Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare, clinically aggressive hematologic malignancy characterized by cutaneous lesions, bone marrow involvement, and leukemic dissemination. Blastic plasmacytoid dendritic cell neoplasm terminology and the underlying pathophysiology process have evolved over decades. It was previously known as acute agranular CD4+ natural killer (NK) cell leukemia in 1995.[1] After it was discovered that the cell of origin is the plasmacytoid dendritic cell in 2008, the World Health Organization (WHO) coined the term blastic plasmacytoid dendritic cell neoplasm to classify hematological malignancies.[2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
No known environmental or genetic risk factors predispose to the development of BPDCN. It can occur as an isolated neoplasm or with other hematologic malignancies. A prior history of hematologic malignancies, such as myelodysplastic syndrome, chronic myeloid leukemia, chronic myelomonocytic leukemia, and acute myeloid leukemia, has been reported in 10% to 20% of patients diagnosed with BPDCN.[3][4][5] Despite this, the biological association between BPDCN and other myeloid malignancies remains elusive.
Physiologically, plasmacytoid dendritic cells are part of the lymphoid system where they normally reside. They are uncommonly found in the skin in physiologic states but migrate to that location in response to infectious (microbial or viral) or inflammatory stimuli.[6] However, a metagenomic analysis of BPDCN to identify an infectious trigger found no bacterial or viral RNA suggesting specific inciting pathogens in the skin or bone marrow.[7]
Epidemiology
BPDCN is a rare hematologic neoplasm, and the exact incidence is unknown. Estimating the incidence is difficult due to changing nomenclature and lack of defining criteria before the 2008 WHO classification system. BPDCN represents 0.7% of primary cutaneous skin malignancies.[8] However, the incidence could be underestimated since it can present without skin involvement.[3]
BPDCN affects people of all races and geographic regions. BPDCN has been reported in patients of all ages, but it is most prevalent in older adults; the median age at diagnosis is 65 to 67 years. There is a slight male predominance, with a male-to-female ratio of about 2.5:1.[4][5][8]
Pathophysiology
Plasmacytoid dendritic cells were identified as the normal cellular counterpart of BPDCN in 2008 and are thought to be the progenitor cells.[9][10] Further research revealed that BPDCN is specifically related to resting plasmacytoid dendritic cells of myeloid lineage.[11]
Normal plasmacytoid dendritic cells can be derived from either myeloid or lymphoid lineages. Both common dendritic cell progenitors and common lymphoid progenitors can differentiate into plasmacytoid dendritic cells and produce type 1 interferons (IFN-I). Still, only myeloid-derived plasmacytoid dendritic cells can process and express the antigen.[12]
An intricate transcriptional network generates common dendritic cell progenitors and common lymphoid progenitors from bone marrow hematopoietic stem cells (HSCs). During this process, progressive lineage commitment results in acquiring alternate cell pathways.[13]
Commitment to plasmacytoid dendritic cell lineage is regulated by various transcription factors governing the differentiation of myeloid and lymphoid hematopoietic progenitors.[14] TCF, BCL11A, and IRF8 have been identified as the primary transcription factors determining plasmacytoid dendritic cell formation. TCF4, an E-box transcription factor, regulates both differentiation and maintenance of the plasmacytoid dendritic cell lineage.[15]
The protein known as B-cell lymphoma/leukemia 11A, or BCL11A, is required to form plasmacytoid dendritic cells. In combination with the transcription factor TCF4, BCL11A is used to distinguish the gene expression profile of plasmacytoid dendritic cells from that of myeloid dendritic cells, which lack the BCL11A protein but express a high level of the BCL6 protein.[16] The IFN regulatory factor 8, IRF8, promotes early dendritic cell lineage commitment in lymphoid-primed multipotent progenitors and common dendritic cell progenitors, and its expression is upregulated during this transformation.[14][17]
IRF8 regulates different hematopoietic lineages and, if mutated, may cause plasmacytoid dendritic cell cytopenia and immunodeficiency.[18] Similar to normal plasmacytoid dendritic cells, blastic plasmacytoid dendritic cell neoplasm expresses BCL11A and TCF4, while IRF8 may be mutated or mis-spliced.[13] The functional effects and the tumor's ability to produce IFN-I due to this mutation remain unknown.
