Introduction
Retinal pattern dystrophies are a slowly progressive heterogeneous group of primarily autosomal dominantly inherited macular diseases whose unifying element involves the deposition of pigment in the retinal pigment epithelium (RPE) of the macula.[1]
Although findings are classically and most often centered in the macula, pigment deposition may also occur in the periphery.[1] Depending on the distribution pattern, these pattern dystrophies can be separated into major categories or types. These include reticular dystrophy, fundus pulverulentus, butterfly-shaped pigment dystrophy, adult-onset foveomacular vitelliform dystrophy, and multifocal pattern dystrophy simulating Stargardt disease. Interestingly, within a patient, a given pattern may transform into another pattern. Each eye may have distinct patterns, and the same familial mutation can correspond to different phenotypic patterns.[2][3][4][5]
Visual prognosis is typically good in these patients, but due to the location of the pigment deposition in the macula and its slowly progressive nature, there is always the possibility of central vision loss and secondary complications such as choroidal neovascularization and macular holes.[4][6]
Patients typically remain asymptomatic until the fourth to fifth decade of life, when they may start to notice changes in central vision. The appearance of pattern dystrophies may lead to misdiagnosis as age-related macular degeneration (AMD), especially given that later stages of pattern dystrophies may resemble AMD and pigment deposits can resemble drusen.[6] Distinguishing pattern dystrophies from AMD requires further workup and follow-up.[4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Pattern dystrophies are attributed to mutations in the human retinal degeneration slow (RDS) and peripherin gene on chromosome 6 at position 21.1.[7] There are various mutations, such as multiple missense mutations including Arg172Trp, Lys197Glu, Tryp246Arg, Cys213Ttr, Gly167Asp, Glu208Asp, Trp246Arg, and Cys250Phe, and nonsense mutations such as Tyr285ter and Gln239ter.[6][8][9][10]
The RDS/Peripherin gene is critical for developing a protein, peripherin-2, a cell surface glycoprotein that may be involved in developing and stabilizing photoreceptor outer segment discs.[11][12]
