Introduction
Pentosan polysulfate (PPS) is a semisynthetic pentasaccharide heparinoid with anticoagulant properties. It was initially used in the 1950s as a thrombolytic due to the ability of the molecule to bind the glycocalyx of circulating red blood cells.[1][2] PPS is the only medication approved by the United States Food and Drug Administration to treat interstitial cystitis (IC).[3]
IC is characterized by bladder pain (suprapubic, pelvic, urethral, vaginal, or perineal) caused by filling and relieved by emptying with petechial bladder mucosal hemorrhages on endoscopy and decreased bladder compliance on urodynamics.[3] This disease is very common, affecting over 1 million Americans, the vast majority of whom are female.[4] In the bladder, PPS is postulated to bind to the urothelium and replace disrupted glycosaminoglycans to protect the urothelium.[4] Less frequently, PPS is used for other indications, including irritable bowel syndrome, pelvic pain syndrome, and inner bladder wall cracks.[5] The recommended dosage for PPS is 100 mg, 3 times a day.[4]
Twenty-two years after its approval as a second-line agent for interstitial cystitis, a 6-patient case series described a progressive maculopathy associated with long-term use of the drug, an association that researchers have demonstrated and characterized multiple times.[4][6][7][8][9]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Studies agree that the duration of PPS use, which correlates strongly with cumulative exposure to PPS, might be the most important risk factor for developing PPSM.[9][10] Although PPSM has been seen in a patient with only 325 g and 2.25 years of exposure, most patients develop the disease years later.[11] Specifically, a 2022 review averaging 9 studies calculated an average of 15.0 ± 5.7 years of exposure and a cumulative exposure of 1824 ± 1042 g, data which were corroborated in a subsequent large review.[12] One of these studies noted a prevalence of toxicity of 16% among all affected patients versus a prevalence of 40% in those with cumulative dosages greater than 1000 g and 55% in those with a cumulative dose greater than 1500 g.[5]
These studies included patients from the time of PPSM diagnosis, not when they become symptomatic, so the onset of the disease is likely earlier.[4] When these patients were categorized by disease severity, there was an association between severity, duration of use, and cumulative dose, but not a daily dose of PPS.[5] This association was also borne out in a large-scale study of insurance records, which showed an increased risk of PPSM among those users with more than 3 years of exposure to PPS compared with those with less than 3 years (hazard ratio of 9.5 vs 2.2).[10]
The daily dose and dose per unit of body weight are also not infrequently discussed as potential enablers of toxicity. One study found a higher mean daily dose among affected patients (445 vs 302 g).[9] This finding has been corroborated in a survey study in which patients on 100 mg of PPS for 15 years were less likely to report maculopathy than the patients on 500 mg for a median intake of 5 years.[13] The BMI of affected patients may be on the upper end of normal (24.6 kg/m) but has also been reported to be closer to the middle of the normal range (23.2 kg/m).[12][14]
Epidemiology
Patients with PPSM are predominantly White (93%) and female (90%), with a mean age of 62.2 ± 13.2 years.[4] The prevalence of PPSM among PPS users is difficult to gauge due to differing study designs, varying durations of drug use, differing cumulative (and perhaps daily) doses, mischaracterization of affected patients as possibly having other maculopathies, and the fact that subtle signs and symptoms of the disease may be missed at a point when rigid screening protocols have not yet been widely adopted.
Notwithstanding these limitations, the prevalence of PPSM among those who have used PPS ranges from 0.7% to 2.4% and 3.4% in large-scale retrospective studies from insurance claims databases; however, these studies likely underestimate the true prevalence of PPSM because patients would have to have been diagnosed with another retinal disease, and length of follow-up in the databases limits the ability to find an association between PPS and a retinal disorder.[10][14][15]
Among larger prospective screening studies on known PPS users, the prevalence is estimated to be between 16.5% and 23.1%, although these may overestimate the true prevalence because of selection bias.[5][8]
Pathophysiology
PPS has been proposed to cause this characteristic maculopathy by direct toxicity to either the retinal pigment epithelium (RPE) or the choroid, although other mechanisms have been proposed. RPE toxicity is thought to be due to PPS interfering with the interaction of the retina's extracellular matrix (ECM) or its regulators, notably fibroblast growth factor (FGF). Choroid toxicity is postulated due to changes in the choroidal vasculature, specifically the choriocapillaris. These findings are perhaps best supported by imaging, which consistently finds a primary abnormality in these layers, as well as the finding of progression despite drug cessation.[4][6][16]
The ECM in the retina includes the interphotoreceptor matrix (IPM). This area is responsible for intracellular communication, the delivery of signaling molecules, nutrients, and metabolism, maintenance of retina adhesion, photoreceptor alignment, and the transport regulation of oxygen. These processes are predominantly controlled by the IPM's large glycosylated proteins, including chondroitin sulfate, dermatan sulfate, SPACR, and SPACRCAN.[17][18][19]
PPS has been shown to interact with cartilage proteoglycans in experimental animal models of arthritis, leading to its approval as a disease-modifying antirheumatic agent for veterinarians.[2] In infectious arthritis, PPS has been shown to be associated with decreased levels of ADAMTs5 and TIMP-3 and stable levels of aggrecan, collagen I, and II.[2] The ECM and IPM also contain complement factor H[18]; PPS has been shown to inhibit the alternative and classical pathways of complement activation and could have a harmful effect via this pathway as well.[2] Due to its structural similarity to these glycosylated proteins, PPS could displace the normal IPM constituents, similar to its effect in the bladder urothelium.