Histopathology
Blastic plasmacytoid dendritic cell neoplasm is most commonly characterized by a widespread, monomorphous infiltration of medium-sized blasts with irregular nuclei, fine chromatin, and single or multiple nucleoli.[4][8][19] Giemsa staining typically reveals a narrow, grayish-blue, and agranular cytoplasm rim. The number of mitoses varies, and the Ki-67 rate ranges from 20% to 80%.[13]
There is substantial dermal infiltration in the skin, which extends into the subcutaneous fat. In most cases, the epidermis and adnexa are not involved.[20] Angioinvasion or coagulative necrosis are not identified.[21] There is diffuse involvement of the interfollicular regions and medulla in lymph nodes, with B-cell follicles being more commonly unaffected. Bone marrow involvement could range from slight infiltration to extensive marrow replacement.[8] Neoplastic cells could exhibit cytoplasmic microvacuoles and pseudopodia on peripheral blood and bone marrow smears.[4]
Immunohistochemical stains are crucial for the diagnosis of BPDCN. The 5th edition of the World Health Organization Classification of Hematolymphoid Tumours outlined immunohistochemical criteria for the diagnosis. These include the expression of CD123 and one other plasmacytoid dendritic cell marker (TCF4, TCL1, CD303, or CD304) in addition to CD4 or CD56. Alternatively, staining with 3 plasmacytoid dendritic cell markers plus absent staining of expected negative markers (CD3, CD14, CD19, CD34, lysozyme, or myeloperoxidase) can also establish the diagnosis.[22]
History and Physical
Despite being a hematologic malignancy, the most prominent characteristic of blastic plasmacytoid dendritic cell neoplasm is skin lesions, followed by involvement of the bone marrow and lymphadenopathy (see Image 1. Examples of Cutaneous Manifestations of BPDCN).[4] Skin manifestations are present about 90% of the time, and 50% of cases will have the disease detectable only in the skin. In addition, skin lesions can persist despite bone marrow remission.[7]
Skin lesions are the most common complaint at presentation, although in many of these cases, BPDCN is also present in other deep organs. Nevertheless, BPDCN can rarely present without skin involvement. The skin lesions range from brown to violaceous bruise-like lesions, plaques, or nodules and can be solitary or widespread.[5] It has been hypothesized that the skin may serve as a protective barrier against the initial transmission of disease.[23] The production of skin-migration molecules such as cutaneous lymphoma-associated antigen and CD56 by tumor cells has been proposed to explain the dermatological manifestations of BPDCN.[13]
Other signs and symptoms of blastic plasmacytoid dendritic cell neoplasm include cytopenias, lymphadenopathy, hepatomegaly, and splenomegaly, which result from involvement of the respective organs (bone marrow, lymph nodes, liver, and spleen).[3][4] There have also been reports of involvement of the mucosal membrane, tonsils, paranasal cavities, lungs, eyes, and central nervous system (CNS).[3][4]
Evaluation
Skin biopsy with routine histology and immunohistochemistry is an essential diagnostic step in evaluating patients with skin lesions suspected to be neoplastic, including blastic plasmacytoid dendritic cell neoplasm. However, the absence of skin lesions does not preclude the diagnosis, as a subset of cases could present without skin involvement. As a result, a blastic plasmacytoid dendritic cell neoplasm diagnosis should be considered in any patient with poorly differentiated leukemia and an uncertain immunophenotype. In addition, patients with leukemic dissemination should undergo a bone marrow biopsy to rule out the presence of other hematological malignancies.
Immunohistochemistry or flow cytometry can be used to confirm the immunophenotype of blastic plasmacytoid dendritic cell neoplasm. The tumor cells are positive for CD4, CD56, CD123 (α-chain of interleukin-3) (see Image 2. Most Common Phenotypic Findings in BPDCN), BDCA-2/CD303 (blood dendritic cell antigen-2), TCF4, TCL1, and SPIB.[20][24][25][26][27]
Terminal deoxynucleotidyl transferase (TdT) expression is seen in up to 40% of cases and is expressed in 10% to 80% of tumor cells. In the Golgi zone, CD68 expression is reflected by a dot-like positivity.[8][20][8] CD7 (an antigen on T-cells) and CD33 (an antigen on myeloid cells) are also frequently expressed. However, expression of the Epstein-Barr virus-encoded RNA, CD19, CD20, CD79a, CD3 or CD5 (both T-cell antigens), myeloperoxidase, CD117, lysozyme, CD13, CD16, and CD34 is not seen. Notably, uncommon cases lacking CD56 expression have been described, and blastic plasmacytoid dendritic cell neoplasm can be diagnosed if tumor cells express CD4, CD123, and TCL (see sections on histopathology).
Pre-treatment evaluation includes complete blood counts, liver and kidney function tests, lactate dehydrogenase, hepatitis B, and HIV. Human leukocyte antigen (HLA) typing should be performed on stem cell transplant candidates. Peripheral blood flow cytometry is essential, along with microscopic peripheral smear evaluation. Unilateral bone marrow biopsy and aspirate are recommended for all patients to detect involvement. Computed tomography (CT) with contrast should be done to evaluate for lymphadenopathy and hepatosplenomegaly. Patients with neurologic signs or symptoms should have appropriate imaging studies and cerebrospinal fluid evaluation.
Treatment / Management
There are multiple, non-mutually exclusive treatment options for BPDCN. These include conventional chemotherapy, the more recent biopharmaceutical (biologic) agent tagraxofusp, and stem cell transplantation.