It is theorized that a mutation in the function of this protein may lead to pattern dystrophy disease by compromising the integrity of photoreceptor membranes.[7][11]
Epidemiology
Due to the rarity of the disease, epidemiological data is limited to regional analysis. One study found the prevalence of pattern dystrophies in northern France to be 33,800 in total or a 1 per1490 ratio, and over 300,000 in greater Europe.[13] Another study found the prevalence of adult-onset vitelliform macular dystrophy ranges from 1 in 7400 to 1 in 8200.[14]
History and Physical
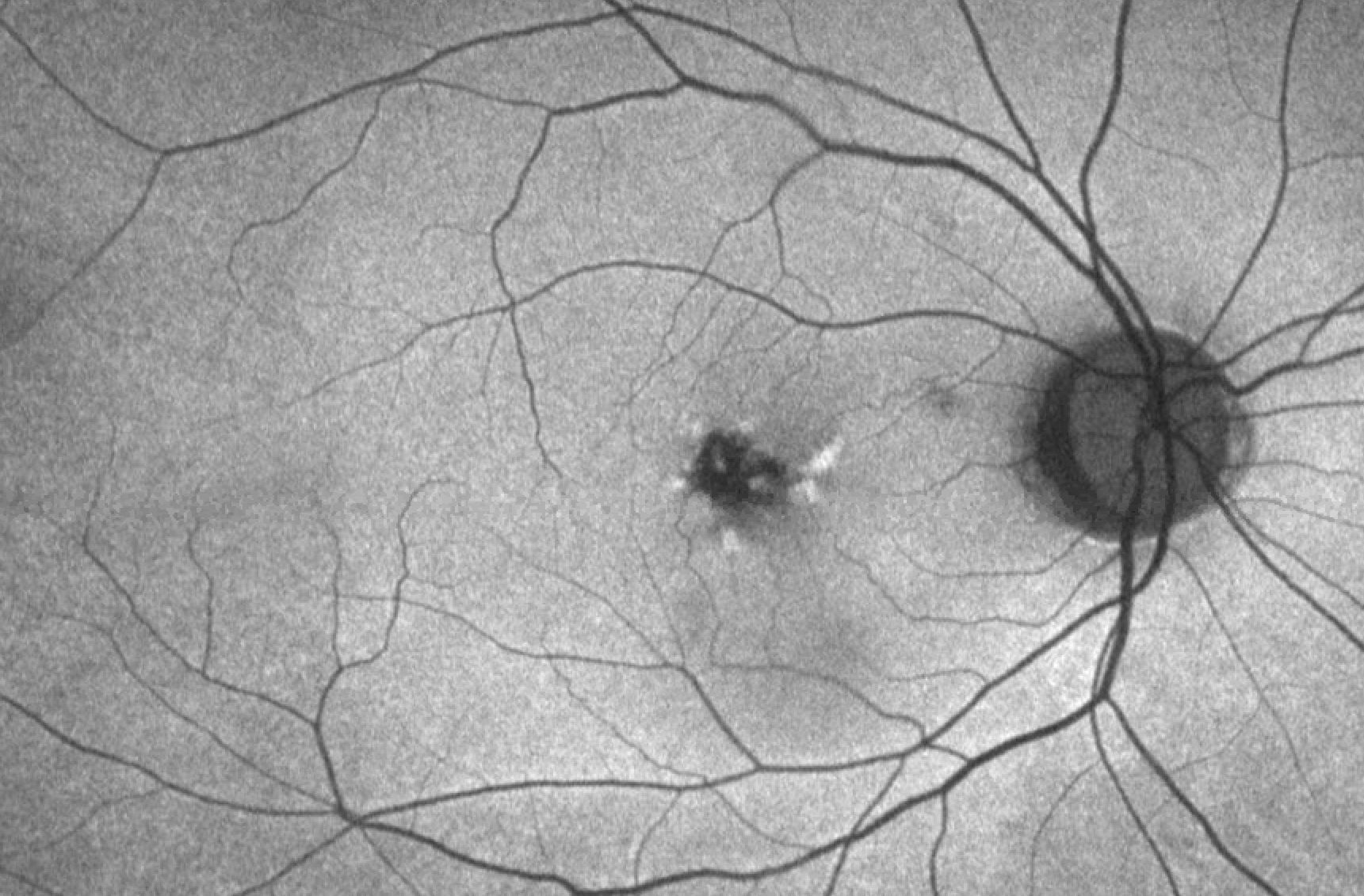
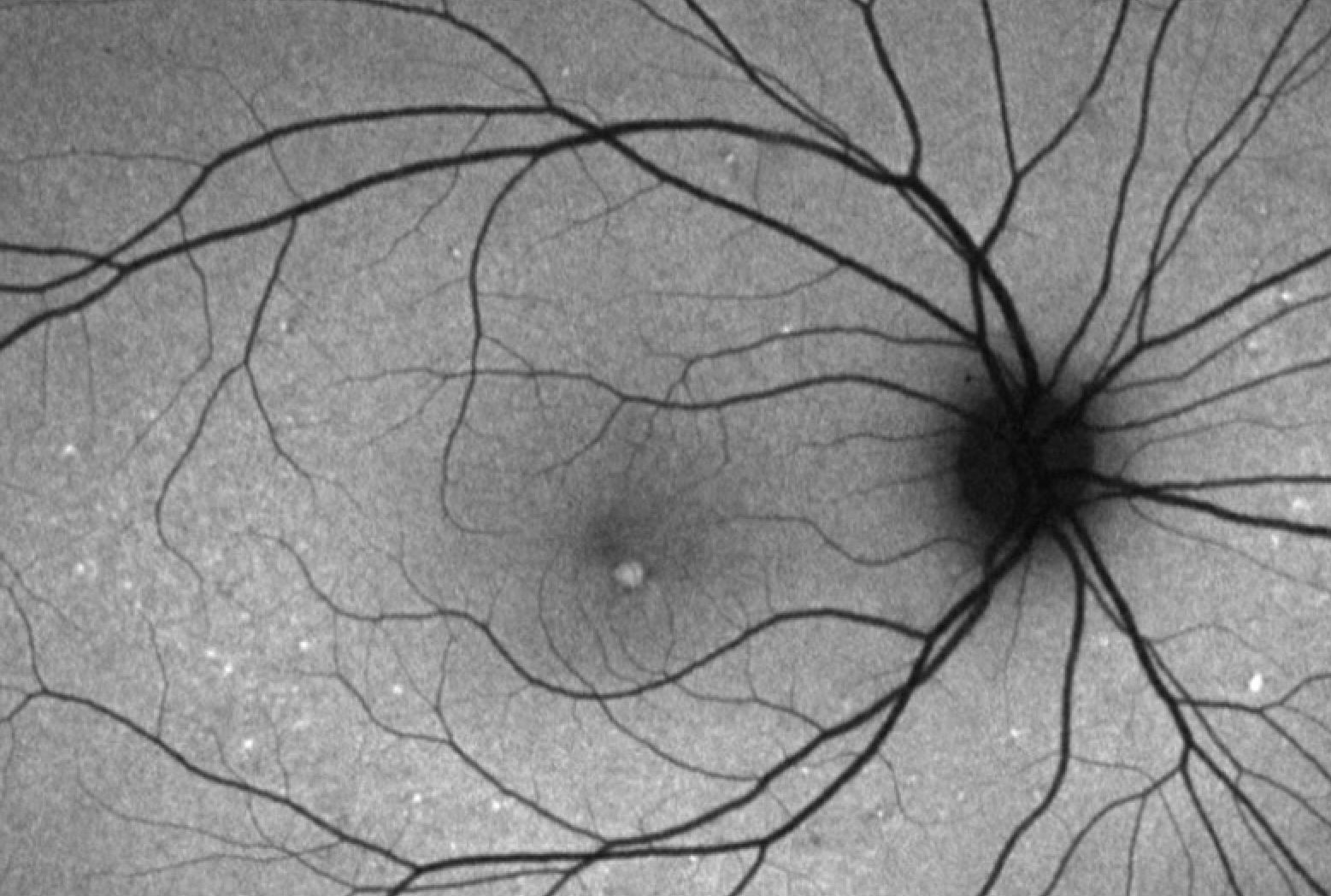
While pattern dystrophies are found on routine screenings for most patients, some patients may present with a decrease in vision and metamorphopsia.[15] On rare occasions, patients may report a history of significant vision loss secondary to complications from pattern dystrophies such as choroidal neovascularization. On physical exam, the fundus exam will reveal a characteristic pattern of pigment deposition in the RPE.
