Introduction
Neovascular glaucoma (NVG) is a secondary glaucoma characterized by new vessels on the iris and angle of the anterior chamber (AC). It is usually associated with a poor visual prognosis.[1][2][3] The mechanism of anterior segment neovascularization is ischemia of the posterior segment of the eye resulting from several ophthalmic and systemic etiologies.[3][4] The most common etiologies include proliferative diabetic retinopathy (PDR), central retinal vein occlusion (CRVO), and ocular ischemic syndrome (OIS).[5]
In 1906, Coats described new vessels over the iris (rubeosis iridis) in a patient with CRVO. Due to the formation of new vessels in the anterior segment of the eye, the rise in intraocular pressure, and the connective tissue growth, the term NVG was coined by Weiss et al.[6] Some other synonyms used for NVG are hemorrhagic glaucoma, thrombotic glaucoma, and congestive glaucoma.[2]
NVG is usually refractory to treatments, and visual morbidity is significant for the patient. There are increasing numbers of NVG cases, probably due to the rise in the number of patients with diabetes and other non-communicable diseases, which are risk factors for retinovascular entities such as diabetic retinopathy, retinal vein occlusions, and ocular ischemic syndromes. Knowledge of the evaluation and management of NVG is crucial for all ophthalmologists.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The inciting factor for NVG is retinal or ocular ischemia.[3][4] All the causes of NVG share the common mechanism of retinal ischemia, which results in the development of new vessels over the iris and the angle of the anterior chamber. The etiological conditions for NVG can be described under the following headings:[4]
|
Retinal Ischemic Disease |
Diabetic retinopathy Central retinal vein occlusion Central retinal artery occlusion Branch retinal vein occlusion Branch retinal artery occlusion Retinal detachment Retinopathy of prematurity Sickle cell retinopathy Hemorrhagic retinal disorders Coat disease Eales disease Leber congenital amaurosis Persistent hyperplastic primary vitreous Retinoschisis Syphilitic posterior uveitis and vasculitis Inherited vitreoretinal degenerations Optic nerve glioma and resulting venous stasis retinopathy |
|
Irradiation |
Photo-radiation External beam radiation Charged particle radiation: Proton, helium ion Plaque brachytherapy |
|
Tumors |
Choroidal melanoma Iris melanoma Ciliary body melanoma Retinoblastoma Hyperviscosity syndromes Myeloproliferative disorders |
|
Inflammatory Diseases |
Chronic uveitis Chronic iridocyclitis Behcet disease Vogt-Koyanagi-Harada syndrome Sympathetic ophthalmia Endophthalmitis Panophthalmitis Crohn disease Giant cell arteritis Trauma |
|
Surgical Causes |
Carotid endarterectomy Pars plana vitrectomy Cataract extraction Nd:YAG Capsulotomy Laser pupiloplasty |
|
Extravascular Disorders |
Ocular ischemic syndrome Carotid artery obstructive disease Carotid-cavernous fistula |
Etiologies of Neovascular Glaucoma
- Among the various causes of NVG, 3 diseases constitute most of the NVG cases encountered in the clinic. These diseases are diabetic retinopathy (33%), ischemic central retinal vein occlusion (33%), and ocular ischemic syndrome (13%).[5][2]
- Up to 60% of the eyes with ischemic CRVO develop neovascularization of the anterior segment of the eye within a few weeks to 2 years after the onset of CRVO.[7] Eyes with non-ischemic CRVO have a risk of conversion to ischemic CRVO, which has been reported to occur at a rate of 3.3% by 4 months and an incidence rate 10 times higher by 3 years.[8]
- Neovascularisation of the iris occurs in 1% to 17% of eyes with diabetic retinopathy. This rate is particularly higher for proliferative diabetic retinopathy.[9][10]
- Ocular ischemic syndrome (OIS) is commonly unilateral, with 20% of cases being bilateral. Global ischemia causes both anterior segment and posterior segment neovascularization in OIS. Severe carotid artery occlusion and diabetic retinopathy are usually associated with OIS.[11]
Epidemiology
The incidence and prevalence of NVG vary in different populations and according to various etiological diseases causing NVG. In a tertiary eye hospital in China, the number of NVG cases was found to be 483 (5.8 %) out of the 8306 glaucoma patients seen over 11 years.[12] The prevalence of NVG among migrant Indians in Singapore was 0.12%.[13] The prevalence of NVG was 0.01% in the population-based Hooghly River Study in West Bengal, India.[14]
The reported proportion of NVG among patients with secondary glaucoma has ranged from 9 to 17.4% in hospital-based studies. Among the eyes with ischemic CRVO, 40 to 45% develop NVG.[15][16] Eyes with ischemic CRVO are more prone to NVG, with an estimated 3,800 patients developing NVG each year.[7]
In eyes with proliferative diabetic retinopathy, 65% of the eyes develop NVI. In eyes with PDR and NVG in 1 eye, the risk of development of NVG in the other eye is 33%.[17] In OIS, 68% of eyes can develop NVG, with an increased risk associated with more advanced carotid stenosis.[18] In the Diabetes Control Complications Trial (DCCT), 24% of NVG over 9 years was found amongst the patients in the standard treatment group compared to 8% in the intensive treatment group.[19]
Pathophysiology