One additional component of the ECM and IPM is the fibroblast growth factors (FGFs) that bind to heparin and heparan sulfate.[20] PPS has been shown to inhibit the FGF-1, -2, and -4 signaling pathways, which play an important role in animal models of the organization and development of the retina, as well as maintenance of retinal health and regeneration.[21]
When FGF signaling is inhibited in transgenic zebrafish, the RPE layer thickens significantly as the cells grow to contact the outer segments of the photoreceptors.[22] In humans, certain chemotherapeutic agents target FGF receptors (FGFRs), including erdafitinib (pan-FGFR inhibitor) and pemigatinib (FGFR 1-3), both of which have been shown to induce an increase in reflectivity and thickening of the ellipsoid and interdigitation zones, with subsequent subretinal fluid (SRF) and serous retinal detachment.[23][24][25][26] SRF from chemotherapeutic targeting starts an average of 21 days after starting the medication. Conversely, SRF will often resolve without discontinuation of PPS. Additionally, in one case report, there were no changes to the choroidal vasculature [24][27]
More recent research using optical coherence tomography–angiography (OCT-A) to characterize choroidal vasculature in PPS-exposed patients who have no other findings of PPSM has shown increased vascular flow deficits within the choriocapillaris and decreased choroidal stroma in the deeper Haller and Sattler layers, leading to an increased choroidal vascular index (CVI). (CVI corresponds to the ratio between the choroidal luminal area and the luminal and stromal areas.) As the authors point out, this could be due to the choriocapillaris being a secondary target of PPSM or a secondary effect of the disrupted RPE.[5][28]
The fact that these flow voids are detectable in patients without other stigmata of the disease lends credence to the choriocapillaris being a primary site of injury, as ischemic changes would only be expected to progress. Further, the evidence that visual acuity is spared despite these changes in the choriocapillaris flow may also support this argument.[7]
Other proposals for the pathogenesis of PPSM include mitochondrial dysfunction (based on phenotypic similarity) and the fact that there may be an undefined common cause of interstitial cystitis, making the maculopathy unrelated to PPS exposure.[1][29][30]
In sum, the ECM of the RPE and the choroidal vasculature contain numerous constituents that have either been shown to be affected by PPS or have theoretical interactions based on the structure of PPS. Therefore, the pathogenesis of PPSM is likely located in this area.[21]
History and Physical
Patients with PPSM will report a history of chronic use of PPS, which they may have already discontinued. If they are unable to report the use of the medication, they may also report having used a drug to help with bladder pain.
Although patients can be asymptomatic, they may also present with the following symptoms in order of decreasing frequency: decreased visual acuity, nyctalopia, metamorphopsia, paracentral scotoma, and delayed dark adaptation.[7][11] These symptoms are generally accepted as having a gradual onset. However, one case report described a patient who transitioned from asymptomatic and without retinal findings to having developed advanced PPSM within 2 years.[31]
Regarding visual acuity, the decrease experienced by patients is typically mild, with many studies reporting an average of 20/25 unless the patchy areas of the atrophic retina coalesce into the central fovea.[4][5][32] However, measures of visual acuity alone fail to characterize the visual disability caused by PPSM. Patient-reported outcomes on the National Eye Institute Visual Functioning Questionnaire (NEI VFQ-39) reflect that patients with moderate and advanced PPSM have worse function than those with intermediate age-related macular degeneration (AMD). Additionally, results on the Low Luminance Questionnaire reflect especially low subjective scores on driving, dim lighting, and extreme lighting.[32]
Delayed dark adaptation has also been studied objectively. Although many patients were found to have an increased rod intercept time, the wide variability of the results caused the test to have a low sensitivity for detecting PPSM.[32]
Patients are unaffected by decreased contrast sensitivity until late in the disease state.[32]
Evaluation
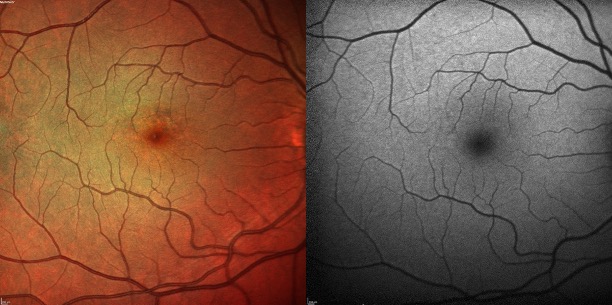
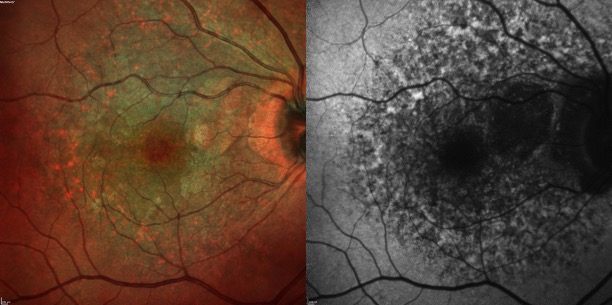