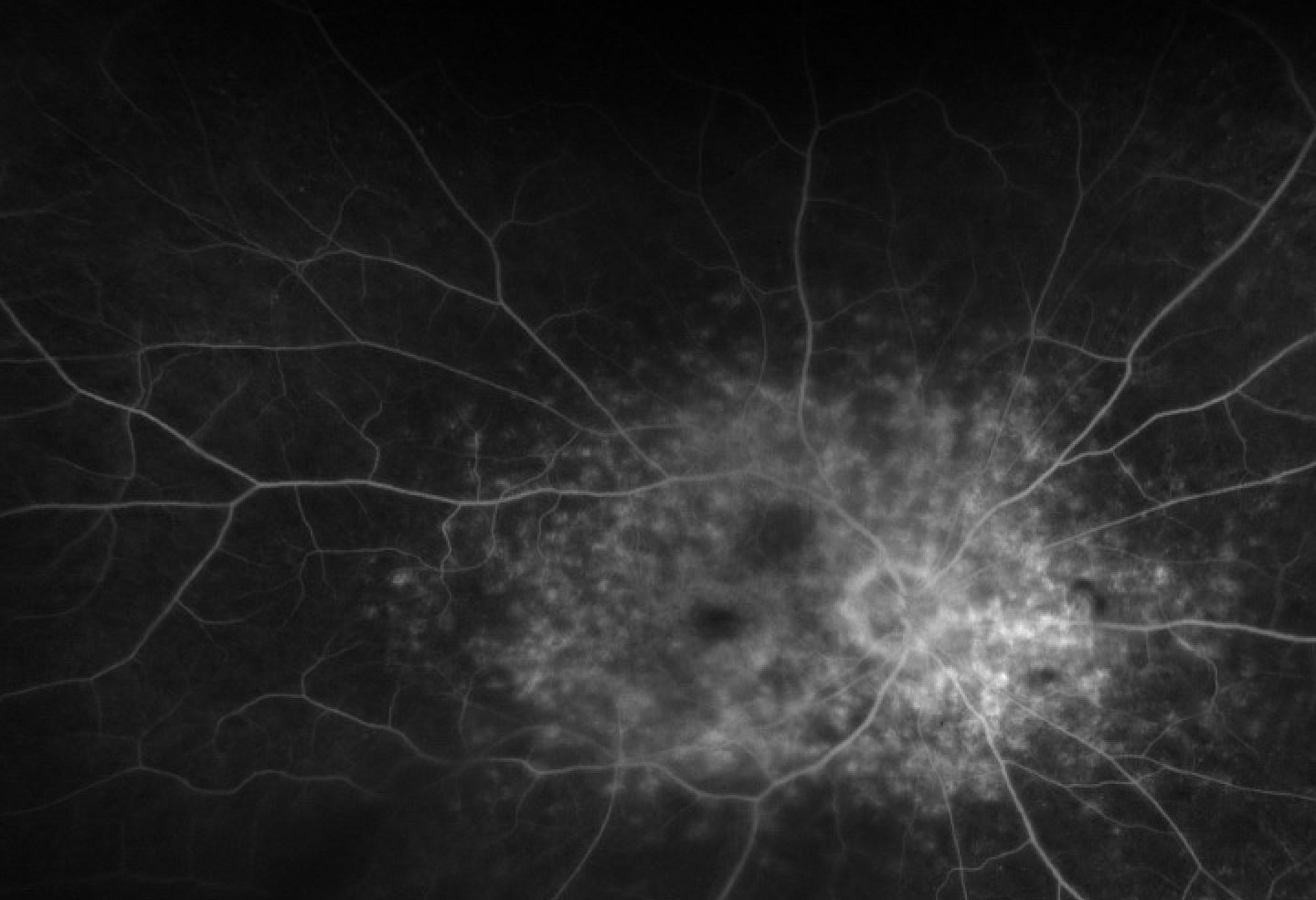
The nature and description of the pattern will define the type of pattern dystrophy the patient has. Imaging modalities such as fluorescein angiography (FA), fundus autofluorescence (FAF), EOG, and ERG may provide additional information to stage and define the pattern dystrophy; however, diagnosing pattern dystrophy is a clinical diagnosis. While all pattern dystrophies are associated with pseudoxanthoma elasticum, fundus pulverulentus has the strongest association.[16]
Additionally, maternally inherited diabetes and deafness have shown to have a significant correlation with pattern dystrophies, with some estimates placing it over 80%.[17]
Evaluation
Butterfly-shaped pattern dystrophy is named after the characteristic bilateral pigmented deposits seen in a butterfly pattern at the RPE. Typically, there is a central area of RPE atrophy, outside of which there is RPE distention from lipofuscin.[7] The color of the pigment ranges from black to yellow or white. On FA, these pigmented areas block fluorescence, and the central lesion is easily visible, distinguishing this dystrophy from others.[7]
FAF will reveal areas of both increased and decreased autofluorescence corresponding to lipofuscin changes. ERG and EOG will reveal mild changes; however, patients maintain normal vision. Patients are able to maintain good vision in the first two to three decades of life; however, fifth decade and beyond, vision changes may occur with corresponding atrophic depigmented lesions seen on FAF and FA.[18]
Reticular dystrophy features a network of pigmented lines and knots in a fish net-like appearance in the RPE. Lesions are up to five disc diameters from the macula and typically fade away with age. Some of the lesions that fade away leave atrophic changes that appear irregular with white dots in the RPE.[18] FA will reveal hypoflourescent segments surrounding areas of hyperfluorescence with normal choroidal vasculature.[19] Similar to butterfly-shaped pattern dystrophy, ERG and EOG are normal or minimally abnormal in reticular dystrophy.
