Retinal Vascular Anomalies (VHL, Cavernous Hemangioma, Wyburn-Mason)
Retinal Vascular Anomalies (VHL, Cavernous Hemangioma, Wyburn-Mason)
Introduction
Retinal vascular anomalies are rare hereditary or sporadic conditions affecting the retina and multiple organ systems. These anomalies include vascular tumors and telangiectasias. The vascular tumors of the retina are retinal capillary hemangiomas, cavernous hemangiomas, racemose hemangiomas, and retinal vasoproliferative tumors. The retinal telangiectasias are observed in conditions such as Coats disease, Leber's miliary aneurysms, and idiopathic juxtafoveal telangiectasias.[1]
Capillary hemangiomas of the retina were first reported by Vigla in 1864 in a patient with central nervous system (CNS) lesions.[2] Retinal capillary hemangiomas are benign vascular tumors originating from the neurosensory retina or optic disc. These tumors are sporadic or associated with Von Hippel-Lindau (VHL) syndrome.[3] VHL syndrome is an inherited autosomal dominant disorder characterized by vascular tumors and cysts in multiple organ systems.[4] Conditions associated with VHL syndrome include CNS and retinal hemangioblastomas, renal cell carcinoma, pheochromocytoma, pancreatic islet tumors, endolymphatic sac tumors, and epididymal, renal, and pancreatic cystadenomas.[5][6] The retinal tumors typically present as a reddish-orange mass in the retinal periphery, supplied by a pair of dilated and tortuous vessels.[7] Vision loss is due to retinal exudation, detachment, vitreous hemorrhage, and neovascular glaucoma.[8]
Cavernous hemangiomas of the retina, also known as retinal cavernomas, were initially described as angiomatosis retinae by Niccol and Moore in 1934.[9][10] These hemangiomas are rare non-progressive retinal vascular hamartomas. In some cases, they may involve the CNS, which can cause significant morbidity and mortality if not diagnosed and treated early.
Retinal capillary hemangiomas can manifest as a solitary lesion or part of Wyburn-Mason syndrome, also known as Bonnet-Dechaume-Blanc syndrome.[11] This rare congenital disorder is sporadic and results in arteriovenous malformations (AVM) in the retina, visual pathways, and midbrain, maxilla, and mandible regions.[12] During ophthalmoscopic examination, dilated and tortuous vessels from the optic disc to the retinal periphery are observed.[13] The visual acuity upon presentation ranges from normal to poor, depending on the associated complications.[14]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The retinal capillary hemangiomas, also called retinal hemangioblastomas, may present in isolation or as a significant manifestation of VHL syndrome. Mutation in the VHL gene at chromosome 3p25.3 is primarily associated with this autosomal dominant syndrome.[15] The VHL gene encodes the VHL protein (pVHL) responsible for the degradation of ubiquitin-mediated hypoxia-inducible factor (HIF).[16] The inactivation of pVHL leads to sustained activation of HIF proteins, triggering angiogenesis and tumor formation in retinal hemangioblastomas.[17]
Retinal cavernous hemangiomas can be sporadic or inherited in an autosomal dominant form.[18] Familial retinal cavernomas have associated cerebral and cutaneous involvement.[19] In combined retinal and cerebral cavernous hemangiomas, multiple cerebral cavernous malformation gene mutations, including CCM1/KRIT1 at chromosome 7q21.2, CCM2/MGC4607 at chromosome 7p13, and CCM3/PDCD10 at chromosome 3q26.1, have been identified.[20] A somatic mutation in the PIK3CA gene at chromosome 3q26.32 has been reported to cause sporadic cerebral cavernous malformation, also called CCM4.[21]
Racemose hemangiomas in Wyburn-Mason syndrome occur due to a developmental abnormality that affects the primitive vascular mesoderm, shared by the developing optic cup and anterior neural tube.[12] The anterior vascular plexus is responsible for the formation of the retinal and hyaloid vessels in the eye and the vasculature of the midbrain.[22]
Epidemiology
Retinal capillary hemangiomas represent the most common and often earliest manifestation of VHL syndrome.[23] The prevalence of retinal capillary hemangiomas in VHL is reported to be up to 1 in 73,080 individuals, with a higher occurrence observed in Europeans compared to non-Europeans.[24][25] Patients with solitary retinal hemangiomas have a 30% to 46% chance of developing VHL at a later stage.[26] The mean age of diagnosis of retinal capillary hemangiomas in VHL is 25 years.[27] Both men and women are equally affected. VHL-associated tumors are typically bilateral or multiple. Evidence of genetic anticipation exists; the earlier the onset, the more severe the presentation is in successive generations.[28] The average life expectancy of patients with VHL syndrome is between ages 40 and 52, with CNS hemangioblastomas being the primary cause of mortality.[29]
Cavernous hemangiomas of the retina are more common in White patients, with no apparent gender predilection. The median age of presentation is 21.[30] Familial type of cavernous malformations has an autosomal dominant inheritance with high penetrance and variable expressivity.[31] The frequency of retinal cavernous hemangiomas is estimated at 5% in patients with familial cerebral cavernous hemangiomas.[10]
The exact prevalence of racemose hemangiomas is not known due to their rarity.[32] No racial or gender preference is recognized. The mean age of presentation is before the third decade of life.[22] Hemodynamic changes in high-flow arteriovenous malformations can result in intra-lesional vessel wall involution, potentially leading to retinal or choroidal ischemia and degeneration.[33] Minor arteriovenous malformations may remain asymptomatic and are diagnosed at a later stage.[34]
Pathophysiology
In VHL syndrome, a mutation in the VHL gene leads to the inactivation of pVHL.[35] This failure of hypoxia-inducible factor degradation creates a pseudo-hypoxia state, leading to dysregulated angiogenesis, cellular proliferation, and a shift in glycolytic metabolism, thereby promoting the formation of hemangioblastomas.[36] The VHL gene exhibits 3 types of mutations, each with distinct characteristics as follows:
- The first type of mutation is a deletion or nonsense mutation, typically observed in isolated hemangioblastomas.
- The second type of mutation is a missense mutation, which is further classified into 3 subtypes—type 2A, type 2B, and type 2C. In type 2A, patients exhibit both hemangioblastomas and pheochromocytomas. In contrast, in type 2B, patients also present with renal cell carcinomas. Type 2C is characterized by the presence of only pheochromocytomas. Missense mutations are predominantly found in patients with retinal cavernous hemangiomas.
- The third type of mutation, known as type 3, is associated with polycythemia.[37][38]
Retinal cavernous hemangiomas have been described as localized vascular hamartomas partly separated from the normal retinal vascular system or as congenital venous malformations with the same growth potential as VHL syndrome.[39] The saccular dilatations in the hemangiomas exhibit the anatomy of normal retinal vessels, explaining the lack of exudation or hemorrhage.[40]
Retinal hemangiomas in Wyburn-Mason syndrome occur due to a developmental defect in the primitive vascular plexus.[41] Disturbing tissue before the seventh week of gestation can result in anomalous vessels in the eye and ipsilateral mesencephalon.[12] However, if the disturbance occurs after the seventh week of pregnancy, it will only affect one of the structures.[42] The direct communication between arterial and venous systems in high-flow arteriovenous malformations leads to turbulent blood flow, damage to the vessel walls, thrombosis, and vessel occlusion.[43] High-flow arteriovenous malformations can sometimes cause edema from capillary leakage or pressure changes.[44]
