually noted. The ectropion uveae may involve all around the pupil or maybe sectoral. Distortion of the round shape of the pupil may be noted in sectoral involvement with iris ectropion. The pupil may be round or oval. The pupil may not be in the center of the iris (corectopia). The size of the pupil may be normal or more significant than the fellow eye.[18]
The pupil may be normally reactive to light or, in some cases, less reactive to light. The cornea is nearly always transparent. Mild ipsilateral ptosis, mild proptosis, and prominent corneal nerves may be noted. The ptosis is usually related to dysfunction of the Müller's muscle which originates from the neural crest cells.[19] Telecanthus and iris coloboma have been reported.[39]
Systemic associations include neurofibromatosis type 1 and Prader Willi syndrome.[14][15] Glaucoma and raised intraocular pressure (IOP) may be present initially or develop at infancy, childhood, early adolescence, or even in the fifth decade.[40]
Gonioscopy shows anterior iris insertion, prominent iris processes, prominent Schwalbe line, pigmentation at the angle, and poorly developed trabecular meshwork. Fundus evaluation may show normal optic discs or varying stages of optic nerve damage due to glaucoma.
Fine iris new vessels, angle neovascularization, and raised intraocular pressure due to neovascular glaucoma may be seen in acquired ectropion. Gonioscopy may show new vessels in the angle of the anterior chamber. In early cases, a magnified examination of the pupillary border is necessary to identify fine tufts of new vessels which usually respond to therapy. The cornea may be clear or show epithelial edema due to raised intraocular pressure. The slit-lamp examination also reveals the cause of ectropion uveae, including inflammation. Fundus evaluation will show the pathology that led to the formation of new vessels, including venous occlusions, proliferative diabetic retinopathy, long-standing retinal detachment, and arterial occlusions. Severe ocular inflammation can also cause NVI.[41]
Evaluation
Clinical examination is often sufficient in congenital or acquired—Iris ectropion syndromes. In bilateral neonatal congenital iris ectropion, genetic evaluation has been done. Slit-lamp biomicroscopy, gonioscopy, intraocular pressure measurement, and fundus evaluation are routinely performed. Anterior segment photography helps in documenting the disease.
Depending on the etiology, ultrasound biomicroscopy, anterior segment optical coherence tomography (OCT), and fundus fluorescein angiography may be done. Specifically, evidence of peripheral capillary nonperfusion has to be looked for in the eyes with ectropion uveae. Ultrawide field fundus fluorescein angiography can pick up the peripheral areas of capillary nonperfusion even in small pupil and media haze.[42]
The management of glaucoma needs evaluation of the central corneal thickness, OCT of the optic disc and retinal nerve fiber layer, posterior segment photo, and Humphrey visual fields.[43]
Treatment / Management
In NO-CEU, early surgical management is needed in the form of trabeculotomy and trabeculectomy.[7] Goniotomy may not be effective.[10][11] Careful and long-term follow-up is required to detect glaucoma, which can develop at any age. In many patients, failure of filtering procedures may occur. Repeated filtering procedures may be required. In older patients, shunt procedures and valve surgeries may be necessary to control the IOP. The outcome is usually worse in NO-CEU compared to primary congenital glaucoma.[44] (B3)
Trabeculectomy with mitomycin C in a three-year-old girl with CEU resulted in good control of IOP and reversal of cupping of the optic nerve. Singh et al. reported good anatomical and visual outcomes in a 10-year-old boy with CEU and glaucoma with trabeculotomy and trabeculectomy with mitomycin C. They advocated early surgical management of glaucoma in patients with CEU. Similarly, early trabeculotomy in a three-month-old infant resulted in good control of IOP in the case reported by Leaks et al.[45] (B3)
Trabeculectomy with mitomycin C resulted in bleb failure in a seven-year-old boy with CEU and glaucoma along with ptosis and facial asymmetry necessitating shunt surgery.[46] Kaushik et al. reported the need for multiple surgeries in children who presented with bilateral congenital ectropion uveae and intractable glaucoma in infancy.(B3)
The management of acquired ectropion uveae should be directed to the cause. Most commonly, posterior segment ischemia and retinal capillary nonperfusion are the causes. The management of peripheral retinal capillary nonperfusion with multiple settings of scatter laser (pan-retinal photocoagulation) usually cause regression of the NVI. Anti-vascular endothelial growth factor (anti-VEGF) agents injected in the vitreous cavity, or the anterior chamber may cause regression of fine NVI though larger vessels usually remain. Neovascular glaucoma is a challenge to treat.