Anterior segment examination is predominantly within normal limits for patients of this age.[5] Fundus examination findings can be subtle and are primarily characterized as densely-packed bilateral paracentral hyperpigmented spots with surrounding yellow subretinal deposits with mottled RPE atrophy.[11][33] Most of these spots are bilateral (97.3%) and confined to the posterior pole; however, wide-field imaging has also shown that 36% of patients had changes in the peripheral retina.[6][12][6]
The pattern is most striking on autofluorescence, which shows a typically well-circumscribed area of small, densely-packed hyper- and hypoautofluorescent spots.[11][34] Fundus autofluorescence (FAF) also discloses that 100% of eyes have a peripapillary hypoautofluorescent halo.[5]
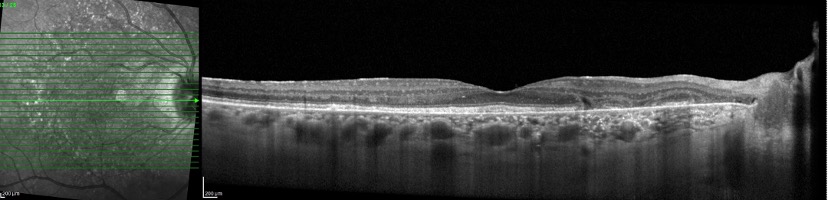
On OCT, the colocalized yellowish pigment on fundus examination and the hyperautofluorescent lesions correspond to nodular RPE abnormalities and excrescences, which cast a shadow onto the underlying choroid (thereby differentiating them from drusen).[4][11] With time, these areas of excrescences and conglomerations change; specifically, overlying retinal layers will progressively thin, and there will be associated RPE atrophy.[6][7][8][11] Cystoid macular edema (CME) occurs less frequently (between 4.2% and 12.9% of patients), as do neovascular membranes (1.4% to 17.6% of patients) and subfoveal vitelliform deposits.[35]
Near-infrared reflectance (NIR), co-acquired with OCT, is often highly effective in showing the characteristic lesions in mild forms of the disease, including when FAF findings are inconclusive.[12] Specifically, similar symmetrical hyperreflectant spots centered on the macula may be seen on NIR, whereas the FAF does not show these lesions. This difference may be due to NIR utilizing a longer wavelength of light (820 nm) than FAF (480 nm), penetrating more avidly into deeper retinal layers.[36] The critical implication of this finding is that patients who are unable to access a center with fundus autofluorescence capabilities can still be screened if OCT is used.
On OCT-A, PPSM has many characteristic findings that may hint at the ultimate pathophysiology of the disease. PPSM is associated with abnormal foveal avascular zone (FAZ) configurations and decreased choriocapillaris perfusion in patients with later stages of the disease. These areas of decreased perfusion were shown to have a flow void average of 54%, compared with 14% in non-PPSM patients, and they correspond to areas of outer retinal atrophy.[5][7]
A separate analysis of the stroma on OCT-A found a decreased stromal choroidal area with a preserved stromal luminal area, yielding an increased choroidal vascularity index. These changes on OCT-A may be utilized to detect patients with forme fruste PPSM, as increased flow voids were found in 15 patients with greater than 1000 g of PPS exposure who had no other findings on retinal imaging.[28] Further, it can help differentiate the condition from AMD, in which the choroidal vascularity index increases.[5] Importantly, OCT-A can also identify patients with choroidal neovascular membranes.[12][37]
When tested on electroretinography (ERG), patients can have nonspecific responses ranging from normal to mild attenuation of amplitude in code and rod responses with variable delay in response.[38] Similarly, multifocal ERG will reflect mild-to-severe attenuation of response amplitude.[6][34] Electro-oculograms reflect predominantly normal Arden ratios, though dark adaptometry is frequently found to be prolonged.[7][11][32]
Multiple studies have included genetic testing for patients with PPSM to rule out other maculopathies. Although none have found clear associations, some wonder if there may be a genetic predisposition to having a more severe phenotype.[5][7][39] For example, a patient in one study had an NPHP1 mutation and severe phenotype, and another had a family history of AMD and a variant of undetermined significance in the RP1 gene who presented with geographic atrophy and count fingers vision.[7][9] Other studies have reported multiple variants of uncertain significance, including ABCA4, ADAM9, IMPG2, MPZ, and TIMP3.[6]
Based on these results, Barnes and colleagues proposed 6 diagnostic criteria:[33]
- Macular hyperpigmented spots with yellow-orange deposits and patchy RPE atrophy on fundus photography
- Densely packed clusters of hyper- and hypoautofluorescent areas within the posterior pole on FAF
- Focal thickening of the RPE with hyperreflectance on NIR
- The peripapillary hypoautofluorescent halo
- Maximum size of the hyperautofluorescent spots of 2 venule widths.