There is a lack of standard of care for the treatment of BPDCN, and treatment historically consisted of intensive chemotherapy regimens borrowed from acute myeloid leukemias (7 days of cytarabine and 3 days of daunorubicin or idarubicin) and acute lymphoid leukemia/lymphoma (HyperCVAD or CHOP regimens). The response rates have been suboptimal, with a median survival of 8.7 months in one multicenter retrospective study of 43 cases.[3] That same study also found an advantage for the acute lymphoid leukemia/lymphoma (ALL/LBL) regimens over the acute myeloid leukemia (AML) regimen, with better complete remission rates (67% vs. 27%, P = .02) and better overall survival (12.3 months vs. 7.1 months, P = .02). However, the relapse rate was higher in the ALL/LBL therapy group. Another study of 42 patients found that treatment with a hyper-CVAD regimen led to a higher complete response than treatment with a CHOP-based regimen (91% versus 50%), but that difference did not achieve statistical significance, and there was no difference in overall survival.[28](B2)
The universal overexpression of CD123 by blastic plasmacytoid dendritic cell neoplasm led to the development of tagraxofusp, which is a CD123-directed cytotoxin consisting of recombinant human interleukin-3 fused to a truncated diphtheria toxin. In 2018, tagraxofusp received US FDA approval for use in adults and children older than 2 years for the initial treatment of BPDCN or relapsed/recurrent disease.[29][30] (B3)
In the prospective, open-label, multicenter cohort of 29 patients with untreated BPDCN, tagraxofusp resulted in a combined rate of complete remission (CR) and complete clinical remission (CRc, defined as absence of detectable disease in blood/bone marrow and lymph nodes, with microscopically detected disease in the skin) in 21 (72%) patients. Survival rates at 18 and 24 months were 59% and 52%, respectively. Elevated liver enzymes, capillary leak syndrome, and hypoalbuminemia were among the most common adverse effects. Dosage was administered via IV once a day at 12 mcg/kg on days 1 through 5 of a 21-day cycle. Treatment is continued until the disease progresses or significant toxicity occurs.[31][32] In the retrospective study previously mentioned by Seongseok Yun[28], they also treated patients with tagraxofusp (N=12). They found a complete response rate of 50%, which is not statistically different from the rates mentioned for Hyper-CVAD and CHOP regimens of chemotherapy. (B3)
Stem cell transplant, both autologous and allogeneic, has been used successfully in treating BPDCN, but optimal timing and type of transplant are still being determined. Patients who undergo allogeneic stem cell transplants have significantly longer survival than those who have not received a transplant (hazard ratio, 0.160; 95% CI, 0.0453-0.56).[28] It is currently considered the only way to achieve durable remission and should be performed in eligible candidates, especially after complete remission.[33] In a systematic review of 4 studies involving 128 patients with BPDCN who underwent allo-SCT, the pooled overall survival (OS) rate was 50% for all patients. Among those who received allografting in the first complete remission (CR1), the pooled OS and progression-free/disease-free survival (PFS/DFS) rates were higher at 67% and 53%, respectively. However, for patients who underwent allografting in > CR1, the pooled OS and PFS/DFS rates were significantly lower at 7% for both outcomes. Additionally, it was found that myeloablative conditioning regimens resulted in lower relapse rates (18%) compared to reduced-intensity regimens (40%).[34](A1)
Management in Children
It is recommended that children (2 to 18 years of age) diagnosed with blastic plasmacytoid dendritic cell neoplasm undergo remission induction therapy with either tagraxofusp or an ALL/LBL-like regimen followed by observation rather than allogeneic HCT in the first remission stage. Allogeneic HCT is associated with significant toxicity and outweighs any potential longer-term favorable outcomes. However, because tagraxofusp is not FDA-authorized for children younger than 2 years, treatment with an ALL/LBL-like regimen is recommended.[35][36][37](B2)
Relapsed or Refractory Cases
The optimal treatment for relapsed or refractory BPDCN is not well defined. Treatment should occur within a randomized controlled trial framework as much as possible. Tagraxofusp salvage therapy followed by allogeneic HCT for patients who had previously received an ALL/LBL-like regimen, and repeat treatment with tagraxofusp or an ALL/LBL-like regimen for individuals previously treated with tagraxofusp is the recommended option. Venetoclax, bendamustine, and biweekly CHOP chemotherapy are other therapeutic options.[38][39][40]
Differential Diagnosis
BPDCN must be differentiated from mature plasmacytoid dendritic cell proliferation (MPDCP). Mature plasmacytoid dendritic cell proliferations are neoplastic but benign proliferations of plasmacytoid dendritic cells most commonly seen in the setting of AML—especially those AMLs with RUNX1 mutation, chronic myelomonocytic leukemia, and less commonly, myelodysplastic syndrome and myeloproliferative neoplasms. They are found in the skin, lymph nodes, and bone marrow and are differentiated from BPDCN by their mature morphology and immunohistochemical profile (CD34 positive, CD56 negative, and low expression of CD123 and TCL1). BPDCN has a blast-like morphology and should be negative for CD34, positive for CD56, and strongly expressed in CD123 and TCL1.[41][42]
Given the blast-like morphology of BPDCN, other immature hematolymphoid and non-hematolymphoid neoplasms can enter the differential diagnosis, and distinction is essential for appropriate therapeutic intervention. These include acute myeloid leukemia, B-ALL, T-ALL, natural killer/T-cell lymphoma nasal type, adult T-cell leukemia/lymphoma, anaplastic large cell lymphoma, angiosarcoma, Kaposi sarcoma, Merkel cell carcinoma, and malignant melanoma.[43] A high degree of suspicion with a thorough history, morphologic evaluation, and immunohistochemical findings will lead to the correct diagnosis.
Prognosis
The prognosis of BPDCN is generally poor as it is an aggressive neoplasm of immature cells that is rapidly fatal if not treated early (see treatment/management section above). Different case series show median survival rates of 12 to 27 months, with longer ranges for pediatric cases or those with only cutaneous involvement.[3] Hematopoietic stem-cell transplantation (HSCT) is the best option to maximize long-term survival.
Younger patients have a more favorable survival rate.[4][44][45] In a study of 25 pediatric patients that combined original patients with previously published case reports, the survival rate after receiving chemotherapy was 72% (64% event-free survival and 9 months to 13 years follow-up range).[46] Another prognostic indicator is the expression of CXCL12, a chemokine ligand associated with the leukemic transformation of skin lesions.[45] Lastly, there appears to be a correlation between the maturity of tumor cells and their responsiveness to therapy, with immature cells being more receptive to treatment than mature cells.[47]
The clinical presentation of the skin lesions (nodular, bruise-like, or disseminated lesions) did not achieve statistical significance for prognostic differences.[5]
Complications
Blastic plasmacytoid dendritic cell neoplasms and their treatment can have complications. The clinical course of this malignancy is lethal without appropriate treatment. Despite treatment, relapses can occur. Therefore, close clinical monitoring and surveillance are recommended. There are known adverse effects that result from treatment with chemotherapy and HSCT, including pancytopenia, liver toxicity, renal dysfunction, an increased risk of infections, and death.