Preoperative anti-VEGF agents may increase the success rate and reduce ocular hemorrhage in eyes with NVG undergoing filtration surgery. Filtration surgery usually has poor success rates, and many cases eventually require glaucoma drainage devices. In eyes with painful blind eye due to NVG, the management options include diode laser cyclophotocoagulation, cyclocryotherapy, retrobulbar injection of absolute alcohol, and in extreme cases with very disturbing pain and enucleation. Many eyes with acquired iris ectropion also have macular edema, which is treated with intravitreal anti-VEGF agents, steroids, or even pars plana vitrectomy.[47][48] However, steroids should be cautiously used in such eyes predisposed to high IOP and NVG. Vitreous hemorrhage may need pars plana vitrectomy or may be treated with intravitreal anti-VEGF agents.[49][50][51] (B3)
Management of carotid artery occlusive disease includes removing carotid plaque and stenting the carotid artery, which can cause a reversal of the ocular ischemic features.[52](B3)
Differential Diagnosis
Congenital iris ectropion is a disorder caused by the arrested development of the neural crest. Conditions that have pigmented epithelium on the anterior surface of the iris are often confused with congenital iris ectropion. Axenfeld Rieger syndrome also may have iris ectropion which may be congenital or acquired. There is a smooth cryptless iris surface and secondary glaucoma. Unlike congenital iris ectropion syndrome, corectopia and iris holes are seen. It is a bilateral condition and has similar poor results with goniotomy.[23]
The iridocorneal endothelial (ICE) syndrome is also unilateral but presents later in life. The proliferation of pigmented epithelium is seen. The endothelial cells behave as epithelial cells that multiply and migrate. This differs from the retained primordial endothelium in congenital iris ectropion and Axenfeld Rieger syndrome.[22][23]
Prognosis
Congenital ectropion associated with glaucoma has a poor prognosis. Congenital glaucoma needs early surgical intervention in the form of trabeculotomy with trabeculectomy. Many times, bleb failure may occur, which leads to repeated surgeries. NO-CEU was associated with bilateral severe congenital glaucoma. There was worse corneal clarity and worse control of IOP without surgery in NO-CEU compared to neonatal-onset primary congenital glaucoma.[53] The prognosis of acquired ectropion uveae depends on the etiology.
Complications
The complications associated with congenital ectropion uveae include:
Deterrence and Patient Education
Congenital iris ectropion requires parent education regarding the need for long-term follow-up as glaucoma may manifest at any age. Management of refractive error, squint, and amblyopia is of paramount importance to improve visual acuity and binocular vision. Acquired ectropion of the iris may be associated with neovascular glaucoma. Urgent management with pan-retinal photocoagulation anti-vascular endothelial growth factor agents is needed to prevent a painful blind eye. Multiple visits may be necessary.
Enhancing Healthcare Team Outcomes
The management of congenital iris ectropion requires a multidisciplinary and interprofessional approach. CEU with glaucoma in children needs pediatric glaucoma specialists and requires the involvement of pediatricians, anesthesiologists, and internists. Pre and postoperative nursing care for the repeated evaluations under anesthesia and help of psychologists to counsel the parents may be required.[55]
The pharmacist helps to prepare correct doses of medications. Nursing staff plays a vital role in confirming compliance with therapy. Acquired iris ectropion has many systemic associations requiring physician evaluation. Collaboration with oncologists is necessary for many situations, such as breast cancer metastasizing to the iris.