- Absence of typical drusen
Further, based on their work, Wang and colleagues proposed the following guidelines for defining disease severity:[9]
- Mild: Speckled pattern on FAF without well-demarcated atrophic lesions on FAF and no evidence of complete RPE and outer retinal atrophy
- Moderate: Well-demarcated, nummular, and colocalized lesions with RPE and outer retinal atrophy; no central foveal involvement
- Severe: Well-demarcated lesions with associated hypoautofluorescent atrophy with RPE and outer retinal atrophy, which involves the central fovea
Hanif and colleagues proposed a similar classification of severity:[6]
- Disease within the vascular arcades and without areas of atrophy
- Disease extending to the vascular arcades but not more than 2 disc diameters beyond or the presence of noncentral atrophy.
- Disease that extends more than 2 disc diameters beyond the temporal arcades or the presence of central foveal atrophy.
Additionally, Wang et al found a correlation between disease severity and cumulative dosage; however, the sample size was too small to draw any conclusions.[5]
Treatment / Management
There is no known treatment for PPSM; therefore, primary prevention is imperative with medication avoidance or using the lowest effective dose if necessary. The sight-threatening sequelae of PPSM can be treated with common medications already used to address those conditions. For example, patients with cystoid macular edema have been successfully treated with carbonic anhydrase inhibitors and anti–vascular endothelial growth factor (VEGF) drugs, and patients with choroidal neovascular membranes have been treated with intravitreal anti-VEGF medications.[37]
Differential Diagnosis
The differential diagnosis of PPSM and the primary methods of differentiating it from these conditions include:
AMD
PPSM and AMD are characterized by patients with similar demographics and pigmentary macular changes, which can progress to geographic atrophy. The 2 entities can be differentiated via multimodal imaging and identification of the typical pattern of PPSM.[40]
Specifically, hyperpigmented macular spots with yellow-orange deposits, which are at the level of the RPE and not below, along with dense packing of hyper- and hypoautofluorescent spots on FAF (especially with a peripapillary autofluorescent halo) best describe PPSM. On the other hand, AMD can be identified by drusen, which is below the RPE. In one study comparing these diagnoses, no macular drusen were found in eyes diagnosed with PPS. In contrast, RPEE pigmentary clumps were more frequently found in AMD eyes of patients with prior PPS exposure.[40]
Pattern Dystrophy
When differentiating PPSM from other pattern dystrophies, the same framework from above is used; however, special attention is paid to three features in patients with borderline imaging.[33] First, the peripapillary hypoautofluorescent halo is extremely useful for differentiating from the ABCA4-retinopathies; however, it is less so when the disease has not yet progressed to encompassing the optic nerve. Second, the density of abnormalities in PPSM is significantly higher than what is seen in hereditary maculopathies. Third, PPSM can involve (but does not necessarily have to) the central fovea early in the disease course.[33]
Inquiring about the family history or performing genetic testing can be useful in cases that are not elucidated by these three findings.
Mitochondrial Dystrophy
There is a phenotypic overlap between PPSM and mitochondrial retinopathies, including reticulated-appearing fundus, peripapillary atrophy, and nyctalopia. One difference that may be seen between these diseases is that PPSM does not necessarily spare the fovea early in the disease, whereas mitochondrial illnesses will. Further, mitochondrial illnesses often have systemic manifestations not seen in PPSM, such as muscle weakness (eg, cardiomyopathy).[41]
Prognosis