Histopathology
Histopathological examination of retinal capillary hemangiomas shows the presence of unusual capillary-like channels occupying all the layers of the retina that are surrounded by foamy cells with vacuoles.[45][46] The tumors do not have endothelial cells; some may have reactive glial proliferation at the edges of larger hemangioblastomas. Chan et al have reported the presence of sporadic tumorlet cells in ocular VHL-associated lesions.[47] These cells form angio-mesenchymal islands and are frequently adjacent to small retinal vessels or within the inner retinal layers. Ocular hemangioblastomas exhibit high levels of cellular markers, including CD133, erythropoietin, and erythropoietin receptor.[48]
Histopathological examination of retinal cavernous hemangioma shows the presence of large-sized vascular spaces lined by normal endothelium involving all the retinal layers. These vessels are surrounded by thick fibroglial septa, occasionally containing glial cells.[49] Upon ultrastructural examination, the vessels are lined by a continuous layer of non-fenestrated endothelial cells, which exhibit terminal bars on the luminal side and a thin, uninterrupted basal membrane. The basal membrane is further surrounded by an interrupted layer of pericytes encased by their basal membrane.[40]
Retinal hemangiomas have a lack of histopathological data. Distinguishing between arteries and veins is complex, and the lesions occupy the entire retina thickness. The abnormal vessels are made of fibromuscular media and acellular adventitious sheath.[34]
History and Physical
The presentation of retinal capillary hemangiomas depends on the size and location of the tumor. Family history plays an essential role in identifying VHL syndrome. The usual complaint is progressive vision loss.[13] Cerebellar hemangioblastomas may present with headache, vomiting, ataxia, and sensory and motor deficits.[50] Endolymphatic sac tumors are rare and typically present with symptoms such as aural fullness, disequilibrium, and hearing loss. Pancreatic and renal cysts are typically asymptomatic.[51] Patients with adrenal pheochromocytomas present with sweating, palpitation, hypertension, pallor, headache, or nausea.[52] Many lesions are detected incidentally on routine dilated fundus examination. Cystadenomas of the epididymis, if present bilaterally, can rarely cause infertility.[53]
Cavernous hemangiomas are usually asymptomatic but can present with a mild-to-moderate decrease in visual acuity.[54] CNS involvement may present with headaches, seizures, and visual disturbances.[10] Retinal hemangiomas of the eye are asymptomatic in smaller arteriovenous malformations and are incidentally detected during a routine examination.[22] Larger arteriovenous malformations may cause severe vision loss, and they present with vitreous hemorrhage, retinal detachment, venous occlusions, optic disc edema, and optic atrophy.[55] Neurological symptoms include headache, seizures, hemiparesis, cranial neuropathies, and hydrocephalus.[12]
Evaluation
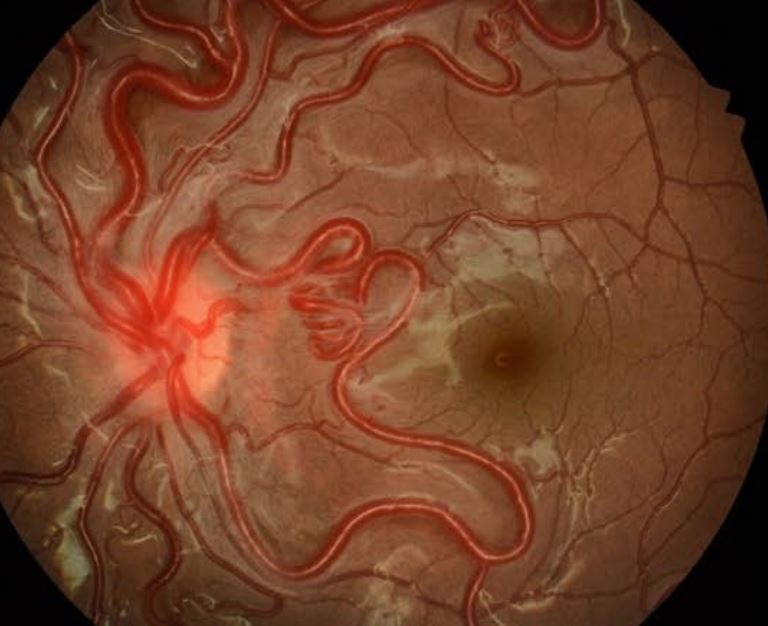
The diagnosis of retinal capillary hemangiomas is made through clinical examination.[56] Dilated ophthalmoscopic examination reveals a reddish-orange mass with dilated and tortuous afferent arterioles and efferent venules (see Image. Retinal Capillary Hemangioma). The most common location is the mid-peripheral temporal quadrant of the retina.[57] However, 15% of the lesions are in the juxtapapillary or peripapillary retina.[7] The incipient lesions appear as small aneurysmal dilatations of retinal capillaries. The optic nerve hemangioblastomas lack the feeding artery and draining vein, as observed in retinal hemangioblastomas, and are often misdiagnosed. Three types of optic nerve hemangioblastomas are endophytic, exophytic, and sessile.[58]
- Endophytic tumors appear as round, reddish lesions projecting into the pre-papillary vitreous.
- Exophytic lesions extend from the disc margin to the sub-retinal space and appear as a yellow, nodular tumor.
- Sessile types are challenging to detect as they appear as a localized thickening at the neuroretinal rim without prominent coloration. Sessile hemangioblastomas at the juxtapapillary retina show vascularity on optical coherence tomography angiography (OCTA).[52][59][60]
Fundus fluorescein angiography (FFA) is the most important diagnostic tool due to the tumor's vascular nature.[61] This technique is useful in detecting subclinical pinpoint tumors as small as the width of a third-order retinal artery and in detecting leakage from the mass into the vitreous cavity and adjacent retina in patients with distant macular edema.[62] Ultrasonography shows an acoustically solid intraocular tumor with or without surrounding subretinal fluid.[52] Optical coherence tomography (OCT) shows intraretinal tumors involving the entire retinal thickness and associated retinal changes such as retinal edema, hard exudates (hyperreflective lesions), and the epiretinal membrane.[63] OCTA helps measure the dimensions and visualize the tumor's structure and response to treatment.[64] On OCTA, the lesions are bright, well-defined, and elevated, involving superficial and deeper retinal layers or surrounded by void areas in the deep capillary plexus.[65]
- CNS hemangioblastomas most frequently occur in the cerebellum (60%), spinal cord (30%), and brainstem (10%) and rarely involve optic pathways and choroidal plexus (1%). Magnetic resonance imaging (MRI) of the brain can show nodular lesions with adjacent cysts.
- Endolymphatic sac tumors are rare and appear as enhancing cysts in MRI.
- Renal manifestations include benign renal cysts and clear cell renal carcinomas diagnosed using MRI.
- Pheochromocytomas are found in approximately 20% of patients with VHL syndrome, and they are diagnosed by testing urinary catecholamines and plasma-free metanephrines compounded with abdominal ultrasound and MRI.
- Pancreatic manifestations, such as cysts, serous cystadenomas, and neuroendocrine tumors, are typically asymptomatic and are identified through MRI.
- Epididymal cysts are found in 25% to 60% of men and can be identified by palpation. Ultrasound demonstrates a solid mass; sometimes, cystic lesions can be noted.[52][66][67][68][69][70]
Genetic testing to identify VHL gene mutation can benefit family members using southern blot and multiplex ligation-dependent probe amplification techniques.[71]
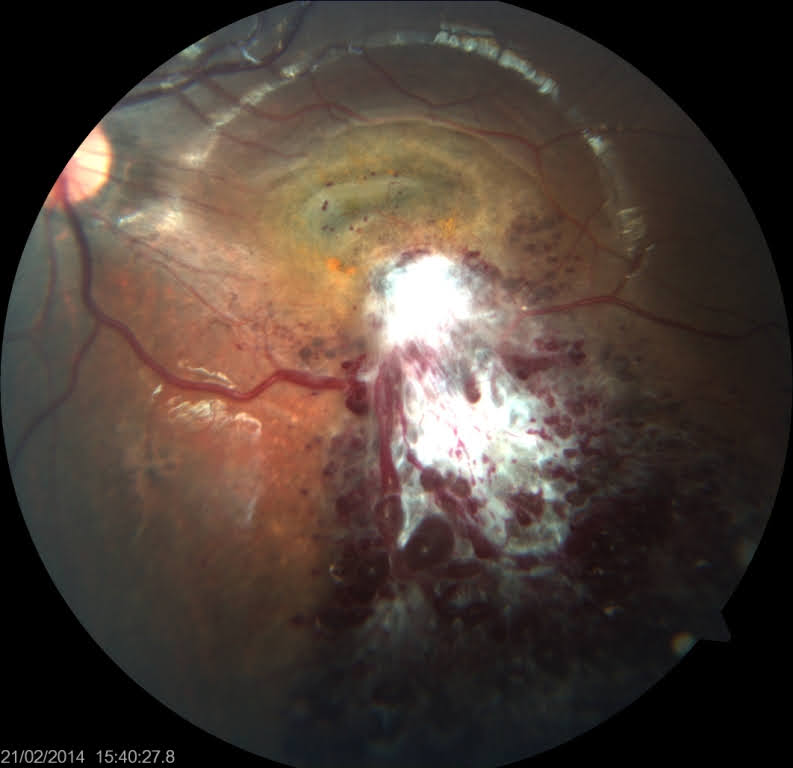
Cavernous vascular hemangioma of the retina can be diagnosed through dilated fundoscopy. The lesions appear as demarcated dark, intraretinal grape-like clusters of dilated and saccular blood vessels along the retinal vein or on the optic disc (see Image. Retinal Cavernous Hemangioma).[72] Aneurysms may be small and subtle or extensive, involving multiple quadrants of the retina.[73] These aneurysms are raised above the retinal surface, with grey and white glial zones covering the surface due to prior recurrent hemorrhages.[74] FFA typically shows hypofluorescence in the arterial phase with a slow appearance of fluorescein within the venous aneurysms.[32] Aneurysm filling is characterized by a fluorescein-erythrocyte interface. Red blood cells settle at the bottom leaving plasma on the top, creating fluorescein-blood levels.[75] OCT demonstrates a lobulated hyperreflective mass with clear cystic spaces.[76] Ultrasonography shows a well-defined, acoustically solid mass and helps detect large tumors obscured by vitreous hemorrhage. Familial forms of cavernous hemangiomas are associated with cutaneous and cerebral cavernous hemangiomas. Cerebral cavernous hemangioma presents with headaches, seizures, and transient visual disturbances. Cerebral cavernous hemangiomas are 3 times more common in the supratentorial part of the brain compared to in the infratentorial part of the brain.[32] Brain MRI is needed to identify CNS cavernomas.[30] Genetic testing for CCM genes should be completed in appropriate clinical settings in case of multiple hemangiomas or positive family history.[77]