The inciting event for neovascular glaucoma is retinal ischemia.[4] All the diseases underlying NVG result in retinal ischemia due to the disease process. Retinal ischemia causes an imbalance between the pro-angiogenic and anti-angiogenic factors. The major pro-angiogenic factors include vascular endothelial growth factor (VEGF), hepatocyte growth factor, tumor necrosis factor, insulin-like growth factor, and inflammatory cytokines like interleukin-6. The major anti-angiogenic factors include pigment epithelium-derived factors, transforming growth factor-beta, somatostatin, and thrombospondin.[20][21][22][23]
VEGF can cause mitosis and migration of vascular endothelial cells and leukocyte adhesion at the endothelial cells. These events can lead to a breakdown of the blood-retinal barrier.[24] Retinal ischemia and consequent events result in the formation of new leaky vessels over the iris and angle of the anterior chamber. It has also been postulated that oxygen from the aqueous humor diffuses posteriorly in the eyes with hypoxic retina. This, in turn, causes the release of VEGF from the non-pigmented epithelium of the ciliary body, which causes new blood vessel formation in the anterior segment of the eye.[25] This stage is known as the stage of rubeosis iridis. Factors like transforming growth factor-beta (1 and 2) and fibroblast growth factor contribute to the development of fibrovascular membranes over the surface of the iris and the angle of the anterior chamber.[26]
This fibrovascular membrane has proliferated myofibroblasts. This membrane can cover the surface of the iris and the angle of the anterior chamber, obstructing the flow of aqueous humor through the trabecular meshwork. This is known as the secondary open-angle stage of NVG. Over some time, this fibrovascular membrane contracts to cause secondary angle-closure glaucoma. This is known as the secondary closed-angle stage of NVG. Both the secondary open-angle and angle-closure stages are associated with raised IOP.
Ischemia of the retina and optic nerve head results in deleterious effects on the vision of the eyes with NVG. Ocular perfusion pressure is the difference between arterial Blood pressure (BP) and intraocular pressure (IOP).[27] Arterial BP is diastolic BP + 1/3 (systolic BP – diastolic BP). A low arterial BP can further aggravate the ischemia of the retina and the optic nerve head; thus, a low systemic arterial blood pressure should be avoided in patients with NVG.[15]
Histopathology
Histologically, new blood vessels are seen over the surface of the iris and rarely on the iris stroma. These blood vessels are formed due to the budding of endothelial cells from the capillaries. These vessels arise from the vessels over the iris' root or the major arterial circle. These vessels are devoid of a muscular layer and have little adventitial tissue. The fibrovascular membrane comprises myofibroblast and proliferating smooth muscle tissue and can extend over the surface of the iris and the angle of the anterior chamber.[28][29]
History and Physical
The etiology of NVG is multifactorial, and the clinical history should cover the detailed aspects of the particular etiology. Patients with diabetes mellitus should be evaluated thoroughly regarding their glycemic control and other associated comorbidities like hypertension, hyperlipidemia, anemia, nephropathy, and cardiopathy.
Patients with CRVO should be evaluated for various etiologies of thrombotic events. The systemic etiologies include diabetes, hypertension, hyperlipidemia, hyperviscosity syndromes, myeloproliferative disorders, protein C and protein S deficiency, Factor V Leiden mutation, smoking, oral contraceptive pills, and pregnancy. An important associated ocular factor is glaucoma. Patients with OIS may have a history of transient ischemic attack or stroke. Detailed neurological evaluation is necessary for these patients.
Patients with prior ocular surgeries, such as pars plana vitrectomy, glaucoma filtration surgeries, and cataract surgeries, should be evaluated for previous intra-operative complications, if any. Intra-operative posterior capsule tear and vitreous loss are associated with a higher rate of NVG in the eyes of patients with PDR. Similarly, eyes with aphakia are at higher risk of NVG than pseudophakia. Recurrent retinal detachments in the eyes and having undergone vitreoretinal surgeries are risk factors for NVG.
Patients coming from geographic locations or ethnicities with significant rates of sickle cell disease and other hemoglobinopathies need to be evaluated regarding the possibility of sickle cell anemia, prior blood transfusions, and pain in the extremities.
Pediatric age group patients should be evaluated for low birth weight, premature birth, neonatal septicemia, twin babies, and neonatal oxygen administration. All these predispose to retinopathy of prematurity (ROP) and can cause NVG. Children should be evaluated for retinoblastoma, and a family history of retinoblastoma needs to be taken. Examination for pediatric conditions such as persistent hyperplastic primary vitreous (PHPV) or Coat disease should be performed.
Patients with a prior history of uveitis need to be evaluated regarding the frequency and chronicity of red and painful eyes. Any history of ocular tumors such as choroidal melanoma, retinoblastoma, iris melanoma, and ciliary body melanoma should be elicited. Similarly, a history of ocular or periocular radiation therapy for tumors should be elicited.