PPSM is a progressive maculopathy that leads to areas of RPE atrophy that can decrease visual acuity, causing legal blindness. Other visually debilitating symptoms include nyctalopia, metamorphopsia, a paracentral scotoma, and delayed dark adaptation.[4] The disease may progress despite discontinuation; however, cessation may help slow or reverse the disease.[7][42][43][44][45]
Complications
In addition to decreased visual acuity from RPE atrophy, the sight-threatening sequelae of PPSM include CME, subfoveal vitelliform deposits, and macular neovascular membranes.[12][35][37][46]
Deterrence and Patient Education
In addition to educating urologists and urogynecologists on PPSM, various proposals for screening guidelines for the condition have been proposed. All generally agree on baseline testing at the initiation of treatment with PPS, annual testing after that, and collaborative decision-making about drug discontinuation when patients have a cumulative dose of greater than 1500 g. At each screening appointment with the ophthalmologist, various imaging modalities should be used, including NIR, FAF, and OCT-A, if possible.[28][47] The need to screen patients has successfully been integrated into an electronic medical record system at a single institution.[48]
Enhancing Healthcare Team Outcomes
Although no large-scale interventional studies to discontinue PPS have been conducted, experts agree that communication between urologists, urogynecologists, and ophthalmologists should be codified to screen PPS patients continuously.[47] Pharmacists also play a crucial role in patient education on the drug and its proper use, monitoring, and dosing. They can coordinate with nursing staff as part of an interprofessional ophthalmological care team.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)

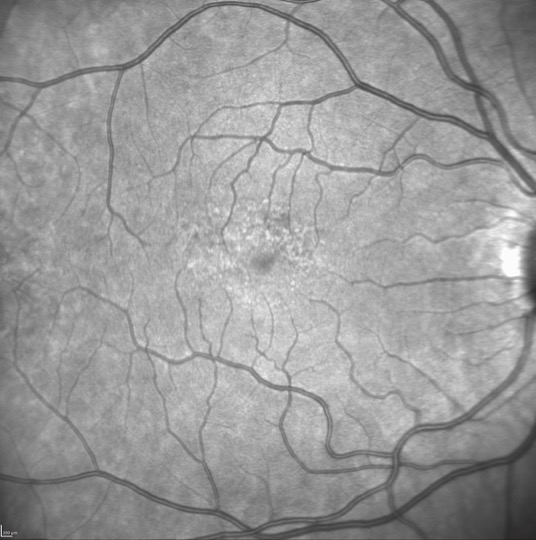
Mild pentosan polysulfate maculopathy without imaging findings on color fundus photography, fundus autofluorescence, or optical coherence tomography is seen here on OCT-Angiography as increased flow deficits (examples highlighted in orange), lending a moth-eaten appearance. Used with permission from David Sarraf, MD
(Click Image to Enlarge)
References
Paredes Mogica JA, De EJB. Pentosan Polysulfate Maculopathy: What Urologists Should Know in 2020. Urology. 2021 Jan:147():109-118. doi: 10.1016/j.urology.2020.08.072. Epub 2020 Oct 10 [PubMed PMID: 33045286]
Herrero LJ, Foo SS, Sheng KC, Chen W, Forwood MR, Bucala R, Mahalingam S. Pentosan Polysulfate: a Novel Glycosaminoglycan-Like Molecule for Effective Treatment of Alphavirus-Induced Cartilage Destruction and Inflammatory Disease. Journal of virology. 2015 Aug:89(15):8063-76. doi: 10.1128/JVI.00224-15. Epub 2015 May 27 [PubMed PMID: 26018160]
Nickel JC, Moldwin R. FDA BRUDAC 2018 Criteria for Interstitial Cystitis/Bladder Pain Syndrome Clinical Trials: Future Direction for Research. The Journal of urology. 2018 Jul:200(1):39-42. doi: 10.1016/j.juro.2018.02.011. Epub 2018 Feb 13 [PubMed PMID: 29452126]
Level 3 (low-level) evidenceLindeke-Myers A, Hanif AM, Jain N. Pentosan polysulfate maculopathy. Survey of ophthalmology. 2022 Jan-Feb:67(1):83-96. doi: 10.1016/j.survophthal.2021.05.005. Epub 2021 May 14 [PubMed PMID: 34000253]
Level 3 (low-level) evidenceWang D, Velaga SB, Grondin C, Au A, Nittala M, Chhablani J, Vupparaboina KK, Gunnemann F, Jung J, Kim JH, Ip M, Sadda S, Sarraf D. Pentosan Polysulfate Maculopathy: Prevalence, Spectrum of Disease, and Choroidal Imaging Analysis Based on Prospective Screening. American journal of ophthalmology. 2021 Jul:227():125-138. doi: 10.1016/j.ajo.2021.02.025. Epub 2021 Feb 27 [PubMed PMID: 33651989]
Hanif AM, Armenti ST, Taylor SC, Shah RA, Igelman AD, Jayasundera KT, Pennesi ME, Khurana RN, Foote JE, O'Keefe GA, Yang P, Hubbard GB 3rd, Hwang TS, Flaxel CJ, Stein JD, Yan J, Jain N. Phenotypic Spectrum of Pentosan Polysulfate Sodium-Associated Maculopathy: A Multicenter Study. JAMA ophthalmology. 2019 Nov 1:137(11):1275-1282. doi: 10.1001/jamaophthalmol.2019.3392. Epub [PubMed PMID: 31486843]