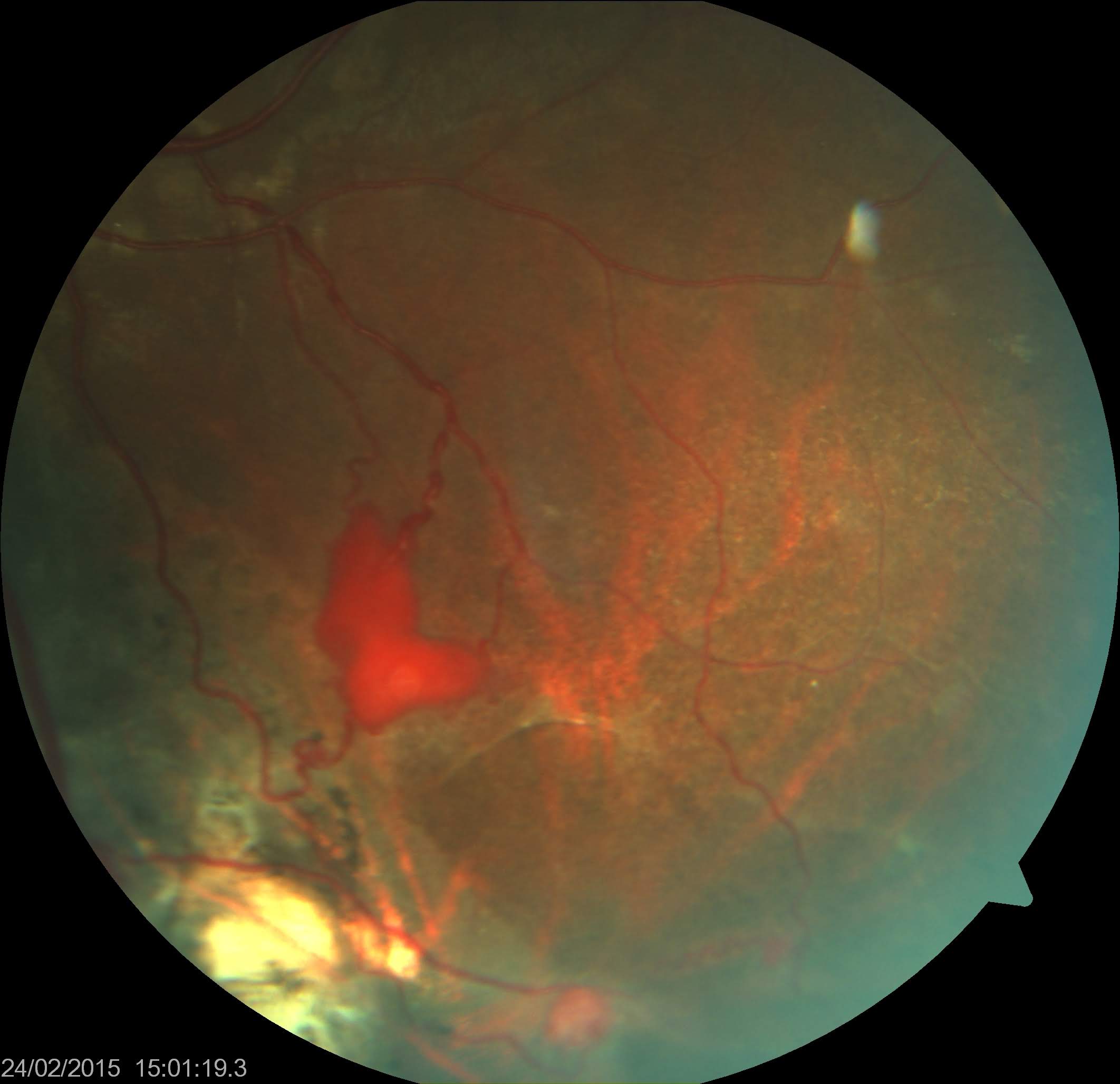
Ophthalmoscopic examination of retinal racemose angiomas shows a large, dilated, tortuous retinal artery extending from the optic disc and directly connecting to the retinal vein without any intervening capillaries (see Image. Retinal Racemose Angioma).[13][78] Arteriovenous communications of the retina or retinal arteriovenous malformations can be classified into three groups based on their characteristics.[33][79]
- Group 1 involves an arteriolar or abnormal capillary plexus between the retinal artery and vein.
- Group 2 involves direct arteriovenous communication without an intervening capillary plexus. Type 1 and type 2 lesions typically do not involve the CNS.
- Group 3 involves arteriovenous shunts with large convoluted vessels. The retinal arteries and veins cannot be differentiated. Such lesions are typical of congenital retinocephalofacial vascular malformation syndrome or Wyburn Mason syndrome, with a high incidence of central nervous system lesions.[80][81]
Typically, no exudation or hemorrhage is present. FFA findings show rapid filling of the affected blood vessels without any leakage. OCTA is a better tool for detecting the vessel of origin and resolving associated leaking macroaneurysms following treatment (focal laser). Some associated macroaneurysms may have spontaneous thrombosis.[82] Vascular lesions in the CNS commonly affect the midbrain and tend to occur on the same side as the affected eye.[32] Intracranial arteriovenous malformations are diagnosed using imaging techniques such as computed tomography (CT), MRI, and magnetic resonance angiography (MRA).
Treatment / Management
Treatment of capillary hemangiomas is decided based on the tumor location, number, size, and secondary effects. Inactive fibrotic lesions can be left untreated. Laser photocoagulation is an effective treatment modality for smaller capillary hemangiomas with basal diameters up to 4 mm.[83] Alternatively, cryotherapy can manage tumors up to 5 mm but requires multiple sessions to achieve optimal results.[84] However, cryotherapy can cause a transient increase in exudative or tractional vitreoretinopathy.[85] More extensive tumors require external beam radiotherapy, plaque brachytherapy, or proton beam therapy.[86] Advanced cases with exudative and tractional retinal detachment may need vitreoretinal surgery.[7] Surgical endoresection of the tumor can improve visual outcomes with minimal sequelae. Endodiathermy or feeder vessel ligation may reduce the risk of intraoperative bleeding and provide better tumor control.[87] Juxtapapillary retinal hemangiomas can pose a significant challenge to treat due to their location in the papillomacular bundle.[88] However, verteporfin photodynamic therapy has shown promise as an effective treatment option for these lesions.[89] The ability to cause selective vascular occlusion with lesser damage to the optic nerve makes it a viable alternative.[90] (A1)
The results of several case reports have suggested that combined anti-vascular endothelial growth factor (anti-VEGF) agents and photodynamic therapy may hold promise as a therapeutic option for certain retinal hemangiomas.[91] Belzutifan is a HIF-2-α inhibitor and is the first Food and Drug Administration (FDA)-approved systemic oral drug for VHL-associated tumors such as renal cell carcinoma, CNS hemangioblastoma, and pancreatic neuroendocrine tumors. The most common adverse effects include anemia, nausea, headache, and raised blood glucose, and it is harmful in pregnancy.[92] Sunitinib, a tyrosine kinase inhibitor, has shown promising results in treating renal carcinomas in VHL syndrome but has not been effective in treating hemangioblastomas.[93] However, disease progression, fatigue, and hand-foot syndrome were noted in patients with advanced VHL disease.[94] Dovitinib, a tyrosine kinase inhibitor of the VEGF receptor and fibroblast growth factor, also resulted in adverse effects such as maculopapular rash, diarrhea, and fatigue.[95] Pazopanib has demonstrated clinical responses in renal tumors of VHL despite adverse effects such as diarrhea, hypertension, fatigue, and transaminitis.[96] (B3)
Retinal cavernous hemangiomas typically remain stable and asymptomatic and, therefore, do not require intervention.[30] Mild vitreous or retinal bleeding generally resolves spontaneously without medical intervention.[32] However, if the subretinal or vitreous hemorrhage is severe, cryotherapy and laser photocoagulation may be recommended as potential treatment options.[97] Systemic infliximab infusion has been reported to cause temporary tumor regression.[98] Recently, photodynamic therapy has proven to be a safer option for patients with symptomatic hemangiomas.[99]
The management approach for retinal hemangiomas depends on the location of the arteriovenous malformations and the associated symptoms. Conservative management is typically recommended for asymptomatic arteriovenous malformations. However, symptomatic cases with complications, such as retinal ischemia, may necessitate laser photocoagulation, pars plana vitrectomy to address non-clearing vitreous hemorrhage, or cyclo-destructive procedures for painful blind eye caused by neovascular glaucoma.[100] Intracranial arteriovenous malformations can be treated with embolization.[101]
Differential Diagnosis
The differential diagnosis of retinal capillary hemangiomas includes cavernous hemangiomas, retinal hemangiomas, retinal macroaneurysms, and retinal vasoproliferative tumors.
Vasoproliferative tumors of the retina are benign reactive vascular proliferations secondary to retinal ischemia or injury. This condition is typically non-familial and has no systemic association.[102] Most tumors are idiopathic, whereas the rest occur secondary to other ocular conditions such as retinitis pigmentosa, Coats disease, uveitis, and retinal detachment.[103] The lesions are yellowish-red and typically occur in the inferotemporal peripheral retina.[104] Differentiation from capillary hemangiomas is possible due to their location at the extreme periphery in the inferior retinal quadrant and the absence of prominent feeder vessels.[32] Management of vasoproliferative tumors depends on the presence of visual disturbances and the lesion size. The smaller and peripheral lesions require only observation. The treatment modalities include laser photocoagulation, transpupillary thermotherapy, cryotherapy, intravitreal anti-VEGF injections, or vitreoretinal surgery, depending on the complications.[105]
Differentiating cavernous hemangiomas of the retina from other idiopathic retinal telangiectasias, such as Coats disease and Leber's miliary aneurysm, is less challenging. These conditions are characterized by progressive vascular dilatation resulting in intraretinal and subretinal exudation.[13] The aneurysms are typically single and located along the retinal vessels adjacent to the areas of ischemia. Fluorescein angiography can assist in diagnosis by detecting early leakage from capillaries with adjacent capillary non-perfusion areas.[106]
Retinal arteriovenous malformations associated with facial arteriovenous malformations should be differentiated from Sturge-Weber Syndrome if port-wine stains appear on the face or are associated with congenital glaucoma.