A unique and serious adverse effect of tagraxofusp is capillary leak syndrome (CLS). CLS occurs 5 days after onset of therapy and is characterized by hypoalbuminemia, weight gain, edema, and hypotension. In phase 1 and 2 trials of the drug, CLS occurred in 19% of patients and was the cause of death in 2 patients. A decrease in albumin at the start of the treatment was strongly associated with the subsequent development of CLS.[21][31]
Deterrence and Patient Education
Patients must be counseled about the clinical course of blastic plasmacytoid dendritic cell neoplasm, the various treatment options, complications, and the risk of cancer recurrence. In addition, the patient must be well-informed on the disease's prognosis, and treatment adherence should be prioritized. In the event of unfavorable treatment effects, the patient must be strongly encouraged to contact their oncologist and undergo a comprehensive evaluation promptly. In recurrent BPDCN, the possibility of participating in ongoing clinical trials should also be considered.
The contraceptive and lactation needs of female patients should be explicitly reviewed. Patients should be instructed to weigh themselves daily on the same scale. Women of childbearing age receiving tagraxofusp should use an effective contraception method during therapy and for at least a week after completing their last treatment. Female patients who become pregnant should contact their doctor to seek alternate treatment choices. Waiting at least 48 hours following the previous dose before restarting breastfeeding is recommended. Although no studies on pregnant women have been conducted, there is a possible risk to the fetus.
Enhancing Healthcare Team Outcomes
An interprofessional healthcare team strategy is required for the most effective treatment of blastic plasmacytoid dendritic cell neoplasm, which may include a hematologist-oncologist, oncology nurse practitioner, oncology pharmacist, infectious disease physician, emergency physician, primary care physician, dermatologist, pathologist, blood bank specialist, case manager, psychiatrist/psychologist, and occupational and physical therapists. The prompt referral of the patient to a specialist and open communication among the various specialists involved is essential to achieving the best possible outcomes for the patient.
All clinicians must contribute from their specialty as part of a holistic approach to patient care. Given the first-in-class nature of tagraxofusp, a specialized oncology pharmacist is a valuable adjunct consult, in addition to performing usual medication reconciliation and patient medication counseling. Nurses administer the drug, report to the clinical team on patient response and possible adverse events, and counsel the patient. The team should be mindful of cultural inclusion concerning the patient and other team members. The interprofessional team approach will yield the best outcomes with the fewest adverse events.
Ethical considerations come into play when determining treatment options and respecting patient autonomy in decision-making. Responsibilities within the interprofessional team should be clearly defined, with each member contributing their specialized knowledge and skills to optimize patient care. Effective interprofessional communication fosters a collaborative environment where information is shared, questions are encouraged, and concerns are addressed promptly.
Lastly, care coordination is pivotal in ensuring seamless and efficient patient care. Physicians, advanced practitioners, nurses, pharmacists, and other healthcare professionals must work together to streamline the patient's journey, from diagnosis through treatment and follow-up. This coordination minimizes errors, reduces delays, and enhances patient safety, ultimately leading to improved outcomes and patient-centered care that prioritizes the well-being and satisfaction of those affected by blastic plasmacytoid dendritic cell neoplasm.
Media
(Click Image to Enlarge)
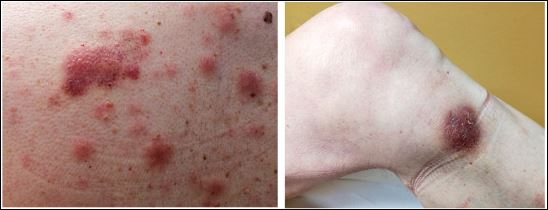