Level 2 (mid-level) evidenceAbou-Jaoude MM, Davis AM, Fraser CE, Leys M, Hinkle D, Odom JV, Maldonado RS. New Insights Into Pentosan Polysulfate Maculopathy. Ophthalmic surgery, lasers & imaging retina. 2021 Jan 1:52(1):13-22. doi: 10.3928/23258160-20201223-04. Epub [PubMed PMID: 33471910]
Vora RA, Patel AP, Melles R. Prevalence of Maculopathy Associated with Long-Term Pentosan Polysulfate Therapy. Ophthalmology. 2020 Jun:127(6):835-836. doi: 10.1016/j.ophtha.2020.01.017. Epub 2020 Jan 17 [PubMed PMID: 32085877]
Wang D, Au A, Gunnemann F, Hilely A, Scharf J, Tran K, Sun M, Kim JH, Sarraf D. Pentosan-associated maculopathy: prevalence, screening guidelines, and spectrum of findings based on prospective multimodal analysis. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2020 Apr:55(2):116-125. doi: 10.1016/j.jcjo.2019.12.001. Epub 2020 Jan 20 [PubMed PMID: 31973791]
Bae SS, Sodhi M, Maberley D, Kezouh A, Etminan M. Risk of maculopathy with pentosan polysulfate sodium use. British journal of clinical pharmacology. 2022 Jul:88(7):3428-3433. doi: 10.1111/bcp.15303. Epub 2022 Mar 22 [PubMed PMID: 35277990]
Abou-Jaoude M, Fraser C, Maldonado RS. Update on maculopathy secondary to pentosan polysulfate toxicity. Current opinion in ophthalmology. 2021 May 1:32(3):233-239. doi: 10.1097/ICU.0000000000000754. Epub [PubMed PMID: 33710012]
Level 3 (low-level) evidenceJain N, Liao A, Garg SJ, Patel SN, Wykoff CC, Yu HJ, London NJS, Khurana RN, Zacks DN, Macula Society Pentosan Polysulfate Maculopathy Study Group. Expanded Clinical Spectrum of Pentosan Polysulfate Maculopathy: A Macula Society Collaborative Study. Ophthalmology. Retina. 2022 Mar:6(3):219-227. doi: 10.1016/j.oret.2021.07.004. Epub 2021 Jul 21 [PubMed PMID: 34298229]
Uner OE, Shah MK, Jain N. PENTOSAN POLYSULFATE AND VISION: Findings from an International Survey of Exposed Individuals. Retina (Philadelphia, Pa.). 2021 Jul 1:41(7):1562-1569. doi: 10.1097/IAE.0000000000003078. Epub [PubMed PMID: 33332810]
Level 3 (low-level) evidenceLudwig CA, Vail D, Callaway NF, Pasricha MV, Moshfeghi DM. Pentosan Polysulfate Sodium Exposure and Drug-Induced Maculopathy in Commercially Insured Patients in the United States. Ophthalmology. 2020 Apr:127(4):535-543. doi: 10.1016/j.ophtha.2019.10.036. Epub 2019 Nov 5 [PubMed PMID: 31899034]
Jain N, Li AL, Yu Y, VanderBeek BL. Association of macular disease with long-term use of pentosan polysulfate sodium: findings from a US cohort. The British journal of ophthalmology. 2020 Aug:104(8):1093-1097. doi: 10.1136/bjophthalmol-2019-314765. Epub 2019 Nov 6 [PubMed PMID: 31694837]
Shah R, Simonett JM, Lyons RJ, Rao RC, Pennesi ME, Jain N. Disease Course in Patients With Pentosan Polysulfate Sodium-Associated Maculopathy After Drug Cessation. JAMA ophthalmology. 2020 Aug 1:138(8):894-900. doi: 10.1001/jamaophthalmol.2020.2349. Epub [PubMed PMID: 32644147]
Brandl C, Schulz HL, Charbel Issa P, Birtel J, Bergholz R, Lange C, Dahlke C, Zobor D, Weber BHF, Stöhr H. Mutations in the Genes for Interphotoreceptor Matrix Proteoglycans, IMPG1 and IMPG2, in Patients with Vitelliform Macular Lesions. Genes. 2017 Jun 23:8(7):. doi: 10.3390/genes8070170. Epub 2017 Jun 23 [PubMed PMID: 28644393]
Ishikawa M, Sawada Y, Yoshitomi T. Structure and function of the interphotoreceptor matrix surrounding retinal photoreceptor cells. Experimental eye research. 2015 Apr:133():3-18. doi: 10.1016/j.exer.2015.02.017. Epub [PubMed PMID: 25819450]
Clark SJ, Keenan TD, Fielder HL, Collinson LJ, Holley RJ, Merry CL, van Kuppevelt TH, Day AJ, Bishop PN. Mapping the differential distribution of glycosaminoglycans in the adult human retina, choroid, and sclera. Investigative ophthalmology & visual science. 2011 Aug 17:52(9):6511-21. doi: 10.1167/iovs.11-7909. Epub 2011 Aug 17 [PubMed PMID: 21746802]
Burgess WH, Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annual review of biochemistry. 1989:58():575-606 [PubMed PMID: 2549857]