Other differential diagnoses include:
Prognosis
The visual prognosis of retinal capillary hemangiomas depends upon several factors, including the size, location, number, and amount of exudation.[101] Patients with multiple tumors are at a greater risk of developing new lesions and thus require more frequent follow-up.[112] Approximately 25% of cases may result in permanent visual loss, and roughly 20% of patients experience visual acuity of 20/100 or less in at least 1 eye.[57] The type of mutations in the VHL gene has been found to affect the visual prognosis.[113] Individuals with truncated VHL proteins had lesser visual morbidity compared to those with missense mutations, who developed more aggressive and multiple retinal cavernous hemangiomas with higher complication rates.[114]
Retinal cavernous hemangiomas are generally considered asymptomatic and non-progressive. In rare cases, vitreous hemorrhage may occur, which is typically self-limiting.[32] However, cerebral cavernous hemangiomas may result in severe complications such as seizures, intracranial hemorrhages, and even death.[18] Routine neuroimaging tests are recommended for early detection and management of the condition.[30]
The prognosis of hemangiomas of the retina is good unless patients develop complications such as macular edema, vitreous hemorrhage, and neovascular glaucoma.[115] Vision loss occurs due to the orbital-cranial arteriovenous malformations causing compressive optic neuropathy.[116] Patients with intracranial arteriovenous malformations have poor outcomes after surgical resection if the lesion is located around the optic chiasma.[117]
Complications
The most common complication of retinal capillary hemangiomas is the formation of epiretinal membrane and subretinal fluid.[37] Retinal exudation and proliferative gliosis are the significant causes of visual impairment in about 50% of the affected individuals.[118] A retrospective analysis of 18 patients revealed that eyes with tractional or exudative retinal detachment exhibited poor visual acuity at follow-up.[119] Visual loss in retinal capillary hemangioma is due to retinal exudation, fibrovascular proliferation, rhegmatogenous or tractional retinal detachment, macular hole, and glaucoma.[8]
Retinal cavernous hemangiomas are non-progressive with minimal complications, the most common being vitreous hemorrhage.[39] Exudation is rare, and fibroglial tissue may develop over the tumor surface, which can cause visual impairment if the tumor is located at the macula.[40][120]
Retinal arteriovenous malformations in Wyburn-Mason syndrome can result in various complications, including macular edema, retinal ischemia, retinal vein occlusion, vitreous hemorrhage, and neovascular glaucoma.[121] Arteriovenous malformations in the maxillary and mandibular region can lead to profuse bleeding during dental or maxillofacial surgical procedures.[122]
Deterrence and Patient Education
Early detection of retinal vascular anomalies and frequent follow-up are crucial for avoiding severe complications. Individuals with a proven VHL mutation or a positive family history should undergo annual screening for blood pressure and visual, auditory, and neurological manifestations from the first year of age. Urinary or blood metanephrines can be tested annually from age 5 to rule out pheochromocytomas.[123] MRI of the brain and spine and abdominal ultrasonography is recommended every 2 years from age 16.[51] In symptomatic patients, an MRI of the internal auditory canal can detect endolymphatic sac tumors.[124] Ocular screening should be performed every 6 to 12 months until age 30 and yearly.[125] Familial forms of cavernous hemangiomas of the retina are typically bilateral and associated with cutaneous and CNS hemangiomas, making it necessary to screen at-risk family members for cerebral hemangiomas with MRI brain.[74]
Pearls and Other Issues
Patients with retinal vascular anomalies should be aware of the possibility of systemic involvement and the importance of periodic screenings for ocular and systemic abnormalities. Such screenings can be crucial in detecting life-threatening conditions. Patients with a positive genetic test for VHL should be referred to a clinical geneticist for appropriate management, and those planning for pregnancy should receive education on fertility issues and the need for prenatal genetic testing. Screening first-degree relatives of patients with multiple organ involvement is also imperative. In cases of cavernous or racemose hemangiomas, ruling out cerebral involvement is essential, which can result in life-threatening intracranial hemorrhage.[126] Asymptomatic lesions can be closely monitored without the need for intracranial surgeries.
Enhancing Healthcare Team Outcomes
Retinal vascular anomalies are rare, but early identification and management are critical for reducing significant mortality and morbidity. A dedicated nurse can be assigned as a case manager to assist the treating clinician in providing psychosocial care and ensuring that the patient attends the clinic on the scheduled appointment dates and follows the screening protocols. A supportive multi-disciplinary team comprising physicians, nurses, radiologists, neurologists, geneticists, ophthalmologists, endocrinologists, and ear-nose-throat specialists can improve care in such patients.[34]
All healthcare professionals involved in patient care should possess the clinical skills and knowledge to manage the condition and be aware of the potential complications. In addition, a strategic approach according to evidence-based guidelines is essential for the management and screening of the condition. Ethical considerations must guide decision-making for treatment, and patients should receive thorough counseling regarding the available treatment options to ensure that treatment aligns with their preferences. Effective coordination, communication, and teamwork are crucial for delivering expert patient care, from screening to management.[127][128]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
Retinal Racemose Angioma. The image shows a large, dilated, tortuous retinal artery extending from the optic disc and directly connecting to the retinal vein without any intervening capillaries.
Ravani R, Patel C, Tripathy K. Optical coherence tomography of racemose angioma. Clin Case Rep. 2020;8(7):1299-1300. doi: 10.1002/ccr3.2841.
References
Gupta A, Paulbuddhe VS, Shukla UV, Tripathy K. Exudative Retinitis (Coats Disease). StatPearls. 2024 Jan:(): [PubMed PMID: 32809517]
Venkatesh P, Takkar B. Proposed Classification System for Retinal Capillary Angiomatosis. Ophthalmic research. 2019:61(2):115-119. doi: 10.1159/000494498. Epub 2018 Nov 29 [PubMed PMID: 30497078]
Karimi S, Arabi A, Shahraki T, Safi S. Von Hippel-Lindau Disease and the Eye. Journal of ophthalmic & vision research. 2020 Jan-Mar:15(1):78-94. doi: 10.18502/jovr.v15i1.5950. Epub 2020 Feb 2 [PubMed PMID: 32095212]
Crespigio J, Berbel LCL, Dias MA, Berbel RF, Pereira SS, Pignatelli D, Mazzuco TL. Von Hippel-Lindau disease: a single gene, several hereditary tumors. Journal of endocrinological investigation. 2018 Jan:41(1):21-31. doi: 10.1007/s40618-017-0683-1. Epub 2017 Jun 6 [PubMed PMID: 28589383]
Tsang SH, Sharma T. Von Hippel-Lindau Disease. Advances in experimental medicine and biology. 2018:1085():201-203. doi: 10.1007/978-3-319-95046-4_42. Epub [PubMed PMID: 30578515]
Level 3 (low-level) evidenceLiu P, Li M, Guan X, Yu A, Xiao Q, Wang C, Hu Y, Zhu F, Yin H, Yi X, Liu L. Clinical Syndromes and Genetic Screening Strategies of Pheochromocytoma and Paraganglioma. Journal of kidney cancer and VHL. 2018:5(4):14-22. doi: 10.15586/jkcvhl.2018.113. Epub 2018 Dec 27 [PubMed PMID: 30613466]