Patients with acquired ectropion uveae have multiple comorbidities such as diabetes mellitus, hypertension, cardiovascular abnormalities, and in some cases, metastasis due to primary carcinomas elsewhere. Endocrinologists, cardiologists, internists, and oncologists will need to be consulted.
Interprofessional care coordination will drive improved patient outcomes with fewer adverse events. [Level 5]
Media
(Click Image to Enlarge)
References
Ritch R, Forbes M, Hetherington J Jr, Harrison R, Podos SM. Congenital ectropion uveae with glaucoma. Ophthalmology. 1984 Apr:91(4):326-31 [PubMed PMID: 6425761]
Level 3 (low-level) evidenceMarkovic V, Vukovic D, Radosavljevic A, Marjanovic I. Acquired ectropion uveae and secondary glaucoma due to trauma: report of 3 cases. European journal of ophthalmology. 2017 Jan 19:27(1):e1-e4. doi: 10.5301/ejo.5000893. Epub 2017 Jan 19 [PubMed PMID: 27768224]
Level 3 (low-level) evidenceHarasymowycz PJ, Papamatheakis DG, Eagle RC Jr, Wilson RP. Congenital ectropion uveae and glaucoma. Archives of ophthalmology (Chicago, Ill. : 1960). 2006 Feb:124(2):271-3 [PubMed PMID: 16476899]
Level 3 (low-level) evidenceYangzes S, Gupta A, Thakur A, Handa S. Congenital iris flocculi. QJM : monthly journal of the Association of Physicians. 2020 Jan 1:113(1):63. doi: 10.1093/qjmed/hcz113. Epub [PubMed PMID: 31125089]
Wilson ME. Congenital iris ectropion and a new classification for anterior segment dysgenesis. Journal of pediatric ophthalmology and strabismus. 1990 Jan-Feb:27(1):48-55 [PubMed PMID: 2324918]
Shifa JZ, Nkomazana O, Bekele NA, Kassa MW. A young Botswana patient with congenital iris ectropion uvea. The Pan African medical journal. 2016:25():42. doi: 10.11604/pamj.2016.25.42.10593. Epub 2016 Sep 29 [PubMed PMID: 28154731]
Kaushik S, Choudhary S, Kaur A, Srivastava P, Pokharel B, Akella M, Pandav SS. Neonatal-Onset Congenital Ectropion Uveae May Be Caused by a Distinct CYP1B1 Pathologic Variant. American journal of ophthalmology. 2022 Jul:239():54-65. doi: 10.1016/j.ajo.2022.01.014. Epub 2022 Jan 24 [PubMed PMID: 35085548]
Hertzberg R. Congenital ectropion uveae and glaucoma. Australian and New Zealand journal of ophthalmology. 1985 Feb:13(1):45-8 [PubMed PMID: 4015878]
Level 3 (low-level) evidencePoliti F, Sachs R, Barishak R. Neurofibromatosis and congenital glaucoma. A case report. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1977:176(3):155-9 [PubMed PMID: 418370]
Level 3 (low-level) evidenceWOLTER JR, BUTLER RG. PIGMENT SPOTS OF THE IRIS AND ECTROPION UVEAE. American journal of ophthalmology. 1963 Dec:56():964-73 [PubMed PMID: 14088677]
Mandal AK. Late-onset unilateral primary developmental glaucoma associated with iridotrabecular dysgenesis, congenital ectropion uveae and thickened corneal nerves: a new neural crest syndrome? Ophthalmic surgery and lasers. 1999 Jul-Aug:30(7):567-70 [PubMed PMID: 10929982]
Level 3 (low-level) evidenceLim FPM, Ho CL. Long-term treatment outcomes for congenital ectropion uveae with ptosis and glaucoma. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2020 Dec:24(6):369-371. doi: 10.1016/j.jaapos.2020.06.015. Epub 2020 Oct 24 [PubMed PMID: 33573764]