Symptoms
An eye with NVG is typically chronic, painful, and red. Sometimes, the intensity of pain and redness may be less pronounced, particularly in young patients with good endothelial reserve. Light sensitivity and blurry vision may be the initial symptoms in some patients.
Signs
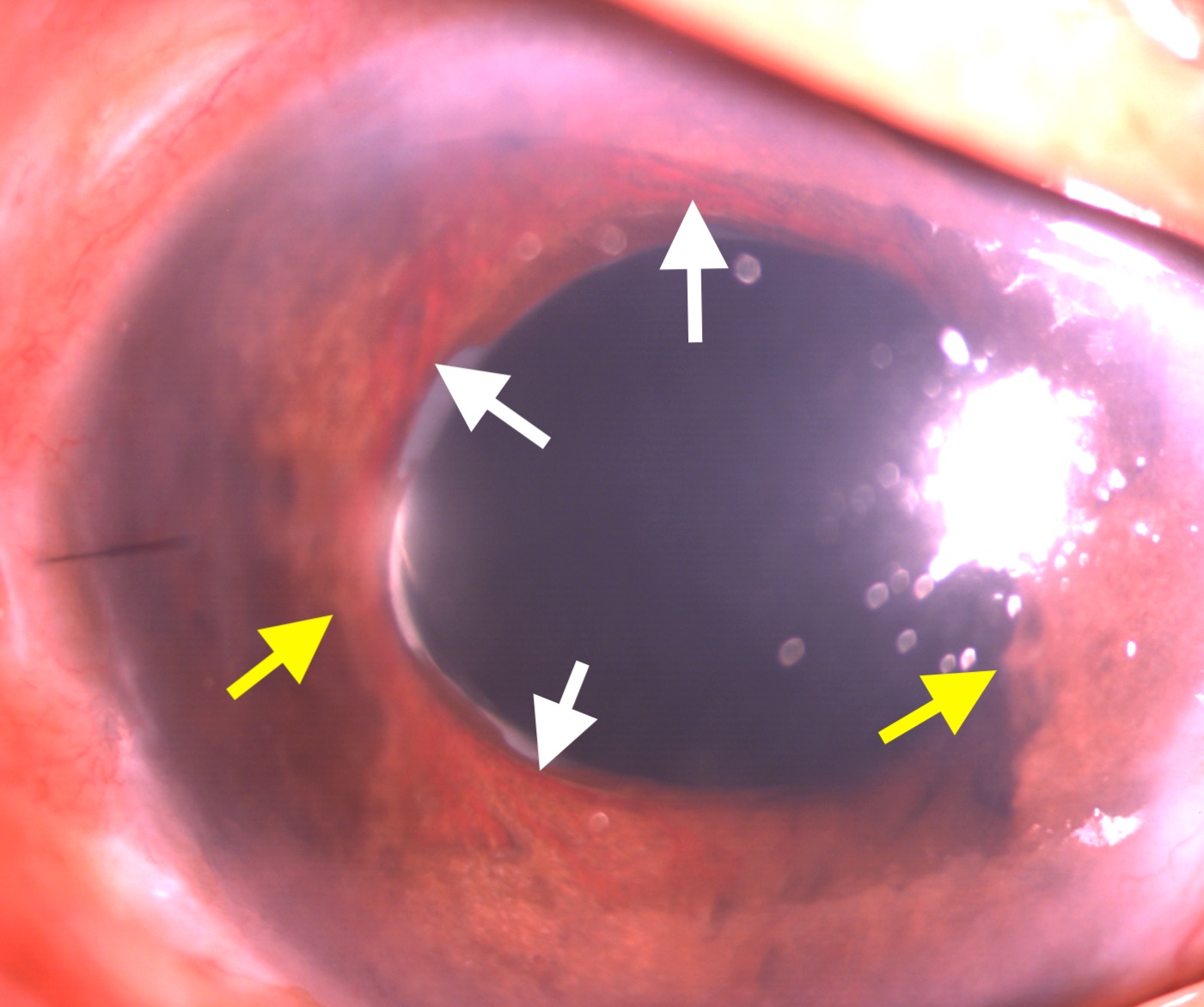
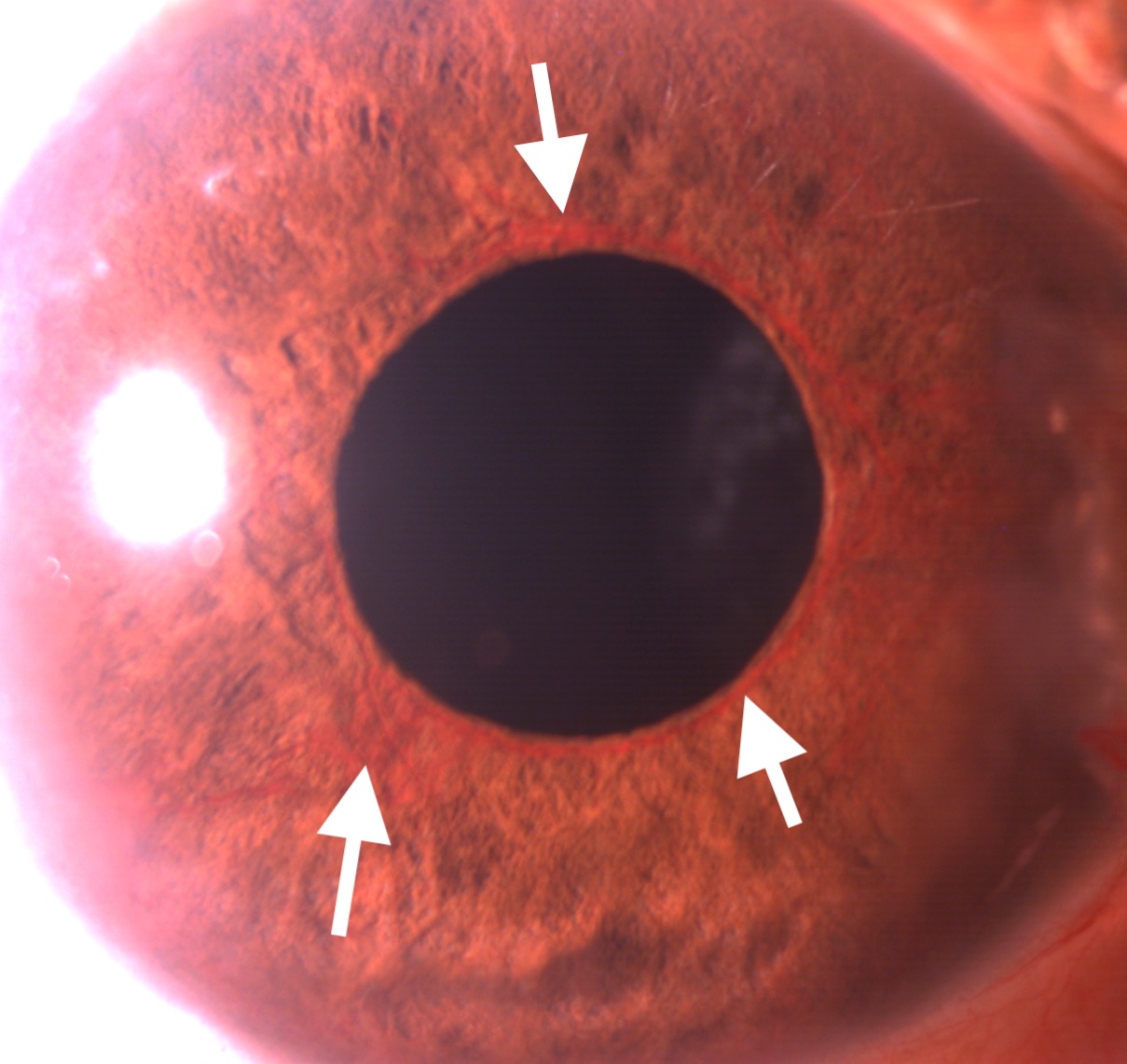
The intraocular pressure in the eyes with NVG is high, often over 50 mm Hg. Corneal edema may or may not be present. The hallmark signs of NVG on anterior segment examination are neovascularization of the iris (NVI) and neovascularization of the angle (NVA). The earliest detection of NVI can be done by the leakage of dye noticed after intravenous injection of fluorescein dye. The NVI appears as fine; new blood vessels are typically present at the border of the pupillary margin (see Image. Slit-lamp Examination Showing Neovascularization of Iris). Sometimes, these vessels can be found at the margin of the iridotomy. These vessels can be differentiated from the normal vessels originating from the ciliary body present over the iris. The latter originates from the root of the iris, travels towards the pupillary border, and disappears in the iris stroma midway. NVA is a finding of gonioscopy. NVA appears as thin vessels over the trabecular meshwork crossing the scleral spur. Though isolated NVA without NVI is rare, sometimes NVA can be found before developing clinically detectable NVI.[30]
NVG can be divided into 4 stages. First is the pre-rubeotic stage, where the new vessels over the iris are not clinically detectable but can be found on fluorescein angiography by the leakage of dye from the margin of the pupillary border. The second is the rubeotic stage, where the new blood vessels can be detected in the form of NVI or NVA. The third is the open-angle stage, where the fibrovascular membrane covering the anterior chamber and iris angle obstructs the aqueous outflow. Fourth is the closed-angle stage due to the contraction of the fibrovascular membrane over the angle of the anterior chamber.[2]
Evaluation
Investigations into the diagnosis of NVG can be broadly categorized as ophthalmic and systemic investigations.
Ophthalmic Investigations
Slit-lamp biomicroscopy: Slit-lamp biomicroscopy is indispensable for detecting NVI and NVA during the workup of a patient with NVG. The fine new vessels can be found over the iris surface, mainly near the pupillary margin. Sometimes, peripheral anterior synechia can be found in the eyes with NVI (see image. Neovascularization of Iris and Peripheral Anterior Synechia). Red blood cells can sometimes be seen in the anterior chamber.