Khan HA, Shahzad MA, Iqbal F, Awan MA, Khan QA, Saatci AO, Abbass A, Hussain F, Hussain SA, Ali A, Ali W. Ophthalmological Aspects of von-Hippel-Lindau Syndrome. Seminars in ophthalmology. 2021 Oct 3:36(7):531-540. doi: 10.1080/08820538.2021.1897851. Epub 2021 Mar 29 [PubMed PMID: 33780299]
Chew EY. Ocular manifestations of von Hippel-Lindau disease: clinical and genetic investigations. Transactions of the American Ophthalmological Society. 2005:103():495-511 [PubMed PMID: 17057815]
Niccol W, Moore RF. A CASE OF ANGIOMATOSIS RETINAE. The British journal of ophthalmology. 1934 Aug:18(8):454-7 [PubMed PMID: 18169216]
Level 3 (low-level) evidenceLabauge P, Krivosic V, Denier C, Tournier-Lasserve E, Gaudric A. Frequency of retinal cavernomas in 60 patients with familial cerebral cavernomas: a clinical and genetic study. Archives of ophthalmology (Chicago, Ill. : 1960). 2006 Jun:124(6):885-6 [PubMed PMID: 16769843]
Yamauchi K, Suzuki Y, Tanaka-Gonome T, Adachi K, Maeda N, Nakazawa M. Racemose hemangioma complicated with macular macroaneurysm rupture. American journal of ophthalmology case reports. 2021 Jun:22():101053. doi: 10.1016/j.ajoc.2021.101053. Epub 2021 Mar 5 [PubMed PMID: 33786403]
Level 3 (low-level) evidenceBhattacharya JJ, Luo CB, Suh DC, Alvarez H, Rodesch G, Lasjaunias P. Wyburn-Mason or Bonnet-Dechaume-Blanc as Cerebrofacial Arteriovenous Metameric Syndromes (CAMS). A New Concept and a New Classification. Interventional neuroradiology : journal of peritherapeutic neuroradiology, surgical procedures and related neurosciences. 2001 Mar 30:7(1):5-17 [PubMed PMID: 20663326]
Knutsson KA, De Benedetto U, Querques G, Del Turco C, Bandello F, Lattanzio R. Primitive retinal vascular abnormalities: tumors and telangiectasias. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2012:228(2):67-77. doi: 10.1159/000338230. Epub 2012 Jun 23 [PubMed PMID: 22738997]
Pangtey BPS, Kohli P, Ramasamy K. Wyburn-Mason syndrome presenting with bilateral retinal racemose hemangioma with unilateral serous retinal detachment. Indian journal of ophthalmology. 2018 Dec:66(12):1869-1871. doi: 10.4103/ijo.IJO_455_18. Epub [PubMed PMID: 30451208]
Richard S, Beigelman C, Gerber S, Van Effenterre R, Gaudric A, Sahel M, Binaghi M, De Kersaint-Gilly A, Houtteville JP, Brunon JP. [Does hemangioblastoma exist outside von Hippel-Lindau disease?]. Neuro-Chirurgie. 1994:40(3):145-54 [PubMed PMID: 7723921]
Ruppert MD, Gavin M, Mitchell KT, Peiris AN. Ocular Manifestations of von Hippel-Lindau Disease. Cureus. 2019 Aug 4:11(8):e5319. doi: 10.7759/cureus.5319. Epub 2019 Aug 4 [PubMed PMID: 31588386]
Wang Y, Abu-Asab MS, Shen D, Zhuang Z, Chew EY, Chan CC. Upregulation of hypoxia-inducible factors and autophagy in von Hippel-Lindau-associated retinal hemangioblastoma. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2014 Aug:252(8):1319-27. doi: 10.1007/s00417-014-2660-0. Epub 2014 May 24 [PubMed PMID: 24859386]
Dobyns WB, Michels VV, Groover RV, Mokri B, Trautmann JC, Forbes GS, Laws ER Jr. Familial cavernous malformations of the central nervous system and retina. Annals of neurology. 1987 Jun:21(6):578-83 [PubMed PMID: 3606045]
Labauge P, Enjolras O, Bonerandi JJ, Laberge S, Dandurand M, Joujoux JM, Tournier-Lasserve E. An association between autosomal dominant cerebral cavernomas and a distinctive hyperkeratotic cutaneous vascular malformation in 4 families. Annals of neurology. 1999 Feb:45(2):250-4 [PubMed PMID: 9989629]
Reddy S, Gorin MB, McCannel TA, Tsui I, Straatsma BR. Novel KRIT1/CCM1 mutation in a patient with retinal cavernous hemangioma and cerebral cavernous malformation. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2010 Sep:248(9):1359-61. doi: 10.1007/s00417-010-1329-6. Epub 2010 Mar 20 [PubMed PMID: 20306072]
Peyre M, Miyagishima D, Bielle F, Chapon F, Sierant M, Venot Q, Lerond J, Marijon P, Abi-Jaoude S, Le Van T, Labreche K, Houlston R, Faisant M, Clémenceau S, Boch AL, Nouet A, Carpentier A, Boetto J, Louvi A, Kalamarides M. Somatic PIK3CA Mutations in Sporadic Cerebral Cavernous Malformations. The New England journal of medicine. 2021 Sep 9:385(11):996-1004. doi: 10.1056/NEJMoa2100440. Epub [PubMed PMID: 34496175]
Dayani PN, Sadun AA. A case report of Wyburn-Mason syndrome and review of the literature. Neuroradiology. 2007 May:49(5):445-56 [PubMed PMID: 17235577]
Level 3 (low-level) evidenceJesberg DO, Spencer WH, Hoyt WF. Incipient lesions of von Hippel-Lindau disease. Archives of ophthalmology (Chicago, Ill. : 1960). 1968 Nov:80(5):632-40 [PubMed PMID: 5693353]
Binderup MLM, Stendell AS, Galanakis M, Møller HU, Kiilgaard JF, Bisgaard ML. Retinal hemangioblastoma: prevalence, incidence and frequency of underlying von Hippel-Lindau disease. The British journal of ophthalmology. 2018 Jul:102(7):942-947. doi: 10.1136/bjophthalmol-2017-310884. Epub 2017 Sep 28 [PubMed PMID: 28972023]
Azimi F, Aghajani A, Khakpour G, Chaibakhsh S. A meta-analysis of different von Hippel Lindau mutations: are they related to retinal capillary hemangioblastoma? Molecular genetics and genomics : MGG. 2022 Nov:297(6):1615-1626. doi: 10.1007/s00438-022-01940-z. Epub 2022 Aug 25 [PubMed PMID: 36006455]
Level 1 (high-level) evidenceSingh A, Shields J, Shields C. Solitary retinal capillary hemangioma: hereditary (von Hippel-Lindau disease) or nonhereditary? Archives of ophthalmology (Chicago, Ill. : 1960). 2001 Feb:119(2):232-4 [PubMed PMID: 11176984]
Maher ER, Yates JR, Harries R, Benjamin C, Harris R, Moore AT, Ferguson-Smith MA. Clinical features and natural history of von Hippel-Lindau disease. The Quarterly journal of medicine. 1990 Nov:77(283):1151-63 [PubMed PMID: 2274658]
Ning XH, Zhang N, Li T, Wu PJ, Wang X, Li XY, Peng SH, Wang JY, Chen JC, Gong K. Telomere shortening is associated with genetic anticipation in Chinese Von Hippel-Lindau disease families. Cancer research. 2014 Jul 15:74(14):3802-9. doi: 10.1158/0008-5472.CAN-14-0024. Epub 2014 Jul 1 [PubMed PMID: 24986515]
Lonser RR, Butman JA, Huntoon K, Asthagiri AR, Wu T, Bakhtian KD, Chew EY, Zhuang Z, Linehan WM, Oldfield EH. Prospective natural history study of central nervous system hemangioblastomas in von Hippel-Lindau disease. Journal of neurosurgery. 2014 May:120(5):1055-62. doi: 10.3171/2014.1.JNS131431. Epub 2014 Feb 28 [PubMed PMID: 24579662]
Wang W, Chen L. CAVERNOUS HEMANGIOMA OF THE RETINA: A Comprehensive Review of the Literature (1934-2015). Retina (Philadelphia, Pa.). 2017 Apr:37(4):611-621. doi: 10.1097/IAE.0000000000001374. Epub [PubMed PMID: 27820777]
Goldberg RE, Pheasant TR, Shields JA. Cavernous hemangioma of the retina. A four-generation pedigree with neurocutaneous manifestations and an example of bilateral retinal involvement. Archives of ophthalmology (Chicago, Ill. : 1960). 1979 Dec:97(12):2321-4 [PubMed PMID: 229814]
Singh AD, Rundle PA, Rennie I. Retinal vascular tumors. Ophthalmology clinics of North America. 2005 Mar:18(1):167-76, x [PubMed PMID: 15763202]
Archer DB, Deutman A, Ernest JT, Krill AE. Arteriovenous communications of the retina. American journal of ophthalmology. 1973 Feb:75(2):224-41 [PubMed PMID: 4697179]
So JM, Mishra C, Holman RE. Wyburn-Mason Syndrome. StatPearls. 2024 Jan:(): [PubMed PMID: 29630270]
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999 May 20:399(6733):271-5 [PubMed PMID: 10353251]
Singer EA, Bratslavsky G, Middelton L, Srinivasan R, Linehan WM. Impact of genetics on the diagnosis and treatment of renal cancer. Current urology reports. 2011 Feb:12(1):47-55. doi: 10.1007/s11934-010-0156-y. Epub [PubMed PMID: 21128028]