Examples of cutaneous manifestations of BPDCN (a) Erythematous-cyanotic plaques on the back of a patient with widespread disease. (b) Erythematous-purplish single nodule on the leg of a patient with localized disease. Sapienza MR, Pileri A, Derenzini E, Melle F, Motta G, Fiori S, Calleri A, Pimpinelli N, Tabanelli V, Pileri S. Blastic Plasmacytoid Dendritic Cell Neoplasm: State of the Art and Prospects. Cancers. 2019; 11(5):595. https://doi.org/10.3390/cancers11050595. MDPI open access policy and permission guidelines- https://www.mdpi.com/openaccess
(Click Image to Enlarge)
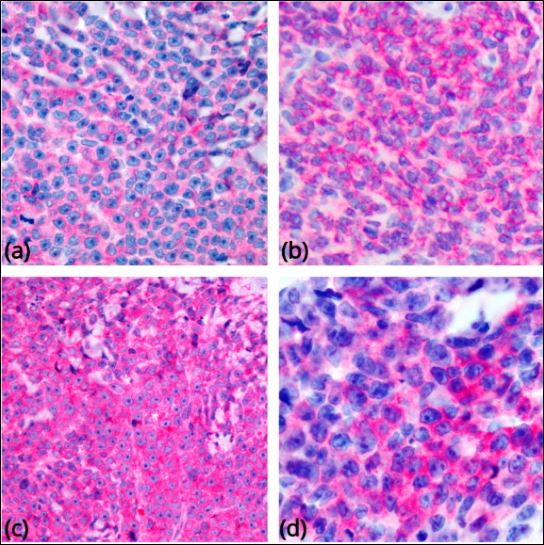
Most common phenotypic findings in BPDCN. Tumor cells show immunoreactivity for CD4 (a), CD56 (b), CD123 (c), and CD303 (d) (original magnification 400×). Sapienza MR, Pileri A, Derenzini E, Melle F, Motta G, Fiori S, Calleri A, Pimpinelli N, Tabanelli V, Pileri S. Blastic Plasmacytoid Dendritic Cell Neoplasm: State of the Art and Prospects. Cancers. 2019; 11(5):595. https://doi.org/10.3390/cancers11050595 MDPI open access policy and permission guideliens - https://www.mdpi.com/openaccess
References
Brody JP, Allen S, Schulman P, Sun T, Chan WC, Friedman HD, Teichberg S, Koduru P, Cone RW, Loughran TP Jr. Acute agranular CD4-positive natural killer cell leukemia. Comprehensive clinicopathologic studies including virologic and in vitro culture with inducing agents. Cancer. 1995 May 15:75(10):2474-83 [PubMed PMID: 7736391]
Level 3 (low-level) evidenceArber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016 May 19:127(20):2391-405. doi: 10.1182/blood-2016-03-643544. Epub 2016 Apr 11 [PubMed PMID: 27069254]
Pagano L, Valentini CG, Pulsoni A, Fisogni S, Carluccio P, Mannelli F, Lunghi M, Pica G, Onida F, Cattaneo C, Piccaluga PP, Di Bona E, Todisco E, Musto P, Spadea A, D'Arco A, Pileri S, Leone G, Amadori S, Facchetti F, GIMEMA-ALWP (Gruppo Italiano Malattie EMatologiche dell'Adulto, Acute Leukemia Working Party). Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica. 2013 Feb:98(2):239-46. doi: 10.3324/haematol.2012.072645. Epub 2012 Oct 12 [PubMed PMID: 23065521]
Level 2 (mid-level) evidenceFeuillard J, Jacob MC, Valensi F, Maynadié M, Gressin R, Chaperot L, Arnoulet C, Brignole-Baudouin F, Drénou B, Duchayne E, Falkenrodt A, Garand R, Homolle E, Husson B, Kuhlein E, Le Calvez G, Sainty D, Sotto MF, Trimoreau F, Béné MC. Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood. 2002 Mar 1:99(5):1556-63 [PubMed PMID: 11861268]
Julia F, Petrella T, Beylot-Barry M, Bagot M, Lipsker D, Machet L, Joly P, Dereure O, Wetterwald M, d'Incan M, Grange F, Cornillon J, Tertian G, Maubec E, Saiag P, Barete S, Templier I, Aubin F, Dalle S. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. The British journal of dermatology. 2013 Sep:169(3):579-86. doi: 10.1111/bjd.12412. Epub [PubMed PMID: 23646868]
Level 2 (mid-level) evidenceReizis B. Plasmacytoid Dendritic Cells: Development, Regulation, and Function. Immunity. 2019 Jan 15:50(1):37-50. doi: 10.1016/j.immuni.2018.12.027. Epub [PubMed PMID: 30650380]
Nomburg J, Bullman S, Chung SS, Togami K, Walker MA, Griffin GK, Morgan EA, LeBoeuf NR, DeCaprio JA, Meyerson M, Lane AA. Comprehensive metagenomic analysis of blastic plasmacytoid dendritic cell neoplasm. Blood advances. 2020 Mar 24:4(6):1006-1011. doi: 10.1182/bloodadvances.2019001260. Epub [PubMed PMID: 32182365]
Level 3 (low-level) evidencePetrella T, Bagot M, Willemze R, Beylot-Barry M, Vergier B, Delaunay M, Meijer CJ, Courville P, Joly P, Grange F, De Muret A, Machet L, Dompmartin A, Bosq J, Durlach A, Bernard P, Dalac S, Dechelotte P, D'Incan M, Wechsler J, Teitell MA. Blastic NK-cell lymphomas (agranular CD4+CD56+ hematodermic neoplasms): a review. American journal of clinical pathology. 2005 May:123(5):662-75 [PubMed PMID: 15981806]
Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. The Journal of experimental medicine. 1997 Mar 17:185(6):1101-11 [PubMed PMID: 9091583]
Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nature immunology. 2000 Oct:1(4):305-10 [PubMed PMID: 11017101]
Sapienza MR, Fuligni F, Agostinelli C, Tripodo C, Righi S, Laginestra MA, Pileri A Jr, Mancini M, Rossi M, Ricci F, Gazzola A, Melle F, Mannu C, Ulbar F, Arpinati M, Paulli M, Maeda T, Gibellini D, Pagano L, Pimpinelli N, Santucci M, Cerroni L, Croce CM, Facchetti F, Piccaluga PP, Pileri SA, AIRC 5xMille consortium ‘Genetics-driven targeted management of lymphoid malignancies and the Italian Registry on Blastic Plasmacytoid Dendritic Cell Neoplasm. Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia. 2014 Aug:28(8):1606-16. doi: 10.1038/leu.2014.64. Epub 2014 Feb 7 [PubMed PMID: 24504027]