Multifocal pattern dystrophy, resembling Stargardt or Fundus Flavimaculatus, demonstrates multiple irregular white-yellow flecks scattered throughout the macula, similar to the flecks seen in Stargardt disease. Lesions range from fleck deposits to confluent lesions of chorioretinal atrophy.[15] FA is key in distinguishing between Stargardts and this pattern dystrophy, as in multifocal pattern dystrophy, there is no dark choroid. Flecks are hyperfluorescent in both early and late stages.[15]
A distinguishing aspect of multifocal dystrophy is that it affects the peripheral retina beyond the macula and can feature constriction of the peripheral visual field. Depending on the severity, this constriction may be debilitating. Additionally, in severe cases, patients may demonstrate full-field ERG abnormalities. Optical coherence tomography (OCT) will reveal the flecks as highly reflective areas of focal thickening.[20] Rare and advanced cases aside, multifocal dystrophy is distinct from Stargardts as it is not as visually devastating, features a distinct inheritance pattern (autosomal dominant), and there is the absence of a dark choroid on FA.[21]
Adult-onset foveomacular vitelliform dystrophy (AFVD) is unique in that it is linked to mutations beyond the peripherin/RDS gene, such as mutations in the VMD2 gene.[22] On exam, it features bilateral, symmetric, round, gray-white lesions in the macula.[23]
Lesions are usually a third to a half of a disc diameter, with larger lesions appearing similar to Best vitelliform macular dystrophy.[24] On FA, the vitelliform lesion will show hypofluorescence with an area of hyperfluorescence surrounding it, while FAF reveals various patterns, including autofluorescent patterns that are patchy, linear, ring-like, and others.[25]
One way to distinguish AVFD from Best is that both EOG and ERG in AVFD are normal.[26] Additionally, vitelliform lesions typically first appear in the fourth decade or later and are smaller. OCT is another imaging modality that allows for the visualization of vitelliform lesions that are located subretinally.[27]
Some patients with multiple drusen may develop vitelliform exudative macular detachments, and this fluid blocks background fluorescence early and stains late on FA. As such, AVFD may be mistaken for choroidal neovascularization. Patients with AVFD, unlike in choroidal neovascularization, will not respond with improvement in visual acuity after receiving anti-vascular endothelial growth factor or photodynamic therapy.
Fundus pulverulentus features a granular appearance and punctiform mottling of the RPE within the macula. Areas of pigment mottling appear as areas of hypofluorescence on FA.[28] Fundus pulverulentus can be mistaken for age-related macular degeneration due to the RPE mottling; however, OCT imaging and follow-up reveal different disease courses. While all pattern dystrophies share an association with pseudoxanthoma elasticum, fundus pulverulentus has the most common association.
Treatment / Management
Visual prognosis is typically good in pattern dystrophies, with gradual visual changes and loss.[15] There is no evidence-based treatment for the pigment deposition and lipofuscin accumulation seen in pattern dystrophies. However, vision loss can be more rapid in some patients due to secondary complications from choroidal neovascularization and macular holes necessitating treatment with anti-VEGF injections and surgical hole repair, respectively.[4][6] (B3)
In cases of slowly progressive vision loss or the less common rapid loss, low vision therapy remains the most prominent option to maximize function.