Gonioscopy: This is a dynamic investigation, preferably done in an undilated pupil. New blood vessels can be found at the angle of the anterior chamber, over the trabecular meshwork, and crossing the scleral spur.
Fundus Fluorescein Angiography (FFA) of the retina and iris: Fundus fluorescein angiography is an invasive test to detect the neovascularization of the optic disc (NVD) neovascularization elsewhere (NVE) and areas of capillary nonperfusion (CNP). The former 2 are hyperfluorescent lesions, and the latter is a hyperfluorescent area. Recent advancements in ultra-wide-field FFA can capture the retinal image up to a 200-degree area of the retina, thus identifying peripheral lesions like NVE and CNP areas better than conventional FFA.[31]
Optical coherence tomography angiography (OCTA) of the retina: OCTA is a rapid, non-invasive modality for diagnosing NVD, NVE, and CNP areas. Wide-field OCTA can capture 12 × 12-mm fovea-centered images.[32] OCTA can also be used to monitor disease status over subsequent visits.
OCTA of iris: Neovascularization of the iris can also be detected using OCTA.[33]
B-scan ultrasound for posterior segment examination: Ultrasonography B scan is helpful in the detection of lesions of the posterior segment of the eyes in certain conditions. B scan can detect the presence of vitreous hemorrhage, additional fibrous proliferation, and tractional or rhegmatogenous retinal detachment. Mass lesions like choroidal melanoma, ciliary body melanoma, and retinoblastoma can be detected. B scan can detect retinal detachment in the advanced stages of ROP with NVG.
Systemic Investigations
Various systemic diseases can be associated with a case of NVG, depending on the specific etiology causing NVG. The investigations for various systemic diseases are as follows:
- Hypertension (associated with retinal venous and arterial occlusion): Blood pressure
- Diabetes (can cause retinal veinous and arterial occlusion, PDR): Blood sugar levels, HbA1c
- Hyperlipidemia (can cause retinal veinous and arterial occlusion): Lipid profile
- OIS: carotid doppler, magnetic resonance angiography
- Uveitis: HLA B-27 assay, treponema serology, Tuberculosis testing (QuantiFERON-TB Gold, Mantoux), Sarcoidosis (ACE, chest X-ray)
- Blood dyscrasia: Complete blood counts, ESR, CRP, Plasma electrophoresis, and specific testing for hyperviscosity syndromes.
Treatment / Management
The principles of management of NVG are as follows:
- Identifying and managing the etiological factors (diabetes, carotid artery obstruction, or other causes as enumerated in Table 1) causing retinal ischemia.
- Retinal ischemia is treated by pan-retinal photocoagulation or intravitreal injections of anti-VEGF agents.
- Control of intraocular pressure medically and surgically.
- Control of inflammation using topical corticosteroid drops.
- Mydriatics such as topical atropine drops
Identifying and managing the etiological factors (as described under the section of etiology) and investigations related to those factors (as described under the section of systemic investigations) are crucial in curtailing the ongoing mechanism of retinal ischemia. This also prevents the contralateral eye from developing NVG in patients with unilateral NVG at presentation. Patients with uncontrolled diabetes, hypertension, hyperlipidemia, and nephropathy should be managed with the help of a physician.
Patients with severe carotid artery obstruction may need carotid endarterectomy, as per the discretion of the vascular surgeon. Blood dyscrasias and hematological abnormalities must be managed as per the internist and hematologist. Patients with uveitis need corticosteroid treatments, cycloplegics, and management of the specific etiology of uveitis. Intra-ocular tumors such as choroidal melanoma or retinoblastoma require treatments specific to the tumor characteristics and available treatments.
The treatment of retinal ischemia is comprised of pan-retinal photocoagulation (PRP) and intravitreal anti-VEGF injections. PRP does not immediately reduce neovascularization but has a longer treatment effect when compared to anti-VEGF injections. As a result, a combination of these treatments is often given initially. Typical settings of PRP include spot size of 400 to 500 microns, laser burns spaced 1 burn-width apart, grade 3 laser burns, and 1200 to 1600 burns per sitting if planned in 2 sittings. Usually, the PRP is completed in 2 or 3 sittings, separated 5 days apart (see Image. Regressed Neovascularization of the Disc and Neovascularization Elsewhere in an Eye With Proliferative Diabetic Retinopathy). However, the presence of media haze, including cornea edema due to raised IOP, cataract, and vitreous hemorrhage, precludes the delivery of PRP.