Rodrigues PF, Alberti-Servera L, Eremin A, Grajales-Reyes GE, Ivanek R, Tussiwand R. Distinct progenitor lineages contribute to the heterogeneity of plasmacytoid dendritic cells. Nature immunology. 2018 Jul:19(7):711-722. doi: 10.1038/s41590-018-0136-9. Epub 2018 Jun 20 [PubMed PMID: 29925996]
Sapienza MR, Pileri A, Derenzini E, Melle F, Motta G, Fiori S, Calleri A, Pimpinelli N, Tabanelli V, Pileri S. Blastic Plasmacytoid Dendritic Cell Neoplasm: State of the Art and Prospects. Cancers. 2019 Apr 28:11(5):. doi: 10.3390/cancers11050595. Epub 2019 Apr 28 [PubMed PMID: 31035408]
Lee J, Zhou YJ, Ma W, Zhang W, Aljoufi A, Luh T, Lucero K, Liang D, Thomsen M, Bhagat G, Shen Y, Liu K. Lineage specification of human dendritic cells is marked by IRF8 expression in hematopoietic stem cells and multipotent progenitors. Nature immunology. 2017 Aug:18(8):877-888. doi: 10.1038/ni.3789. Epub 2017 Jun 26 [PubMed PMID: 28650480]
Ghosh HS, Cisse B, Bunin A, Lewis KL, Reizis B. Continuous expression of the transcription factor e2-2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity. 2010 Dec 14:33(6):905-16. doi: 10.1016/j.immuni.2010.11.023. Epub 2010 Dec 9 [PubMed PMID: 21145760]
Level 3 (low-level) evidenceIppolito GC, Dekker JD, Wang YH, Lee BK, Shaffer AL 3rd, Lin J, Wall JK, Lee BS, Staudt LM, Liu YJ, Iyer VR, Tucker HO. Dendritic cell fate is determined by BCL11A. Proceedings of the National Academy of Sciences of the United States of America. 2014 Mar 18:111(11):E998-1006. doi: 10.1073/pnas.1319228111. Epub 2014 Mar 3 [PubMed PMID: 24591644]
Level 3 (low-level) evidenceKurotaki D, Kawase W, Sasaki H, Nakabayashi J, Nishiyama A, Morse HC 3rd, Ozato K, Suzuki Y, Tamura T. Epigenetic control of early dendritic cell lineage specification by the transcription factor IRF8 in mice. Blood. 2019 Apr 25:133(17):1803-1813. doi: 10.1182/blood-2018-06-857789. Epub 2019 Feb 22 [PubMed PMID: 30796024]
Bigley V, Maisuria S, Cytlak U, Jardine L, Care MA, Green K, Gunawan M, Milne P, Dickinson R, Wiscombe S, Parry D, Doffinger R, Laurence A, Fonseca C, Stoevesandt O, Gennery A, Cant A, Tooze R, Simpson AJ, Hambleton S, Savic S, Doody G, Collin M. Biallelic interferon regulatory factor 8 mutation: A complex immunodeficiency syndrome with dendritic cell deficiency, monocytopenia, and immune dysregulation. The Journal of allergy and clinical immunology. 2018 Jun:141(6):2234-2248. doi: 10.1016/j.jaci.2017.08.044. Epub 2017 Nov 8 [PubMed PMID: 29128673]
Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Advances in anatomic pathology. 2009 Nov:16(6):392-404. doi: 10.1097/PAP.0b013e3181bb6bc2. Epub [PubMed PMID: 19851130]
Level 3 (low-level) evidenceAssaf C, Gellrich S, Whittaker S, Robson A, Cerroni L, Massone C, Kerl H, Rose C, Chott A, Chimenti S, Hallermann C, Petrella T, Wechsler J, Bagot M, Hummel M, Bullani-Kerl K, Bekkenk MW, Kempf W, Meijer CJ, Willemze R, Sterry W. CD56-positive haematological neoplasms of the skin: a multicentre study of the Cutaneous Lymphoma Project Group of the European Organisation for Research and Treatment of Cancer. Journal of clinical pathology. 2007 Sep:60(9):981-9 [PubMed PMID: 17018683]
Jain A, Sweet K. Blastic Plasmacytoid Dendritic Cell Neoplasm. Journal of the National Comprehensive Cancer Network : JNCCN. 2023 May:21(5):515-521. doi: 10.6004/jnccn.2023.7026. Epub [PubMed PMID: 37156483]
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, Chen W, Chen X, Chng WJ, Choi JK, Colmenero I, Coupland SE, Cross NCP, De Jong D, Elghetany MT, Takahashi E, Emile JF, Ferry J, Fogelstrand L, Fontenay M, Germing U, Gujral S, Haferlach T, Harrison C, Hodge JC, Hu S, Jansen JH, Kanagal-Shamanna R, Kantarjian HM, Kratz CP, Li XQ, Lim MS, Loeb K, Loghavi S, Marcogliese A, Meshinchi S, Michaels P, Naresh KN, Natkunam Y, Nejati R, Ott G, Padron E, Patel KP, Patkar N, Picarsic J, Platzbecker U, Roberts I, Schuh A, Sewell W, Siebert R, Tembhare P, Tyner J, Verstovsek S, Wang W, Wood B, Xiao W, Yeung C, Hochhaus A. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022 Jul:36(7):1703-1719. doi: 10.1038/s41375-022-01613-1. Epub 2022 Jun 22 [PubMed PMID: 35732831]