Differential Diagnosis
Pattern dystrophies as a whole may resemble AMD and must be worked up appropriately to ensure AMD is not present. A key differential for multifocal pattern dystrophy is Stargardt disease due to the similar appearance of the flecks in the macula and beyond. FA and genetic testing can help distinguish between the two. Best disease and choroidal neovascularization are differentials for AVFD and can be ruled out based on FA and EOG findings, respectively.
Prognosis
Patients can be reassured that pattern dystrophies do not typically negatively impact their vision significantly, and progression is gradual. Due to the challenges of distinguishing pattern dystrophies from AMD, patients are often mistaken for having AMD and have received treatments targeting AMD such as vitamins, anti-VEGF injections, and PDT.
Areas of atrophy may develop in older patients and affect central vision; however, patients can maintain reading vision in most cases. The majority of patients will not require intervention or treatment and can be followed over time.
Complications
Rarely may patients experience complications secondary to choroidal neovascularization, which is responsive to anti-VEGF therapy.[29] PDT should be avoided as it may negatively impact vision in patients with pattern dystrophy, especially those with AVFD.[30] Additionally, some patients with AVFD may develop macular holes. Unfortunately, vitrectomy for these patients is not effective.
Deterrence and Patient Education
Although most patients will maintain good vision and the disease is slowly progressive, there still needs to be follow-up and a full workup to ensure there is no concurrent AMD. Some may experience a more rapid course of vision loss, requiring low vision therapy. Rarely can patients experience complications such as choroidal neovascularization or macular holes, making it critically important to seek medical care if experiencing vision loss.
Enhancing Healthcare Team Outcomes
Retinal pattern dystrophies are a group of diseases characterized by various patterns of pigment deposition, primarily in the macula. Various forms of mutation in the RDS/peripherin gene form the genetic basis for these dystrophies. It is critical to distinguish pattern dystrophies from age-related macular degeneration by utilizing a complete workup including fluorescein angiography, fundus autofluorescence, electroretinogram, and electrooculogram.
While most patients will experience a slowly progressive course with good visual acuity, a few will have a more rapid course causing a decline in central vision. There is no treatment for the pigment deposition; however, low vision aids are available for those few. Additionally, a minority of patients may experience complications such as macular holes or choroidal neovascularization, necessitating treatment with vitrectomy or anti-vascular endothelial growth factor injections.
It is important to refer patients suspected of retinal pattern dystrophy to an ophthalmologist for further evaluation and testing so that the diagnosis can be confirmed, the patient appropriately followed, age-related macular degeneration ruled out, and complications managed should they arise.
Due to the complexity of diagnosis and potentially devastating consequences of complications such as choroidal neovascularization, the healthcare professional's consultation with ophthalmology should be done promptly in cases of suspected pattern dystrophy.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Deutman AF,van Blommestein JD,Henkes HE,Waardenburg PJ,Solleveld-van Driest E, Butterfly-shaped pigment dystrophy of the fovea. Archives of ophthalmology (Chicago, Ill. : 1960). 1970 May; [PubMed PMID: 5442145]