Dollfus H, Massin P, Taupin P, Nemeth C, Amara S, Giraud S, Béroud C, Dureau P, Gaudric A, Landais P, Richard S. Retinal hemangioblastoma in von Hippel-Lindau disease: a clinical and molecular study. Investigative ophthalmology & visual science. 2002 Sep:43(9):3067-74 [PubMed PMID: 12202531]
Calzada MJ. Von Hippel-Lindau syndrome: molecular mechanisms of the disease. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2010 Mar:12(3):160-5. doi: 10.1007/s12094-010-0485-9. Epub [PubMed PMID: 20231120]
Gass JD. Cavernous hemangioma of the retina. A neuro-oculo-cutaneous syndrome. American journal of ophthalmology. 1971 Apr:71(4):799-814 [PubMed PMID: 5553009]
Messmer E, Font RL, Laqua H, Höpping W, Naumann GO. Cavernous hemangioma of the retina. Immunohistochemical and ultrastructural observations. Archives of ophthalmology (Chicago, Ill. : 1960). 1984 Mar:102(3):413-8 [PubMed PMID: 6538410]
Risau W. Mechanisms of angiogenesis. Nature. 1997 Apr 17:386(6626):671-4 [PubMed PMID: 9109485]
Ponce FA, Han PP, Spetzler RF, Canady A, Feiz-Erfan I. Associated arteriovenous malformation of the orbit and brain: a case of Wyburn-Mason syndrome without retinal involvement. Case report. Journal of neurosurgery. 2001 Aug:95(2):346-9 [PubMed PMID: 11780909]
Level 3 (low-level) evidenceSchatz H, Chang LF, Ober RR, McDonald HR, Johnson RN. Central retinal vein occlusion associated with retinal arteriovenous malformation. Ophthalmology. 1993 Jan:100(1):24-30 [PubMed PMID: 8433820]
Onder HI, Alisan S, Tunc M. Serous retinal detachment and cystoid macular edema in a patient with Wyburn-Mason syndrome. Seminars in ophthalmology. 2015 Mar:30(2):154-6. doi: 10.3109/08820538.2013.835832. Epub 2013 Oct 30 [PubMed PMID: 24171831]
Chan CC, Lee YS, Zhuang Z, Hackett J, Chew EY. Von Hippel-Lindau gene deletion and expression of hypoxia-inducible factor and ubiquitin in optic nerve hemangioma. Transactions of the American Ophthalmological Society. 2004:102():75-9; discussion 79-81 [PubMed PMID: 15747747]
Grossniklaus HE, Thomas JW, Vigneswaran N, Jarrett WH 3rd. Retinal hemangioblastoma. A histologic, immunohistochemical, and ultrastructural evaluation. Ophthalmology. 1992 Jan:99(1):140-5 [PubMed PMID: 1741127]
Vortmeyer AO, Chan CC, Chew EY, Matteson DM, Shen DF, Wellmann A, Weil R, Zhuang Z. Morphologic and genetic analysis of retinal angioma associated with massive gliosis in a patient with von Hippel-Lindau disease. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1999 Jun:237(6):513-7 [PubMed PMID: 10379614]
Chan CC, Chew EY, Shen D, Hackett J, Zhuang Z. Expression of stem cells markers in ocular hemangioblastoma associated with von Hippel-Lindau (VHL) disease. Molecular vision. 2005 Sep 1:11():697-704 [PubMed PMID: 16163267]
Pierro L, Guarisco L, Zaganelli E, Freschi M, Brancato R. Capillary and cavernous hemangioma of the optic disc. Echographic and histological findings. Acta ophthalmologica. Supplement. 1992:(204):102-6 [PubMed PMID: 1332383]
Jagannathan J, Lonser RR, Smith R, DeVroom HL, Oldfield EH. Surgical management of cerebellar hemangioblastomas in patients with von Hippel-Lindau disease. Journal of neurosurgery. 2008 Feb:108(2):210-22. doi: 10.3171/JNS/2008/108/2/0210. Epub [PubMed PMID: 18240914]
Findeis-Hosey JJ, McMahon KQ, Findeis SK. Von Hippel-Lindau Disease. Journal of pediatric genetics. 2016 Jun:5(2):116-23. doi: 10.1055/s-0036-1579757. Epub 2016 Apr 4 [PubMed PMID: 27617152]
Aronow ME, Wiley HE, Gaudric A, Krivosic V, Gorin MB, Shields CL, Shields JA, Jonasch EW, Singh AD, Chew EY. VON HIPPEL-LINDAU DISEASE: Update on Pathogenesis and Systemic Aspects. Retina (Philadelphia, Pa.). 2019 Dec:39(12):2243-2253. doi: 10.1097/IAE.0000000000002555. Epub [PubMed PMID: 31095066]
Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, van Leeuwaarde RS, Ahmad S, Links TP, Giles RH. Von Hippel-Lindau Syndrome. GeneReviews(®). 1993:(): [PubMed PMID: 20301636]
De Laey JJ, Hanssens M, Brabant P, Decq L, De Gersem R, Hoste A, Huyghe P, Lenaerts V, Leys M, Pollet L. Vascular tumors and malformations of the ocular fundus. Bulletin de la Societe belge d'ophtalmologie. 1990:225 Pt 1():1-241 [PubMed PMID: 2128617]
Mansour AM, Wells CG, Jampol LM, Kalina RE. Ocular complications of arteriovenous communications of the retina. Archives of ophthalmology (Chicago, Ill. : 1960). 1989 Feb:107(2):232-6 [PubMed PMID: 2644928]
Tripathy K,Sharma YR, Retinal vascular lesions. Journal of paediatrics and child health. 2017 Jan [PubMed PMID: 28070944]
Webster AR, Maher ER, Moore AT. Clinical characteristics of ocular angiomatosis in von Hippel-Lindau disease and correlation with germline mutation. Archives of ophthalmology (Chicago, Ill. : 1960). 1999 Mar:117(3):371-8 [PubMed PMID: 10088816]
Gass JD, Braunstein R. Sessile and exophytic capillary angiomas of the juxtapapillary retina and optic nerve head. Archives of ophthalmology (Chicago, Ill. : 1960). 1980 Oct:98(10):1790-7 [PubMed PMID: 7425905]
Augsburger JJ, Shields JA, Goldberg RE. Classification and management of hereditary retinal angiomas. International ophthalmology. 1981 Aug:4(1-2):93-106 [PubMed PMID: 7298262]
Custo Greig EP, Duker JS. Retinal hemangioblastoma vascular detail elucidated on swept source optical coherence tomography angiography. American journal of ophthalmology case reports. 2021 Mar:21():101005. doi: 10.1016/j.ajoc.2020.101005. Epub 2020 Dec 23 [PubMed PMID: 33385098]
Level 3 (low-level) evidenceRuia S, Tripathy K. Fluorescein Angiography. StatPearls. 2024 Jan:(): [PubMed PMID: 35015403]
Sagar P, Rajesh R, Shanmugam M, Konana VK, Mishra D. Comparison of optical coherence tomography angiography and fundus fluorescein angiography features of retinal capillary hemangioblastoma. Indian journal of ophthalmology. 2018 Jun:66(6):872-876. doi: 10.4103/ijo.IJO_1199_17. Epub [PubMed PMID: 29786009]
Heimann H, Jmor F, Damato B. Imaging of retinal and choroidal vascular tumours. Eye (London, England). 2013 Feb:27(2):208-16. doi: 10.1038/eye.2012.251. Epub 2012 Nov 30 [PubMed PMID: 23196648]
Sagar P, Shanmugam PM, Konana VK, Ramanjulu R, Mishra KCD, Simakurthy S. Optical coherence tomography angiography in assessment of response to therapy in retinal capillary hemangioblastoma and diffuse choroidal hemangioma. Indian journal of ophthalmology. 2019 May:67(5):701-703. doi: 10.4103/ijo.IJO_1429_18. Epub [PubMed PMID: 31007251]
de Paula A, Abdolrahimzadeh S, Fragiotta S, Di Pippo M, Scuderi G. Current concepts on ocular vascular abnormalities in the phakomatoses. Seminars in ophthalmology. 2021 Oct 3:36(7):549-560. doi: 10.1080/08820538.2021.1900284. Epub 2021 Mar 23 [PubMed PMID: 33755531]
Wanebo JE, Lonser RR, Glenn GM, Oldfield EH. The natural history of hemangioblastomas of the central nervous system in patients with von Hippel-Lindau disease. Journal of neurosurgery. 2003 Jan:98(1):82-94 [PubMed PMID: 12546356]
Schmid S, Gillessen S, Binet I, Brändle M, Engeler D, Greiner J, Hader C, Heinimann K, Kloos P, Krek W, Krull I, Stoeckli SJ, Sulz MC, van Leyen K, Weber J, Rothermundt C, Hundsberger T. Management of von hippel-lindau disease: an interdisciplinary review. Oncology research and treatment. 2014:37(12):761-71. doi: 10.1159/000369362. Epub 2014 Nov 17 [PubMed PMID: 25531723]
Gläsker S, Vergauwen E, Koch CA, Kutikov A, Vortmeyer AO. Von Hippel-Lindau Disease: Current Challenges and Future Prospects. OncoTargets and therapy. 2020:13():5669-5690. doi: 10.2147/OTT.S190753. Epub 2020 Jun 16 [PubMed PMID: 32606780]