Pileri A, Delfino C, Grandi V, Agostinelli C, Pileri SA, Pimpinelli N. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): the cutaneous sanctuary. Giornale italiano di dermatologia e venereologia : organo ufficiale, Societa italiana di dermatologia e sifilografia. 2012 Dec:147(6):603-8 [PubMed PMID: 23149706]
Marafioti T, Paterson JC, Ballabio E, Reichard KK, Tedoldi S, Hollowood K, Dictor M, Hansmann ML, Pileri SA, Dyer MJ, Sozzani S, Dikic I, Shaw AS, Petrella T, Stein H, Isaacson PG, Facchetti F, Mason DY. Novel markers of normal and neoplastic human plasmacytoid dendritic cells. Blood. 2008 Apr 1:111(7):3778-92. doi: 10.1182/blood-2007-10-117531. Epub 2008 Jan 24 [PubMed PMID: 18218851]
Herling M, Teitell MA, Shen RR, Medeiros LJ, Jones D. TCL1 expression in plasmacytoid dendritic cells (DC2s) and the related CD4+ CD56+ blastic tumors of skin. Blood. 2003 Jun 15:101(12):5007-9 [PubMed PMID: 12576313]
Montes-Moreno S, Ramos-Medina R, Martínez-López A, Barrionuevo Cornejo C, Parra Cubillos A, Quintana-Truyenque S, Rodriguez Pinilla SM, Pajares R, Sanchez-Verde L, Martinez-Torrecuadrada J, Roncador G, Piris MA. SPIB, a novel immunohistochemical marker for human blastic plasmacytoid dendritic cell neoplasms: characterization of its expression in major hematolymphoid neoplasms. Blood. 2013 Jan 24:121(4):643-7. doi: 10.1182/blood-2012-08-447599. Epub 2012 Nov 19 [PubMed PMID: 23165482]
Petrella T, Meijer CJ, Dalac S, Willemze R, Maynadié M, Machet L, Casasnovas O, Vergier B, Teitell MA. TCL1 and CLA expression in agranular CD4/CD56 hematodermic neoplasms (blastic NK-cell lymphomas) and leukemia cutis. American journal of clinical pathology. 2004 Aug:122(2):307-13 [PubMed PMID: 15323148]
Yun S, Chan O, Kerr D, Vincelette ND, Idrees A, Mo Q, Sweet K, Lancet JE, Kharfan-Dabaja MA, Zhang L, Sokol L. Survival outcomes in blastic plasmacytoid dendritic cell neoplasm by first-line treatment and stem cell transplant. Blood advances. 2020 Jul 28:4(14):3435-3442. doi: 10.1182/bloodadvances.2020001875. Epub [PubMed PMID: 32722779]
Level 3 (low-level) evidencePemmaraju N, Konopleva M. Approval of tagraxofusp-erzs for blastic plasmacytoid dendritic cell neoplasm. Blood advances. 2020 Aug 25:4(16):4020-4027. doi: 10.1182/bloodadvances.2019000173. Epub [PubMed PMID: 32841341]
Level 3 (low-level) evidenceTesta U, Pelosi E, Frankel A. CD 123 is a membrane biomarker and a therapeutic target in hematologic malignancies. Biomarker research. 2014 Feb 10:2(1):4. doi: 10.1186/2050-7771-2-4. Epub 2014 Feb 10 [PubMed PMID: 24513123]
Pemmaraju N, Lane AA, Sweet KL, Stein AS, Vasu S, Blum W, Rizzieri DA, Wang ES, Duvic M, Sloan JM, Spence S, Shemesh S, Brooks CL, Balser J, Bergstein I, Lancet JE, Kantarjian HM, Konopleva M. Tagraxofusp in Blastic Plasmacytoid Dendritic-Cell Neoplasm. The New England journal of medicine. 2019 Apr 25:380(17):1628-1637. doi: 10.1056/NEJMoa1815105. Epub [PubMed PMID: 31018069]
Hammond D, Pemmaraju N. Tagraxofusp for Blastic Plasmacytoid Dendritic Cell Neoplasm. Hematology/oncology clinics of North America. 2020 Jun:34(3):565-574. doi: 10.1016/j.hoc.2020.01.005. Epub 2020 Mar 18 [PubMed PMID: 32336420]
Taylor J, Haddadin M, Upadhyay VA, Grussie E, Mehta-Shah N, Brunner AM, Louissaint A Jr, Lovitch SB, Dogan A, Fathi AT, Stone RM, Tallman MS, Rampal RK, Neuberg DS, Stevenson KE, Horwitz SM, Lane AA. Multicenter analysis of outcomes in blastic plasmacytoid dendritic cell neoplasm offers a pretargeted therapy benchmark. Blood. 2019 Aug 22:134(8):678-687. doi: 10.1182/blood.2019001144. Epub 2019 Jun 26 [PubMed PMID: 31243042]
Kharfan-Dabaja MA, Reljic T, Murthy HS, Ayala E, Kumar A. Allogeneic Hematopoietic Cell Transplantation Is an Effective Treatment for Blastic Plasmacytoid Dendritic Cell Neoplasm in First Complete Remission: Systematic Review and Meta-analysis. Clinical lymphoma, myeloma & leukemia. 2018 Nov:18(11):703-709.e1. doi: 10.1016/j.clml.2018.07.295. Epub 2018 Aug 2 [PubMed PMID: 30145196]
Level 1 (high-level) evidenceAlfayez M, Konopleva M, Pemmaraju N. Role of tagraxofusp in treating blastic plasmacytoid dendritic cell neoplasm (BPDCN). Expert opinion on biological therapy. 2020 Feb:20(2):115-123. doi: 10.1080/14712598.2020.1701651. Epub 2019 Dec 17 [PubMed PMID: 31801379]