Weleber RG,Carr RE,Murphey WH,Sheffield VC,Stone EM, Phenotypic variation including retinitis pigmentosa, pattern dystrophy, and fundus flavimaculatus in a single family with a deletion of codon 153 or 154 of the peripherin/RDS gene. Archives of ophthalmology (Chicago, Ill. : 1960). 1993 Nov; [PubMed PMID: 8240110]
Level 3 (low-level) evidenceKim RY,Dollfus H,Keen TJ,Fitzke FW,Arden GB,Bhattacharya SS,Bird AC, Autosomal dominant pattern dystrophy of the retina associated with a 4-base pair insertion at codon 140 in the peripherin/RDS gene. Archives of ophthalmology (Chicago, Ill. : 1960). 1995 Apr; [PubMed PMID: 7710395]
Yang Z,Li Y,Jiang L,Karan G,Moshfeghi D,O'Connor S,Li X,Yu Z,Lewis H,Zack D,Jacobson S,Zhang K, A novel RDS/peripherin gene mutation associated with diverse macular phenotypes. Ophthalmic genetics. 2004 Jun; [PubMed PMID: 15370544]
Level 3 (low-level) evidenceFossarello M,Bertini C,Galantuomo MS,Cao A,Serra A,Pirastu M, Deletion in the peripherin/RDS gene in two unrelated Sardinian families with autosomal dominant butterfly-shaped macular dystrophy. Archives of ophthalmology (Chicago, Ill. : 1960). 1996 Apr; [PubMed PMID: 8602784]
Francis PJ, Schultz DW, Gregory AM, Schain MB, Barra R, Majewski J, Ott J, Acott T, Weleber RG, Klein ML. Genetic and phenotypic heterogeneity in pattern dystrophy. The British journal of ophthalmology. 2005 Sep:89(9):1115-9 [PubMed PMID: 16113362]
Zhang K,Garibaldi DC,Li Y,Green WR,Zack DJ, Butterfly-shaped pattern dystrophy: a genetic, clinical, and histopathological report. Archives of ophthalmology (Chicago, Ill. : 1960). 2002 Apr; [PubMed PMID: 11934323]
Level 3 (low-level) evidenceNichols BE,Sheffield VC,Vandenburgh K,Drack AV,Kimura AE,Stone EM, Butterfly-shaped pigment dystrophy of the fovea caused by a point mutation in codon 167 of the RDS gene. Nature genetics. 1993 Mar; [PubMed PMID: 8485574]
Level 3 (low-level) evidenceDownes SM,Fitzke FW,Holder GE,Payne AM,Bessant DA,Bhattacharya SS,Bird AC, Clinical features of codon 172 RDS macular dystrophy: similar phenotype in 12 families. Archives of ophthalmology (Chicago, Ill. : 1960). 1999 Oct; [PubMed PMID: 10532447]
Kohl S,Christ-Adler M,Apfelstedt-Sylla E,Kellner U,Eckstein A,Zrenner E,Wissinger B, RDS/peripherin gene mutations are frequent causes of central retinal dystrophies. Journal of medical genetics. 1997 Aug; [PubMed PMID: 9279751]
Connell G,Bascom R,Molday L,Reid D,McInnes RR,Molday RS, Photoreceptor peripherin is the normal product of the gene responsible for retinal degeneration in the rds mouse. Proceedings of the National Academy of Sciences of the United States of America. 1991 Feb 1; [PubMed PMID: 1992463]
Level 3 (low-level) evidenceTravis GH,Sutcliffe JG,Bok D, The retinal degeneration slow (rds) gene product is a photoreceptor disc membrane-associated glycoprotein. Neuron. 1991 Jan; [PubMed PMID: 1986774]
Level 3 (low-level) evidencePuech B,Kostrubiec B,Hache JC,François P, [Epidemiology and prevalence of hereditary retinal dystrophies in the Northern France]. Journal francais d'ophtalmologie. 1991; [PubMed PMID: 1918822]
Level 2 (mid-level) evidenceDalvin LA,Pulido JS,Marmorstein AD, Vitelliform dystrophies: Prevalence in Olmsted County, Minnesota, United States. Ophthalmic genetics. 2017 Mar-Apr; [PubMed PMID: 27120116]