Level 3 (low-level) evidenceGreenlee T, Hom G, Conti T, Babiuch AS, Singh R. Re: Pearce et al.: Pigmentary maculopathy associated with chronic exposure to pentosan polysulfate sodium (Ophthalmology. 2018;125:1793-1802). Ophthalmology. 2019 Jul:126(7):e51. doi: 10.1016/j.ophtha.2018.12.037. Epub [PubMed PMID: 31229012]
Hochmann S, Kaslin J, Hans S, Weber A, Machate A, Geffarth M, Funk RH, Brand M. Fgf signaling is required for photoreceptor maintenance in the adult zebrafish retina. PloS one. 2012:7(1):e30365. doi: 10.1371/journal.pone.0030365. Epub 2012 Jan 26 [PubMed PMID: 22291943]
Level 3 (low-level) evidenceBloom WR, Edakkunnathu A, Kondapalli SS, Bloom TD. Transient pemigatinib-induced subretinal fluid accumulation and serous retinal detachment. Clinical & experimental optometry. 2023 Jul:106(5):560-563. doi: 10.1080/08164622.2022.2086792. Epub 2022 Jul 6 [PubMed PMID: 35793865]
Fasolino G, Moschetta L, De Grève J, Nelis P, Lefesvre P, Ten Tusscher M. Choroidal and Choriocapillaris Morphology in Pan-FGFR Inhibitor-Associated Retinopathy: A Case Report. Diagnostics (Basel, Switzerland). 2022 Jan 20:12(2):. doi: 10.3390/diagnostics12020249. Epub 2022 Jan 20 [PubMed PMID: 35204340]
Level 3 (low-level) evidenceParikh D, Eliott D, Kim LA. Fibroblast Growth Factor Receptor Inhibitor-Associated Retinopathy. JAMA ophthalmology. 2020 Oct 1:138(10):1101-1103. doi: 10.1001/jamaophthalmol.2020.2778. Epub [PubMed PMID: 32789485]
Patel SN, Camacci ML, Bowie EM. Reversible Retinopathy Associated with Fibroblast Growth Factor Receptor Inhibitor. Case reports in ophthalmology. 2022 Jan-Apr:13(1):57-63. doi: 10.1159/000519275. Epub 2022 Feb 11 [PubMed PMID: 35350233]
Level 3 (low-level) evidenceFrancis JH, Harding JJ, Schram AM, Canestraro J, Haggag-Lindgren D, Heinemann M, Kriplani A, Jhaveri K, Voss MH, Bajorin D, Abou-Alfa GK, Iyer G, Drilon A, Rosenberg J, Abramson DH. Clinical and Morphologic Characteristics of Fibroblast Growth Factor Receptor Inhibitor-Associated Retinopathy. JAMA ophthalmology. 2021 Oct 1:139(10):1126-1130. doi: 10.1001/jamaophthalmol.2021.3331. Epub [PubMed PMID: 34473206]
Fogel Levin M, Santina A, Corradetti G, Au A, Lu A, Abraham N, Somisetty S, Romero Morales V, Wong A, Sadda S, Sarraf D. Pentosan Polysulfate Sodium-Associated Maculopathy: Early Detection Using OCT Angiography and Choriocapillaris Flow Deficit Analysis. American journal of ophthalmology. 2022 Dec:244():38-47. doi: 10.1016/j.ajo.2022.07.015. Epub 2022 Jul 25 [PubMed PMID: 35901995]
Yusuf IH, Charbel Issa P, Lotery AJ. Pentosan Polysulfate Maculopathy-Prescribers Should Be Aware. JAMA ophthalmology. 2020 Aug 1:138(8):900-902. doi: 10.1001/jamaophthalmol.2020.2364. Epub [PubMed PMID: 32644128]
Jain N. Re: Pentosan Polysulfate Maculopathy: What Urologists Should Know in 2020. Urology. 2021 Jun:152():205-206. doi: 10.1016/j.urology.2021.01.027. Epub 2021 Jan 22 [PubMed PMID: 33493509]
Somisetty S, Santina A, Abraham N, Lu A, Morales VR, Sarraf D. RAPID PENTOSAN POLYSULFATE SODIUM MACULOPATHY PROGRESSION. Retinal cases & brief reports. 2023 Nov 1:17(6):660-663. doi: 10.1097/ICB.0000000000001273. Epub [PubMed PMID: 35385434]
Level 3 (low-level) evidenceLyons RJ, Brower J, Jain N. Visual Function in Pentosan Polysulfate Sodium Maculopathy. Investigative ophthalmology & visual science. 2020 Nov 2:61(13):33. doi: 10.1167/iovs.61.13.33. Epub [PubMed PMID: 33231621]
Barnes AC, Hanif AM, Jain N. Pentosan Polysulfate Maculopathy versus Inherited Macular Dystrophies: Comparative Assessment with Multimodal Imaging. Ophthalmology. Retina. 2020 Dec:4(12):1196-1201. doi: 10.1016/j.oret.2020.05.008. Epub 2020 May 21 [PubMed PMID: 32446908]
Level 2 (mid-level) evidencePearce WA, Chen R, Jain N. Pigmentary Maculopathy Associated with Chronic Exposure to Pentosan Polysulfate Sodium. Ophthalmology. 2018 Nov:125(11):1793-1802. doi: 10.1016/j.ophtha.2018.04.026. Epub 2018 May 22 [PubMed PMID: 29801663]