Shanbhogue KP, Hoch M, Fatterpaker G, Chandarana H. von Hippel-Lindau Disease: Review of Genetics and Imaging. Radiologic clinics of North America. 2016 May:54(3):409-22. doi: 10.1016/j.rcl.2015.12.004. Epub 2016 Mar 21 [PubMed PMID: 27153780]
Choyke PL, Glenn GM, Walther MM, Patronas NJ, Linehan WM, Zbar B. von Hippel-Lindau disease: genetic, clinical, and imaging features. Radiology. 1995 Mar:194(3):629-42 [PubMed PMID: 7862955]
Binderup ML, Bisgaard ML, Harbud V, Møller HU, Gimsing S, Friis-Hansen L, Hansen Tv, Bagi P, Knigge U, Kosteljanetz M, Bøgeskov L, Thomsen C, Gerdes AM, Ousager LB, Sunde L, Danish vHL Coordination Group. Von Hippel-Lindau disease (vHL). National clinical guideline for diagnosis and surveillance in Denmark. 3rd edition. Danish medical journal. 2013 Dec:60(12):B4763 [PubMed PMID: 24355456]
Shields JA, Eagle RC Jr, Ewing MQ, Lally SE, Shields CL. Retinal cavernous hemangioma: fifty-two years of clinical follow-up with clinicopathologic correlation. Retina (Philadelphia, Pa.). 2014 Jun:34(6):1253-7. doi: 10.1097/IAE.0000000000000232. Epub [PubMed PMID: 24849703]
Li J, Li Y, Li H. New Interpretation of Multimodality Fundus Imaging for Retinal Cavernous Hemangioma. Current eye research. 2019 Apr:44(4):423-427. doi: 10.1080/02713683.2018.1549263. Epub 2018 Nov 26 [PubMed PMID: 30433829]
Heimann H, Damato B. Congenital vascular malformations of the retina and choroid. Eye (London, England). 2010 Mar:24(3):459-67. doi: 10.1038/eye.2009.310. Epub 2009 Dec 18 [PubMed PMID: 20019761]
Shanmugam PM, Ramanjulu R. Vascular tumors of the choroid and retina. Indian journal of ophthalmology. 2015 Feb:63(2):133-40. doi: 10.4103/0301-4738.154387. Epub [PubMed PMID: 25827544]
Say EA, Shah SU, Ferenczy S, Shields CL. Optical coherence tomography of retinal and choroidal tumors. Journal of ophthalmology. 2012:2012():385058 [PubMed PMID: 23008756]
Labauge P, Denier C, Bergametti F, Tournier-Lasserve E. Genetics of cavernous angiomas. The Lancet. Neurology. 2007 Mar:6(3):237-44 [PubMed PMID: 17303530]
Ravani R, Patel C, Tripathy K. Optical coherence tomography of racemose angioma. Clinical case reports. 2020 Jul:8(7):1299-1300. doi: 10.1002/ccr3.2841. Epub 2020 Apr 12 [PubMed PMID: 32695379]
Level 3 (low-level) evidenceMansour AM, Walsh JB, Henkind P. Arteriovenous anastomoses of the retina. Ophthalmology. 1987 Jan:94(1):35-40 [PubMed PMID: 3561955]
Liu W, Sharma S. Ophthaproblem. Retinal arteriovenous malformations (type 2). Canadian family physician Medecin de famille canadien. 2005 Feb:51(2):203, 209 [PubMed PMID: 15751561]
Schmidt D, Agostini H, Schumacher M. Twenty-seven years follow-up of a patient with congenital retinocephalofacial vascular malformation syndrome and additional congenital malformations (Bonnet-Dechaume-Blanc syndrome or Wyburn-Mason syndrome). European journal of medical research. 2010 Feb 26:15(2):89-91 [PubMed PMID: 20452891]
Shanmugam PM, Simakurthy S, Konana VK, Ramanjulu R, Mishra KCD. Optical coherence tomography angiography of fleeting macroaneurysm in retinal racemose angioma. Indian journal of ophthalmology. 2018 Sep:66(9):1352-1354. doi: 10.4103/ijo.IJO_267_18. Epub [PubMed PMID: 30127170]
Hajjaj A, van Overdam KA, Gishti O, Ramdas WD, Kiliç E. Efficacy and safety of current treatment options for peripheral retinal haemangioblastomas: a systematic review. Acta ophthalmologica. 2022 Feb:100(1):e38-e46. doi: 10.1111/aos.14865. Epub 2021 Apr 8 [PubMed PMID: 33834636]
Level 1 (high-level) evidenceBornfeld N, Kreusel KM. [Capillary hemangioma of the retina in cases of von Hippel-Lindau syndrome. New therapeutic directions]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 2007 Feb:104(2):114-8 [PubMed PMID: 17256181]
Level 3 (low-level) evidenceWatzke RC. Cryotherapy for retinal angiomatosis. A clinicopathologic report. Archives of ophthalmology (Chicago, Ill. : 1960). 1974 Nov:92(5):399-401 [PubMed PMID: 4429468]
Palmer JD, Gragoudas ES. Advances in treatment of retinal angiomas. International ophthalmology clinics. 1997 Fall:37(4):159-70 [PubMed PMID: 9429939]
Level 3 (low-level) evidenceRaval VR, Agarwal A, Tyagi M. Surgical and visual outcomes after vitreoretinal surgery for complex retinal capillary hemangioblastoma. Indian journal of ophthalmology. 2023 Nov:71(11):3544-3551. doi: 10.4103/IJO.IJO_3325_22. Epub [PubMed PMID: 37870022]
Wiley HE, Krivosic V, Gaudric A, Gorin MB, Shields C, Shields J, Aronow ME, Chew EY. MANAGEMENT OF RETINAL HEMANGIOBLASTOMA IN VON HIPPEL-LINDAU DISEASE. Retina (Philadelphia, Pa.). 2019 Dec:39(12):2254-2263. doi: 10.1097/IAE.0000000000002572. Epub [PubMed PMID: 31259811]
Sachdeva R, Dadgostar H, Kaiser PK, Sears JE, Singh AD. Verteporfin photodynamic therapy of six eyes with retinal capillary haemangioma. Acta ophthalmologica. 2010 Dec:88(8):e334-40. doi: 10.1111/j.1755-3768.2010.02008.x. Epub 2010 Oct 14 [PubMed PMID: 20946329]
Papastefanou VP, Pilli S, Stinghe A, Lotery AJ, Cohen VM. Photodynamic therapy for retinal capillary hemangioma. Eye (London, England). 2013 Mar:27(3):438-42. doi: 10.1038/eye.2012.259. Epub 2013 Jan 4 [PubMed PMID: 23288135]
Mennel S, Meyer CH, Callizo J. Combined intravitreal anti-vascular endothelial growth factor (Avastin) and photodynamic therapy to treat retinal juxtapapillary capillary haemangioma. Acta ophthalmologica. 2010 Aug:88(5):610-3. doi: 10.1111/j.1755-3768.2008.01449.x. Epub 2009 Feb 12 [PubMed PMID: 19222401]
Fallah J, Brave MH, Weinstock C, Mehta GU, Bradford D, Gittleman H, Bloomquist EW, Charlab R, Hamed SS, Miller CP, Dorff SE, Chambers WA, Mixter BD, Dinin J, Pierce WF, Ricks TK, Tang S, Donoghue M, Pazdur R, Amiri-Kordestani L, Ibrahim A, Beaver JA. FDA Approval Summary: Belzutifan for von Hippel-Lindau Disease-Associated Tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2022 Nov 14:28(22):4843-4848. doi: 10.1158/1078-0432.CCR-22-1054. Epub [PubMed PMID: 35727604]
Jonasch E, McCutcheon IE, Waguespack SG, Wen S, Davis DW, Smith LA, Tannir NM, Gombos DS, Fuller GN, Matin SF. Pilot trial of sunitinib therapy in patients with von Hippel-Lindau disease. Annals of oncology : official journal of the European Society for Medical Oncology. 2011 Dec:22(12):2661-2666. doi: 10.1093/annonc/mdr011. Epub [PubMed PMID: 22105611]
Level 3 (low-level) evidenceOudard S, Elaidi R, Brizard M, Le Rest C, Caillet V, Deveaux S, Benoit G, Corréas JM, Benoudiba F, David P, Gaudric A, Hammel P, Joly D, Timsit MO, Méjean A, Richard S. Sunitinib for the treatment of benign and malignant neoplasms from von Hippel-Lindau disease: A single-arm, prospective phase II clinical study from the PREDIR group. Oncotarget. 2016 Dec 20:7(51):85306-85317. doi: 10.18632/oncotarget.13301. Epub [PubMed PMID: 27852035]
Pilié P, Hasanov E, Matin SF, Woodson AHH, Marcott VD, Bird S, Slack RS, Fuller GN, McCutcheon IE, Jonasch E. Pilot study of dovitinib in patients with von Hippel-Lindau disease. Oncotarget. 2018 May 4:9(34):23390-23395. doi: 10.18632/oncotarget.25171. Epub 2018 May 4 [PubMed PMID: 29805741]
Level 3 (low-level) evidenceJonasch E, McCutcheon IE, Gombos DS, Ahrar K, Perrier ND, Liu D, Robichaux CC, Villarreal MF, Weldon JA, Woodson AH, Pilie PG, Fuller GN, Waguespack SG, Matin SF. Pazopanib in patients with von Hippel-Lindau disease: a single-arm, single-centre, phase 2 trial. The Lancet. Oncology. 2018 Oct:19(10):1351-1359. doi: 10.1016/S1470-2045(18)30487-X. Epub 2018 Sep 17 [PubMed PMID: 30236511]
Haller JA, Knox DL. Vitrectomy for persistent vitreous hemorrhage from a cavernous hemangioma of the optic disk. American journal of ophthalmology. 1993 Jul 15:116(1):106-7 [PubMed PMID: 8328530]