Boon CJ,van Schooneveld MJ,den Hollander AI,van Lith-Verhoeven JJ,Zonneveld-Vrieling MN,Theelen T,Cremers FP,Hoyng CB,Klevering BJ, Mutations in the peripherin/RDS gene are an important cause of multifocal pattern dystrophy simulating STGD1/fundus flavimaculatus. The British journal of ophthalmology. 2007 Nov; [PubMed PMID: 17504850]
Agarwal A,Patel P,Adkins T,Gass JD, Spectrum of pattern dystrophy in pseudoxanthoma elasticum. Archives of ophthalmology (Chicago, Ill. : 1960). 2005 Jul; [PubMed PMID: 16009832]
Massin P,Virally-Monod M,Vialettes B,Paques M,Gin H,Porokhov B,Caillat-Zucman S,Froguel P,Paquis-Fluckinger V,Gaudric A,Guillausseau PJ, Prevalence of macular pattern dystrophy in maternally inherited diabetes and deafness. GEDIAM Group. Ophthalmology. 1999 Sep; [PubMed PMID: 10485557]
Level 3 (low-level) evidenceChen MS,Chang CC,Tsai TH,Fan IM,Hou PK, Reticular dystrophy of the retinal pigment epithelium. Journal of the Formosan Medical Association = Taiwan yi zhi. 2007 Jun; [PubMed PMID: 17588843]
Level 3 (low-level) evidenceMarano F,Deutman AF,Pinckers AJ,Aandekerk AL,Rijneveld WJ, Reticular dystrophy of the retinal pigment epithelium and choroidal neovascularization. A fluorescein and ICGV study. Acta ophthalmologica Scandinavica. 1997 Feb; [PubMed PMID: 9088395]
Level 3 (low-level) evidenceGhazi NG,Dibernardo C,Ying HS,Mori K,Gehlbach PL, Optical coherence tomography of enucleated human eye specimens with histological correlation: origin of the outer [PubMed PMID: 16564808]
Tuppurainen K,Mäntyjärvi M, The importance of fluorescein angiography in diagnosing pattern dystrophies of the retinal pigment epithelium. Documenta ophthalmologica. Advances in ophthalmology. 1994; [PubMed PMID: 7835193]
Level 3 (low-level) evidenceFelbor U,Schilling H,Weber BH, Adult vitelliform macular dystrophy is frequently associated with mutations in the peripherin/RDS gene. Human mutation. 1997; [PubMed PMID: 9338584]
Renner AB,Tillack H,Kraus H,Kohl S,Wissinger B,Mohr N,Weber BH,Kellner U,Foerster MH, Morphology and functional characteristics in adult vitelliform macular dystrophy. Retina (Philadelphia, Pa.). 2004 Dec; [PubMed PMID: 15579992]
Level 2 (mid-level) evidenceBoon CJ,Klevering BJ,den Hollander AI,Zonneveld MN,Theelen T,Cremers FP,Hoyng CB, Clinical and genetic heterogeneity in multifocal vitelliform dystrophy. Archives of ophthalmology (Chicago, Ill. : 1960). 2007 Aug; [PubMed PMID: 17698758]
Parodi MB,Iacono P,Pedio M,Pece A,Isola V,Fachin A,Pinto M,Ravalico G, Autofluorescence in adult-onset foveomacular vitelliform dystrophy. Retina (Philadelphia, Pa.). 2008 Jun; [PubMed PMID: 18536595]
Theischen M,Schilling H,Steinhorst UH, [EOG in adult vitelliform macular degeneration, butterfly-shaped pattern dystrophy and Best disease]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 1997 Mar; [PubMed PMID: 9181841]
Level 2 (mid-level) evidencePierro L,Tremolada G,Introini U,Calori G,Brancato R, Optical coherence tomography findings in adult-onset foveomacular vitelliform dystrophy. American journal of ophthalmology. 2002 Nov; [PubMed PMID: 12429242]
Level 2 (mid-level) evidenceBattaglia Parodi M, Choroidal neovascularization in fundus pulverulentus. Acta ophthalmologica Scandinavica. 2002 Oct; [PubMed PMID: 12390174]
Level 3 (low-level) evidenceEmpeslidis T,Vardarinos A,Deane J,Banerjee S, Intravitreal ranibizumab in the treatment of butterfly-shaped pattern dystrophy associated with choroidal neovascularization: a case report. Case reports in ophthalmology. 2012 Jan; [PubMed PMID: 22529806]
Level 3 (low-level) evidenceErgun E,Costa D,Slakter J,Yannuzzi LA,Stur M, Photodynamic therapy and vitelliform lesions. Retina (Philadelphia, Pa.). 2004 Jun; [PubMed PMID: 15187662]
Level 3 (low-level) evidence