Iacovino C, Miraglia E, Moramarco A, Corbo G, Lambiase A, Giustini S. Ectropion Uveae in neurofibromatosis type 1: a new manifestation. La Clinica terapeutica. 2021 May 5:172(3):206-208. doi: 10.7417/CT.2021.2314. Epub [PubMed PMID: 33956037]
Futterweit W, Ritch R, Teekhasaenee C, Nelson ES. Coexistence of Prader-Willi syndrome, congenital ectropion uveae with glaucoma, and factor XI deficiency. JAMA. 1986 Jun 20:255(23):3280-2 [PubMed PMID: 3086578]
Level 3 (low-level) evidenceLibov AJ, Maino DM. Prader-Willi syndrome. Journal of the American Optometric Association. 1994 May:65(5):355-9 [PubMed PMID: 8071507]
Level 3 (low-level) evidenceSethi HS,Pal N,Dada T, Bilateral juvenile glaucoma with iridotrabecular dysgenesis, congenital ectropion uveae, and thickened corneal nerves. Eye (London, England). 2005 Dec; [PubMed PMID: 15618973]
Level 3 (low-level) evidenceBellotti A, Labbé A, Fayol N, El Mahtoufi A, Baudouin C. [OCT and neovascular glaucoma]. Journal francais d'ophtalmologie. 2007 Jun:30(6):586-91 [PubMed PMID: 17646747]
Level 2 (mid-level) evidenceDowling JL Jr, Albert DM, Nelson LB, Walton DS. Primary glaucoma associated with iridotrabecular dysgenesis and ectropion uveae. Ophthalmology. 1985 Jul:92(7):912-21 [PubMed PMID: 4022577]
Level 3 (low-level) evidenceKolomeyer AM, Kim BJ. Bilateral, presumed congenital ectropion uveae in a patient with pathologic myopia. American journal of ophthalmology case reports. 2018 Sep:11():119-120. doi: 10.1016/j.ajoc.2018.06.017. Epub 2018 Jun 20 [PubMed PMID: 30128365]
Level 3 (low-level) evidenceBéchetoille A, Ebran JM, Bigorgne J. [Congenital ectropion of the iris epithelium and glaucoma]. Journal francais d'ophtalmologie. 1985:8(8-9):529-34 [PubMed PMID: 3936868]
Level 3 (low-level) evidenceKumari R, Saha BC, Sinha BP. Congenital Ectropion Uveae with Glaucoma: A Case Report. Journal of clinical and diagnostic research : JCDR. 2017 Jun:11(6):ND04-ND05. doi: 10.7860/JCDR/2017/26198.10104. Epub 2017 Jun 1 [PubMed PMID: 28764212]
Level 3 (low-level) evidenceBahn CF, Falls HF, Varley GA, Meyer RF, Edelhauser HF, Bourne WM. Classification of corneal endothelial disorders based on neural crest origin. Ophthalmology. 1984 Jun:91(6):558-63 [PubMed PMID: 6462621]
Shields MB, Buckley E, Klintworth GK, Thresher R. Axenfeld-Rieger syndrome. A spectrum of developmental disorders. Survey of ophthalmology. 1985 May-Jun:29(6):387-409 [PubMed PMID: 3892740]
Level 3 (low-level) evidence. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995 Oct:102(10):1434-44 [PubMed PMID: 9097789]
Level 1 (high-level) evidenceShukla UV, Tripathy K. Diabetic Retinopathy. StatPearls. 2024 Jan:(): [PubMed PMID: 32809640]
Mishra C, Tripathy K. Retinal Traction Detachment. StatPearls. 2024 Jan:(): [PubMed PMID: 32644378]
Tripathy K, Chawla R, Wadekar BR, Venkatesh P, Sharma YR. Evaluation of rhegmatogenous retinal detachments using Optos ultrawide field fundus fluorescein angiography and comparison with ETDRS 7 field overlay. Journal of current ophthalmology. 2018 Sep:30(3):263-267. doi: 10.1016/j.joco.2018.06.006. Epub 2018 Jul 3 [PubMed PMID: 30197958]
Gupta A, Paulbuddhe VS, Shukla UV, Tripathy K. Exudative Retinitis (Coats Disease). StatPearls. 2024 Jan:(): [PubMed PMID: 32809517]