Japiassú RM, Moura Brasil OF, de Souza EC. Regression of Macular Cavernous Hemangioma with Systemic Infliximab. Ophthalmic surgery, lasers & imaging : the official journal of the International Society for Imaging in the Eye. 2010 Mar 9:():1-3. doi: 10.3928/15428877-20100215-34. Epub 2010 Mar 9 [PubMed PMID: 20337337]
Shanmugam MP, Ramanjulu R, Dwivedi S, Barigali A, Havanje A. Therapeutic surprise! Photodynamic therapy for cavernous haemangioma of the disc. Indian journal of ophthalmology. 2017 Aug:65(8):754-757. doi: 10.4103/ijo.IJO_41_16. Epub [PubMed PMID: 28820168]
Rao P, Thomas BJ, Yonekawa Y, Robinson J, Capone A Jr. Peripheral Retinal Ischemia, Neovascularization, and Choroidal Infarction in Wyburn-Mason Syndrome. JAMA ophthalmology. 2015 Jul:133(7):852-4. doi: 10.1001/jamaophthalmol.2015.0716. Epub [PubMed PMID: 25906291]
Turell ME, Singh AD. Vascular tumors of the retina and choroid: diagnosis and treatment. Middle East African journal of ophthalmology. 2010 Jul:17(3):191-200. doi: 10.4103/0974-9233.65486. Epub [PubMed PMID: 20844673]
Shields CL, Shields JA, Barrett J, De Potter P. Vasoproliferative tumors of the ocular fundus. Classification and clinical manifestations in 103 patients. Archives of ophthalmology (Chicago, Ill. : 1960). 1995 May:113(5):615-23 [PubMed PMID: 7748132]
Rundle P, Shields JA, Shields CL, Singh AD, Peairs R. Vasoproliferative tumour of the ocular fundus associated with Waardenburg's syndrome. Eye (London, England). 2000 Feb:14 ( Pt 1)():105-6 [PubMed PMID: 10755116]
Huang YM, Chen SJ. Clinical characters and treatments of retinal vasoproliferative tumors. Taiwan journal of ophthalmology. 2016 Apr-Jun:6(2):85-88. doi: 10.1016/j.tjo.2016.04.003. Epub 2016 May 24 [PubMed PMID: 29018717]
Honavar SG. Retinal vasoproliferative tumor - A proposal for classification. Indian journal of ophthalmology. 2018 Feb:66(2):185-186. doi: 10.4103/ijo.IJO_128_18. Epub [PubMed PMID: 29380753]
Shienbaum G, Tasman WS. Coats disease: a lifetime disease. Retina (Philadelphia, Pa.). 2006 Apr:26(4):422-4 [PubMed PMID: 16603961]
Temkar S, Natarajan K, Deb AK, Sagar P. Exudative vascular anomalous complex-like lesion of the peripapillary area. European journal of ophthalmology. 2024 Mar:34(2):NP83-NP86. doi: 10.1177/11206721231202895. Epub 2023 Sep 16 [PubMed PMID: 37715627]
Querques G, Kuhn D, Massamba N, Leveziel N, Querques L, Souied EH. Perifoveal exudative vascular anomalous complex. Journal francais d'ophtalmologie. 2011 Oct:34(8):559.e1-4. doi: 10.1016/j.jfo.2011.03.002. Epub 2011 May 7 [PubMed PMID: 21550688]
Singh D, Tripathy K. Retinal Macroaneurysm. StatPearls. 2024 Jan:(): [PubMed PMID: 35015432]
Tripathy K, Bypareddy R, Chawla R. Congenital retinal macrovessel may be associated with unilateral foveal hypoplasia/small foveal avascular zone. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2019 Feb:54(1):139. doi: 10.1016/j.jcjo.2018.06.018. Epub 2018 Aug 25 [PubMed PMID: 30851769]
Chawla R, Bypareddy R, Tripathy K, Daggumili SS, Tomar AS. Optical Coherence Tomography Angiography Imaging of Congenital Retinal Macrovessel. Ophthalmic surgery, lasers & imaging retina. 2016 Oct 1:47(10):972-973. doi: 10.3928/23258160-20161004-14. Epub [PubMed PMID: 27759867]
Schmidt D, Natt E, Neumann HP. Long-term results of laser treatment for retinal angiomatosis in von Hippel-Lindau disease. European journal of medical research. 2000 Feb 28:5(2):47-58 [PubMed PMID: 10720563]
Reich M, Jaegle S, Neumann-Haefelin E, Klingler JH, Evers C, Daniel M, Bucher F, Ludwig F, Nuessle S, Kopp J, Boehringer D, Reinhard T, Lagrèze WA, Lange C, Agostini H, Lang SJ. Genotype-phenotype correlation in von Hippel-Lindau disease. Acta ophthalmologica. 2021 Dec:99(8):e1492-e1500. doi: 10.1111/aos.14843. Epub 2021 Mar 15 [PubMed PMID: 33720516]
Hudler P, Urbancic M. The Role of VHL in the Development of von Hippel-Lindau Disease and Erythrocytosis. Genes. 2022 Feb 17:13(2):. doi: 10.3390/genes13020362. Epub 2022 Feb 17 [PubMed PMID: 35205407]
Effron L, Zakov ZN, Tomsak RL. Neovascular glaucoma as a complication of the Wyburn-Mason syndrome. Journal of clinical neuro-ophthalmology. 1985 Jun:5(2):95-8 [PubMed PMID: 2432092]
Reck SD, Zacks DN, Eibschitz-Tsimhoni M. Retinal and intracranial arteriovenous malformations: Wyburn-Mason syndrome. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2005 Sep:25(3):205-8 [PubMed PMID: 16148629]
Hopen G, Smith JL, Hoff JT, Quencer R. The Wyburn-Mason syndrome. Concomitant chiasmal and fundus vascular malformations. Journal of clinical neuro-ophthalmology. 1983 Mar:3(1):53-62 [PubMed PMID: 6222080]
Singh AD, Nouri M, Shields CL, Shields JA, Perez N. Treatment of retinal capillary hemangioma. Ophthalmology. 2002 Oct:109(10):1799-806 [PubMed PMID: 12359597]
Lee SH, Park KH, Woo SJ, Park SJ, Joo K. Clinical and Genetic Characteristics of Retinal Capillary Hemangioblastoma in Korean Patients. Korean journal of ophthalmology : KJO. 2022 Dec:36(6):543-549. doi: 10.3341/kjo.2022.0079. Epub 2022 Oct 25 [PubMed PMID: 36281577]
Gold AS, Nguyen JT, Murray TG. Macular hole secondary to capillary hemangioblastoma. Optometry and vision science : official publication of the American Academy of Optometry. 2010 Sep:87(9):E705-9. doi: 10.1097/OPX.0b013e3181ea1abe. Epub [PubMed PMID: 20601911]
Shah GK, Shields JA, Lanning RC. Branch retinal vein obstruction secondary to retinal arteriovenous communication. American journal of ophthalmology. 1998 Sep:126(3):446-8 [PubMed PMID: 9744380]
Hoyt WF, Cameron RB. Racemose angioma of the mandible, face, retina, and brain: report of case. Journal of oral surgery (American Dental Association : 1965). 1968 Sep:26(9):596-601 [PubMed PMID: 5302209]
Level 3 (low-level) evidenceLouise M Binderup M, Smerdel M, Borgwadt L, Beck Nielsen SS, Madsen MG, Møller HU, Kiilgaard JF, Friis-Hansen L, Harbud V, Cortnum S, Owen H, Gimsing S, Friis Juhl HA, Munthe S, Geilswijk M, Rasmussen ÅK, Møldrup U, Graumann O, Donskov F, Grønbæk H, Stausbøl-Grøn B, Schaffalitzky de Muckadell O, Knigge U, Dam G, Wadt KA, Bøgeskov L, Bagi P, Lund L, Stochholm K, Ousager LB, Sunde L. von Hippel-Lindau disease: Updated guideline for diagnosis and surveillance. European journal of medical genetics. 2022 Aug:65(8):104538. doi: 10.1016/j.ejmg.2022.104538. Epub 2022 Jun 13 [PubMed PMID: 35709961]
Poulsen ML, Gimsing S, Kosteljanetz M, Møller HU, Brandt CA, Thomsen C, Bisgaard ML. von Hippel-Lindau disease: surveillance strategy for endolymphatic sac tumors. Genetics in medicine : official journal of the American College of Medical Genetics. 2011 Dec:13(12):1032-41. doi: 10.1097/GIM.0b013e31822beab1. Epub [PubMed PMID: 21912262]
Daniels AB, Chang EY, Chew EY, Gombos DS, Gorin MB, Shields CL, Wiley HE. Consensus Guidelines for Ocular Surveillance of von Hippel-Lindau Disease. Ophthalmology. 2024 May:131(5):622-633. doi: 10.1016/j.ophtha.2023.12.014. Epub 2023 Dec 12 [PubMed PMID: 38092079]
Level 3 (low-level) evidenceBokhari MR, Al-Dhahir MA. Brain Cavernous Angiomas. StatPearls. 2024 Jan:(): [PubMed PMID: 28613621]
Mikhail MI, Singh AK. Von Hippel-Lindau Syndrome. StatPearls. 2024 Jan:(): [PubMed PMID: 29083737]
Larcher A, Belladelli F, Fallara G, Rowe I, Capitanio U, Marandino L, Raggi D, Capitanio JF, Bailo M, Lattanzio R, Barresi C, Calloni SF, Barbera M, Andreasi V, Guazzarotti G, Pipitone G, Carrera P, Necchi A, Mortini P, Bandello F, Falini A, Partelli S, Falconi M, De Cobelli F, Salonia A. Multidisciplinary management of patients diagnosed with von Hippel-Lindau disease: A practical review of the literature for clinicians. Asian journal of urology. 2022 Oct:9(4):430-442. doi: 10.1016/j.ajur.2022.08.002. Epub 2022 Sep 10 [PubMed PMID: 36381595]