Corneal edema may necessitate intravitreal injection of anti-VEGF agents and topical and systemic antiglaucoma medications to reduce the IOP and improve the corneal clarity, after which PRP can be performed. In cataracts obscuring the view to the fundus, intravitreal injection of anti-VEGF agents is required to reduce the NVI and NVA. Additionally, topical and systemic antiglaucoma medications need to be used to reduce the IOP. Cataract surgery can be performed after the regression of anterior segment neovascularization starts and there is a decrease in IOP. In the presence of vitreous hemorrhage, intravitreal injection of anti-VEGF agents and topical and systemic antiglaucoma medications should be started immediately, and pars plana vitrectomy should be considered as soon as feasible. Intravitreal injections are useful in decreasing the VEGF drive in the retina of the eyes with NVG and are very important in managing retinal ischemia.
Intraocular pressure management often requires topical and systemic medications initially, and ultimately, surgical management is often necessary. Topical beta-blockers, alpha agonists, and carbonic anhydrase inhibitors are frequently used to manage IOP in eyes with NVG. Prostaglandin analogs can increase inflammation but are still often used as maximum medical treatment may be necessary. Systemic carbonic anhydrase inhibitors are also useful in the short-term management of IOP but must be used cautiously in patients with renal impairment. Surgical management of IOP is indicated where the maximal medical management fails to control the IOP. Surgical management of high IOP in the eyes with NVG includes trabeculectomy or glaucoma drainage device. Though trabeculectomy is less preferred in eyes with NVG due to their high failure rate, there are some studies describing improved success rates of trabeculectomy augmented by anti-metabolites such as mitomycin C (MMC) or 5-Fluorouracil.[34] (B2)
In 1 study, the success rate after trabeculectomy with MMC was reported to be 62% at the end of 1 year post-operatively; however, this decreased to 51.7% at the end of 5 years. Preoperative intravitreal anti-VEGF agents have been shown to reduce the failure rate of trabeculectomy in the eyes with NVG.[35] (B2)
While there is no consensus regarding the surgical approach to NVG, recent studies suggest early implantation of GDD for the successful management of NVG.[36] In 1 study by Noor N A et al, GDD combined with intravitreal bevacizumab could maintain better visual acuity than GDD alone at 3 years post-surgery. However, there was no significant difference in the final surgical success rate.[37] Transscleral cyclophotocoagulation (TSCPC) is a surgical treatment option, particularly for eyes with poor visual potential and IOP elevation refractory to medical treatments.
Eyes with NVG often have associated inflammation, especially in the acute phase, which can be treated with topical corticosteroid eye drops. Prednisolone acetate 1% can be used in tapered doses. The pain and discomfort due to cyclospasm in the eyes with NVG can be mitigated with the help of cycloplegic eye drops such as atropine or cyclopentolate. Eyes with NVG, no perception of light (PL), and no pain do not need treatment and can be observed. However, if these eyes are painful, they must be treated with pressure-lowering drops, cycloplegics, and possibly TSCPC.
Differential Diagnosis
The differential diagnosis of NVG includes diseases with similar clinical presentations and diseases that can be ascribed to the etiologies of retinal ischemia.[15][4]
- Acute angle-closure glaucoma is a close mimicker of NVG where corneal edema, anterior segment inflammation, and minimally engorged iris vessels can be associated with raised IOP. A gonioscopy of the other eye assists in diagnosing the findings of a shallow anterior chamber and occlude angle. Fundus examination may be difficult in the presence of a hazy cornea. Retinal ischemia should be ruled by fundoscopy or, if needed, by fundus fluorescein angiography (FFA).
- Eyes with acute or chronic uveitis may mimic NVG. The presence of keratin precipitates (KPs) lining the endothelium and engorged blood vessels (rather than fine, lacy vessels) over the iris can suggest uveitis as the underlying etiology.
- Eyes in the post-operative period of anterior segment ophthalmic surgeries may present with engorged iris vessels. However, these vessels can be differentiated from neovascularization of the iris. These engorged blood vessels are radial, present over the iris stroma, and do not cross the scleral spur, unlike the arborizing superficial placed NVI, which crosses the scleral spur.
- Eyes with posterior segment tumors may present with engorged iris vessels. These tumors should be diagnosed promptly using fundoscopy, B-scan ultrasound, UBM, gonioscopy, and magnetic resonance imaging (MRI) scans.
- A carotid-cavernous fistula can present with new blood vessels over the iris. Gonioscopy in these eyes can find blood in the Schlemm’s canal. Radiology of the brain and orbits aid in the diagnosis.
- The ocular ischemic syndrome presents with anterior segment neovascularization, possibly associated with normal or low IOP. Carotid doppler study or magnetic resonance angiography helps identify the carotid vessels' stenosis.
- Neovascularization of the anterior segment of the eye can also be seen in anterior segment dysgenesis (especially essential iris atrophy). Corectopia and iris atrophy can help diagnose these eyes. Other conditions, such as pseudoexfoliation syndrome and Fuchs heterochromic iridocyclitis, can also present with new vessels over the anterior segment of the eyes and raised IOP.