Kanukollu VM, Tripathy K. Leukocoria. StatPearls. 2022 Jan:(): [PubMed PMID: 32809629]
Raizada K, Tripathy K. Eales Disease. StatPearls. 2022 Jan:(): [PubMed PMID: 32644547]
Demirci H, Shields CL, Shields JA, Eagle RC Jr, Honavar SG. Diffuse iris melanoma: a report of 25 cases. Ophthalmology. 2002 Aug:109(8):1553-60 [PubMed PMID: 12153810]
Level 2 (mid-level) evidenceSassi H, Ouederni M, Ben Aoun S, Bouguila H, Cheour M. [Breast cancer metastatic to the iris presenting as ectropion uveae]. Journal francais d'ophtalmologie. 2021 Oct:44(8):1297-1299. doi: 10.1016/j.jfo.2020.12.014. Epub 2021 Aug 24 [PubMed PMID: 34452769]
Rezaei L, Ahmadyani R. A Very Rare Association of Fuchs Heterochromic Uveitis and Ectropion Uvea in Usher Syndrome. Advanced biomedical research. 2021:10():50. doi: 10.4103/abr.abr_286_20. Epub 2021 Dec 25 [PubMed PMID: 35127577]
Singh K, Bhattacharyya M, Gotmare N, Joon A, Aggarwal H, Singh A. Congenital ectropion uveae (CEU) with refractory glaucoma: Early trabeculectomy saves vision. European journal of ophthalmology. 2021 Mar:31(2):NP112-NP115. doi: 10.1177/1120672119860379. Epub 2019 Jul 8 [PubMed PMID: 31282208]
Shields CL, Kaliki S, Shah SU, Luo W, Furuta M, Shields JA. Iris melanoma: features and prognosis in 317 children and adults. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2012 Feb:16(1):10-6. doi: 10.1016/j.jaapos.2011.10.012. Epub [PubMed PMID: 22370659]
Level 2 (mid-level) evidenceHjelmeland LM, Stewart MW, Li J, Toth CA, Burns MS, Landers MB 3rd. An experimental model of ectropion uveae and iris neovascularization in the cat. Investigative ophthalmology & visual science. 1992 Apr:33(5):1796-803 [PubMed PMID: 1373124]
Level 3 (low-level) evidenceAnderson DR. The development of the trabecular meshwork and its abnormality in primary infantile glaucoma. Transactions of the American Ophthalmological Society. 1981:79():458-85 [PubMed PMID: 7342408]
Level 3 (low-level) evidenceCompernolle V, Brusselmans K, Franco D, Moorman A, Dewerchin M, Collen D, Carmeliet P. Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1alpha. Cardiovascular research. 2003 Dec 1:60(3):569-79 [PubMed PMID: 14659802]
Level 3 (low-level) evidenceKumar V, Kumar K, Chanana B, Rohatgi J, Gupta VP. Congenital ectropion uveae with iris coloboma and telecanthus. Contact lens & anterior eye : the journal of the British Contact Lens Association. 2011 Jun:34(3):147-8. doi: 10.1016/j.clae.2010.12.007. Epub 2011 Jan 20 [PubMed PMID: 21256075]
Level 3 (low-level) evidenceQuigley HA, Stanish FS. Unilateral congenital iris pigment epithelial hyperplasia associated with late-onset glaucoma. American journal of ophthalmology. 1978 Aug:86(2):182-4 [PubMed PMID: 686120]
Level 3 (low-level) evidenceKuo IC, Cunningham ET Jr. Ocular neovascularization in patients with uveitis. International ophthalmology clinics. 2000 Spring:40(2):111-26 [PubMed PMID: 10791260]
Tripathy K, Chawla R, Vohra R. Evaluation of the fundus in poorly dilating diabetic pupils using ultrawide field imaging. Clinical & experimental optometry. 2017 Nov:100(6):735-736. doi: 10.1111/cxo.12484. Epub 2016 Oct 5 [PubMed PMID: 27704602]
Mahabadi N, Foris LA, Tripathy K. Open Angle Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 28722917]