Wannamaker KW, Sisk RA. Large Subfoveal Vitelliform Lesions in a Case of Pentosan Polysulfate Maculopathy. Ophthalmology. 2020 Dec:127(12):1641. doi: 10.1016/j.ophtha.2020.07.048. Epub [PubMed PMID: 33222775]
Level 3 (low-level) evidenceAbdolrahimzadeh S, Ciancimino C, Grassi F, Sordi E, Fragiotta S, Scuderi G. Near-Infrared Reflectance Imaging in Retinal Diseases Affecting Young Patients. Journal of ophthalmology. 2021:2021():5581851. doi: 10.1155/2021/5581851. Epub 2021 Jul 31 [PubMed PMID: 34373789]
Mishra K, Patel TP, Singh MS. Choroidal Neovascularization Associated with Pentosan Polysulfate Toxicity. Ophthalmology. Retina. 2020 Jan:4(1):111-113. doi: 10.1016/j.oret.2019.08.006. Epub 2019 Aug 23 [PubMed PMID: 31570285]
Vora RA, Patel AP, Yang SS, Melles R. A case of pentosan polysulfate maculopathy originally diagnosed as stargardt disease. American journal of ophthalmology case reports. 2020 Mar:17():100604. doi: 10.1016/j.ajoc.2020.100604. Epub 2020 Jan 25 [PubMed PMID: 32043016]
Level 3 (low-level) evidenceKalaw FGP, Ignacio JCI, Wu CY, Ferreyra H, Nudleman E, Baxter SL, Freeman WR, Borooah S. PENTOSAN POLYSULFATE SODIUM (ELMIRON) MACULOPATHY: A Genetic Perspective. Retina (Philadelphia, Pa.). 2023 Jul 1:43(7):1174-1181. doi: 10.1097/IAE.0000000000003794. Epub [PubMed PMID: 36996461]
Level 3 (low-level) evidenceChristiansen JS, Barnes AC, Berry DE, Jain N. Pentosan polysulfate maculopathy versus age-related macular degeneration: comparative assessment with multimodal imaging. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2022 Feb:57(1):16-22. doi: 10.1016/j.jcjo.2021.02.007. Epub 2021 Mar 12 [PubMed PMID: 33722504]
Level 2 (mid-level) evidenceHanif AM, Yan J, Jain N. Pattern Dystrophy: An Imprecise Diagnosis in the Age of Precision Medicine. International ophthalmology clinics. 2019 Winter:59(1):173-194. doi: 10.1097/IIO.0000000000000262. Epub [PubMed PMID: 30585925]
Barnett JM, Jain N. POTENTIAL NEW-ONSET CLINICALLY DETECTABLE PENTOSAN POLYSULFATE MACULOPATHY YEARS AFTER DRUG CESSATION. Retinal cases & brief reports. 2022 Nov 1:16(6):724-726. doi: 10.1097/ICB.0000000000001090. Epub [PubMed PMID: 33229920]
Level 3 (low-level) evidenceHiggins K, Welch RJ, Bacorn C, Yiu G, Rothschild J, Park SS, Moshiri A. Identification of Patients with Pentosan Polysulfate Sodium-Associated Maculopathy through Screening of the Electronic Medical Record at an Academic Center. Journal of ophthalmology. 2020:2020():8866961. doi: 10.1155/2020/8866961. Epub 2020 Dec 17 [PubMed PMID: 33489347]
Huckfeldt RM, Vavvas DG. Progressive Maculopathy After Discontinuation of Pentosan Polysulfate Sodium. Ophthalmic surgery, lasers & imaging retina. 2019 Oct 1:50(10):656-659. doi: 10.3928/23258160-20191009-10. Epub [PubMed PMID: 31671200]
Jung EH, Lindeke-Myers A, Jain N. Two-Year Outcomes After Variable Duration of Drug Cessation in Patients With Maculopathy Associated With Pentosan Polysulfate Use. JAMA ophthalmology. 2023 Mar 1:141(3):260-266. doi: 10.1001/jamaophthalmol.2022.6093. Epub [PubMed PMID: 36729449]
Dieu AC, Whittier SA, Domalpally A, Pak JW, Voland RP, Boyd KM, Gottlieb JL, Crabtree GS, Giles DL, McAchran SE, Mititelu M. Redefining the Spectrum of Pentosan Polysulfate Retinopathy: Multimodal Imaging Findings from a Cross-Sectional Screening Study. Ophthalmology. Retina. 2022 Sep:6(9):835-846. doi: 10.1016/j.oret.2022.03.016. Epub 2022 Mar 24 [PubMed PMID: 35339727]
Level 2 (mid-level) evidenceSadda SR. A path to the development of screening guidelines for pentosan maculopathy. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2020 Feb:55(1):1-2. doi: 10.1016/j.jcjo.2019.12.003. Epub [PubMed PMID: 32085858]
Hom GL, Kuo BL, Ross JH, Chapman GC, Sharma N, Sastry R, Muste JC, Greenlee TE, Conti TF, Singh RP, Sharma S. Characterization of pentosan polysulfate patients for development of an alert and screening system for ophthalmic monitoring. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2023 Mar 3:():. pii: S0008-4182(23)00041-8. doi: 10.1016/j.jcjo.2023.01.019. Epub 2023 Mar 3 [PubMed PMID: 36878265]