Prognosis
The prognosis of NVG is guarded. The prognosis depends upon prompt prophylaxis or treatment of retinal ischemia. Another important factor for the visual prognosis is the control of IOP. IOP elevation is often recalcitrant to medical treatment. The success rate of trabeculectomy in NVG is limited due to inflammation and hyphema. However, in the eyes that have received prior intravitreal bevacizumab injections, prior pan-retinal photocoagulation of the retina, and concurrent application of mitomycin-C or 5-fluorouracil, the success rate has been reported to be higher. Though the success in the control of IOP in trabeculectomy with MMC is described to be between 62% to 82% at the end of 1 year, the success rate decreases drastically over the following years. Eyes operated on with GDD have been reported to have more successful IOP control.[38][39][37]
The valved and non-valved GDDs have been reported to control the IOP in eyes with NVG effectively.[40] Intravitreal injection of anti-VEGF agents and GDD may have better results in maintaining vision and controlling IOP than GDD alone.[37][39] Early surgery with GDD may be preferred over an initial trabeculectomy in these eyes with NVG. Gnanavelu S et al did not find any difference in the failure rate of the eyes with NVG treated with a GDD among various causes of NVG, such as CRVO, PDR, and other etiologies.[36]
Complications
NVG complications can be categorized as complications of the disease process and complications of the treatment modalities. The complications of the disease process include corneal decompensation due to prolonged elevation of IOP, ectropion uvea due to contracture of the fibrovascular membrane over the surface of the iris, and angle of the anterior chamber, and loss of vision from retinal ischemia or glaucomatous optic neuropathy.
The most common complication of the surgical management of control of IOP is hyphema, which ranges from 4 to 85%.[41][42] Other complications include serous choroidal detachment in up to 20% of eyes and suprachoroidal hemorrhage in up to 5% of eyes.[42]
Though rare, complications such as phlebitis, bleb leak, and endophthalmitis can be expected in eyes undergoing trabeculectomy, irrespective of the indication of the surgery. Complications specific to GDD include erosion of the conjunctiva overlying the GDDs (up to 12.5% of eyes), tube block, and corneal endothelial defects due to touch with the tip of the tube (up to 3 to 14% of the eyes).[43][44] TSCPC complications include phthisis and scleral thinning. All surgical treatments, particularly TSCPC, carry a risk of sympathetic ophthalmia.
Deterrence and Patient Education
NVG is often refractory to treatment and has a poor prognosis in terms of IOP control and maintenance of vision. A delay in presenting to the ophthalmic clinic is associated with worse outcomes for the patient. Patients with NVG need to seek prompt treatment from the ophthalmic caregiver.
Vulnerable patients with risk factors like DM, HTN, hyperlipidemia, carotid vascular diseases, and ocular tumors should be counseled regarding the symptoms of NVG. The ophthalmologists giving primary care to patients with NVG must not hesitate to refer them to a referral center for treatment, such as GDD surgeries and intravitreal anti-VEGF injections. The patients must follow the strict follow-up protocol, as multiple visits are often required. The patients must be counseled about the guarded prognosis related to the control of IOP and visual gain.