Level 3 (low-level) evidenceAbla D, Abboud MR, Noun D, Tarek N, Pemmaraju N. Hyper-CVAD combined with Venetoclax for relapsed pediatric blastic plasmacytoid dendritic cell neoplasm (BPDCN): A case report and literature review. Leukemia research reports. 2022:17():100313. doi: 10.1016/j.lrr.2022.100313. Epub 2022 Apr 12 [PubMed PMID: 35462725]
Level 3 (low-level) evidenceOno K, Ise M, Ikebe D, Sato A, Wang X, Sugawara T, Tsujimura H, Itami M, Kumagai K. [Successful treatment with biweekly CHOP for bone marrow relapse of blastic plasmacytoid dendritic cell neoplasm]. [Rinsho ketsueki] The Japanese journal of clinical hematology. 2017:58(2):150-154. doi: 10.11406/rinketsu.58.150. Epub [PubMed PMID: 28321093]
Montero J, Stephansky J, Cai T, Griffin GK, Cabal-Hierro L, Togami K, Hogdal LJ, Galinsky I, Morgan EA, Aster JC, Davids MS, LeBoeuf NR, Stone RM, Konopleva M, Pemmaraju N, Letai A, Lane AA. Blastic Plasmacytoid Dendritic Cell Neoplasm Is Dependent on BCL2 and Sensitive to Venetoclax. Cancer discovery. 2017 Feb:7(2):156-164. doi: 10.1158/2159-8290.CD-16-0999. Epub 2016 Dec 16 [PubMed PMID: 27986708]
Bétrian S, Guenounou S, Luquet I, Demur C, Huynh A, Ysebaert L, Recher C, Huguet F. Bendamustine for relapsed blastic plasmacytoid dendritic cell leukaemia. Hematological oncology. 2017 Jun:35(2):252-255. doi: 10.1002/hon.2252. Epub 2015 Oct 8 [PubMed PMID: 28620927]
Zalmaï L, Viailly PJ, Biichle S, Cheok M, Soret L, Angelot-Delettre F, Petrella T, Collonge-Rame MA, Seilles E, Geffroy S, Deconinck E, Daguindau E, Bouyer S, Dindinaud E, Baunin V, Le Garff-Tavernier M, Roos-Weil D, Wagner-Ballon O, Salaun V, Feuillard J, Brun S, Drenou B, Mayeur-Rousse C, Okamba P, Dorvaux V, Tichionni M, Rose J, Rubio MT, Jacob MC, Raggueneau V, Preudhomme C, Saas P, Ferrand C, Adotevi O, Roumier C, Jardin F, Garnache-Ottou F, Renosi F. Plasmacytoid dendritic cells proliferation associated with acute myeloid leukemia: phenotype profile and mutation landscape. Haematologica. 2021 Dec 1:106(12):3056-3066. doi: 10.3324/haematol.2020.253740. Epub 2021 Dec 1 [PubMed PMID: 33054115]
Fei F, Liedtke M, Silva O. Case Report: Mature Plasmacytoid Dendritic Cell Proliferation Associated With a Lymphoid Neoplasm. Frontiers in oncology. 2022:12():903113. doi: 10.3389/fonc.2022.903113. Epub 2022 Jul 6 [PubMed PMID: 35875095]
Level 3 (low-level) evidenceSukswai N, Aung PP, Yin CC, Li S, Wang W, Wang SA, Ortega V, Lyapichev K, Nagarajan P, Alfattal R, Angelova E, Tang Z, Loghavi S, Kanagal-Shamanna R, Miranda RN, Pemmaraju N, Bhalla K, Konopleva M, Medeiros LJ, Khoury JD. Dual Expression of TCF4 and CD123 Is Highly Sensitive and Specific For Blastic Plasmacytoid Dendritic Cell Neoplasm. The American journal of surgical pathology. 2019 Oct:43(10):1429-1437. doi: 10.1097/PAS.0000000000001316. Epub [PubMed PMID: 31261288]
Tsagarakis NJ, Kentrou NA, Papadimitriou KA, Pagoni M, Kokkini G, Papadaki H, Pappa V, Marinakis T, Anagnostopoulos NI, Vadikolia C, Anagnostopoulos A, Angelopoulou MK, Terpos E, Poziopoulos C, Anargyrou K, Rontogianni D, Papadaki T, Psarra A, Kontopidou FN, Skoumi D, Papadhimitriou SI, Paterakis G, Hellenic Dendritic Cell Leukemia Study Group. Acute lymphoplasmacytoid dendritic cell (DC2) leukemia: results from the Hellenic Dendritic Cell Leukemia Study Group. Leukemia research. 2010 Apr:34(4):438-46. doi: 10.1016/j.leukres.2009.09.006. Epub 2009 Sep 29 [PubMed PMID: 19793612]
Hashikawa K, Niino D, Yasumoto S, Nakama T, Kiyasu J, Sato K, Kimura Y, Takeuchi M, Sugita Y, Hashimoto T, Ohshima K. Clinicopathological features and prognostic significance of CXCL12 in blastic plasmacytoid dendritic cell neoplasm. Journal of the American Academy of Dermatology. 2012 Feb:66(2):278-91. doi: 10.1016/j.jaad.2010.12.043. Epub 2011 Aug 11 [PubMed PMID: 21835496]
Jegalian AG, Buxbaum NP, Facchetti F, Raffeld M, Pittaluga S, Wayne AS, Jaffe ES. Blastic plasmacytoid dendritic cell neoplasm in children: diagnostic features and clinical implications. Haematologica. 2010 Nov:95(11):1873-9. doi: 10.3324/haematol.2010.026179. Epub 2010 Jul 27 [PubMed PMID: 20663945]
Level 2 (mid-level) evidenceJaye DL, Geigerman CM, Herling M, Eastburn K, Waller EK, Jones D. Expression of the plasmacytoid dendritic cell marker BDCA-2 supports a spectrum of maturation among CD4+ CD56+ hematodermic neoplasms. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2006 Dec:19(12):1555-62 [PubMed PMID: 16998465]