Kaushik S, Dhingra D, Arora A, Singh MP, Kaur S, Joshi G, Snehi S, Gupta G, Pandav SS. Clinical profile and outcome of early surgery in neonatal-onset glaucoma presenting over a 5-year period. The British journal of ophthalmology. 2022 Mar:106(3):368-375. doi: 10.1136/bjophthalmol-2020-317230. Epub 2020 Dec 2 [PubMed PMID: 33268344]
Laaks D, Freeman N. Congenital iris ectropion uveae presenting with glaucoma in infancy. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2013 Apr:17(2):214-6. doi: 10.1016/j.jaapos.2012.11.010. Epub 2013 Mar 21 [PubMed PMID: 23522945]
Level 3 (low-level) evidenceHatami M, Doozandeh A, Feizi M. Glaucoma in Ectropion Uveae Syndrome: A Case Report and Literature Review. Journal of ophthalmic & vision research. 2019 Jul-Sep:14(3):370-375. doi: 10.18502/jovr.v14i3.4793. Epub 2019 Jul 18 [PubMed PMID: 31660115]
Level 3 (low-level) evidenceTripathy K, Sharma YR, R K, Chawla R, Gogia V, Singh SK, Venkatesh P, Vohra R. Recent advances in management of diabetic macular edema. Current diabetes reviews. 2015:11(2):79-97 [PubMed PMID: 25801496]
Level 3 (low-level) evidenceTripathy K. Re: Pars plana vitrectomy for treatment-naïve diabetic macular edema with or without vitreomacular traction. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2018 Oct:53(5):548. doi: 10.1016/j.jcjo.2018.01.015. Epub 2018 Feb 17 [PubMed PMID: 30340728]
Jena S, Tripathy K. Vitreous Hemorrhage. StatPearls. 2024 Jan:(): [PubMed PMID: 32644557]
Tripathy K. Intravitreal Bevacizumab for the Treatment of Vitreous Hemorrhage Due to Proliferative Diabetic Retinopathy. American journal of ophthalmology. 2017 May:177():229. doi: 10.1016/j.ajo.2017.01.034. Epub 2017 Mar 10 [PubMed PMID: 28285715]
Tripathy K. Encouraging results of 25G+ minimally invasive vitrectomy surgery for diabetic tractional retinal detachment. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2017 Sep:255(9):1863-1864. doi: 10.1007/s00417-017-3730-x. Epub 2017 Jun 30 [PubMed PMID: 28667484]
Tripathy K, Mazumdar S. Recurrent retinal and choroidal ischemia in a case of ocular ischemic syndrome. Therapeutic advances in ophthalmology. 2019 Jan-Dec:11():2515841419848926. doi: 10.1177/2515841419848926. Epub 2019 Jul 12 [PubMed PMID: 31321381]
Level 3 (low-level) evidenceKaushik S, Dhingra D, Vibha B, Saini A, Gupta G, Snehi S, Singh N, Thattaruthody F, Pandav SS. Neonatal-Onset Congenital Ectropion Uveae: A Distinct Phenotype of Newborn Glaucoma. American journal of ophthalmology. 2021 Mar:223():83-90. doi: 10.1016/j.ajo.2020.10.001. Epub 2020 Oct 9 [PubMed PMID: 33045217]
Tripathy K, Chawla R, Temkar S, Sagar P, Kashyap S, Pushker N, Sharma YR. Phthisis Bulbi-a Clinicopathological Perspective. Seminars in ophthalmology. 2018:33(6):788-803. doi: 10.1080/08820538.2018.1477966. Epub 2018 Jun 14 [PubMed PMID: 29902388]
Level 3 (low-level) evidencePirlich N, Grehn F, Mohnke K, Maucher K, Schuster A, Wittenmeier E, Schmidtmann I, Hoffmann EM. Anaesthetic protocol for paediatric glaucoma examinations: the prospective EyeBIS Study protocol. BMJ open. 2021 Oct 5:11(10):e045906. doi: 10.1136/bmjopen-2020-045906. Epub 2021 Oct 5 [PubMed PMID: 34610927]