Enhancing Healthcare Team Outcomes
Several systemic diseases can result in NVG. Diseases such as diabetes, hypertension, hyperlipidemia, and blood dyscrasias require management by an internist. However, the ophthalmologist may alert the internist and patient regarding the need for improved control of their systemic disease. The presence of carotid artery obstruction in ocular ischemic syndrome requires prompt evaluation by an interventional cardiologist or endovascular surgeon.
The presence of systemic or ocular malignancies may require treatment coordinated through an oncologist. The assistance of a caregiver or patient advocate is often helpful in ensuring treatment compliance and follow-up of the ocular and systemic conditions associated with NVG. Thus, coordination between different subspecialties should be emphasized rather than isolated ophthalmic care for optimal outcomes of NVG.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
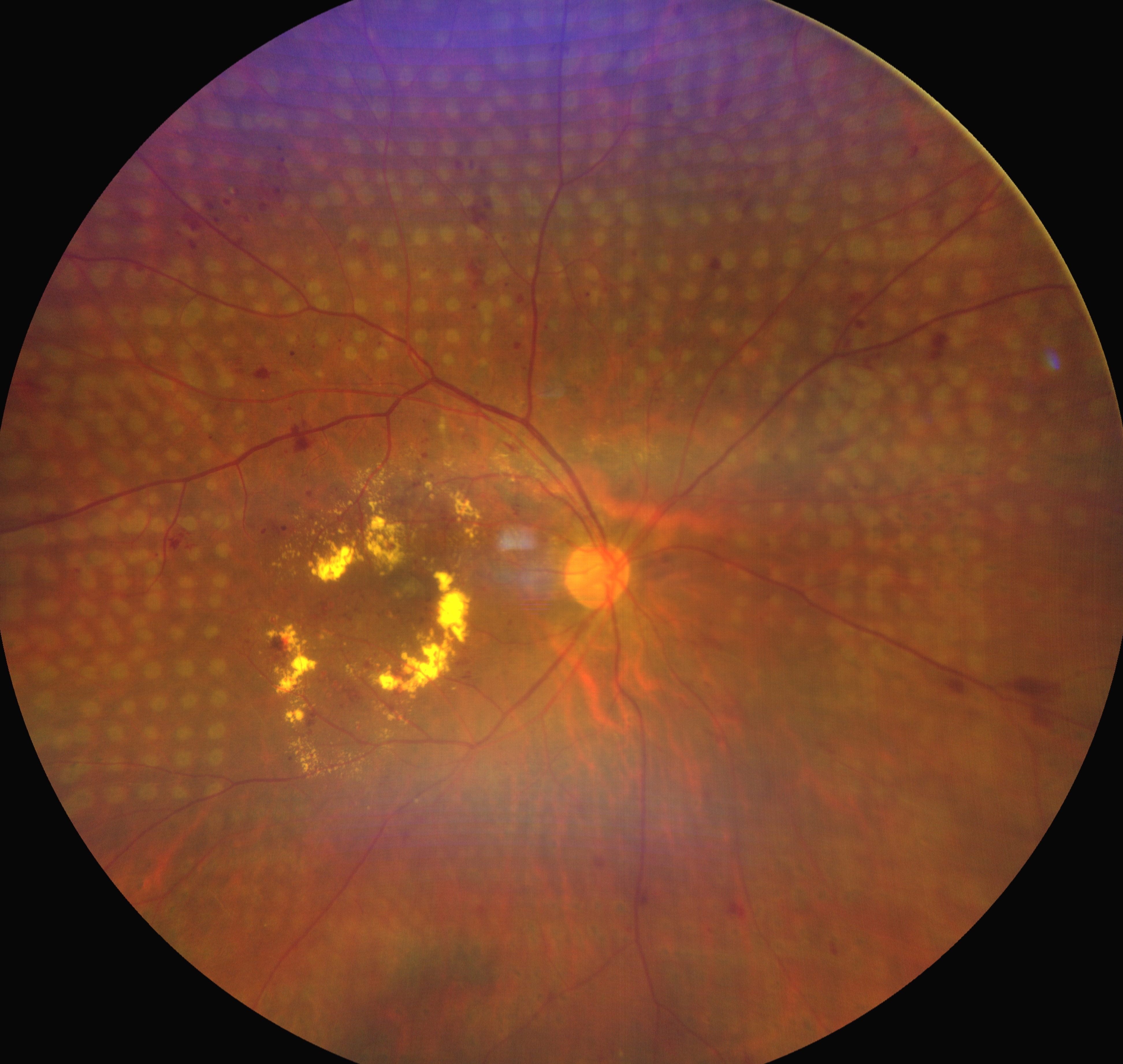
Regressed Neovascularization of the Disc and Neovascularization Elsewhere in an Eye With Proliferative Diabetic Retinopathy. Regressed neovascularization of the disc (NVD) and neovascularization elsewhere (NVE) in an eye with proliferative diabetic retinopathy (PDR) with neovascular glaucoma (NVG) which has undergone pan-retinal photocoagulation (PRP). Please note the PRP laser spots over the retina. Anterior segment examination revealed the regression of neovascularization of the iris (NVI) in this eye.
Contributed by C Mishra, DNB, FICO, MRCS Ed
(Click Image to Enlarge)
References
Rodrigues GB, Abe RY, Zangalli C, Sodre SL, Donini FA, Costa DC, Leite A, Felix JP, Torigoe M, Diniz-Filho A, de Almeida HG. Neovascular glaucoma: a review. International journal of retina and vitreous. 2016:2():26 [PubMed PMID: 27895936]
Senthil S, Dada T, Das T, Kaushik S, Puthuran GV, Philip R, Rani PK, Rao H, Singla S, Vijaya L. Neovascular glaucoma - A review. Indian journal of ophthalmology. 2021 Mar:69(3):525-534. doi: 10.4103/ijo.IJO_1591_20. Epub [PubMed PMID: 33595466]
Yang H, Yu X, Sun X. Neovascular glaucoma: Handling in the future. Taiwan journal of ophthalmology. 2018 Apr-Jun:8(2):60-66. doi: 10.4103/tjo.tjo_39_18. Epub [PubMed PMID: 30038883]
Sivak-Callcott JA,O'Day DM,Gass JD,Tsai JC, Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology. 2001 Oct; [PubMed PMID: 11581047]
Level 1 (high-level) evidenceOhrt V. The frequency of rubeosis iridis in diabetic patients. Acta ophthalmologica. 1971:49(2):301-7 [PubMed PMID: 5109792]
WEISS DI, SHAFFER RN, NEHRENBERG TR. Neovascular gluacoma complicating carotid-cavernous fistula. Archives of ophthalmology (Chicago, Ill. : 1960). 1963 Mar:69():304-7 [PubMed PMID: 13999715]
Mocanu C, Barăscu D, Marinescu F, Lăcrăţeanu M, Iliuşi F, Simionescu C. [Neovascular glaucoma--retrospective study]. Oftalmologia (Bucharest, Romania : 1990). 2005:49(4):58-65 [PubMed PMID: 16524128]
Level 2 (mid-level) evidenceBaseline and early natural history report. The Central Vein Occlusion Study. Archives of ophthalmology (Chicago, Ill. : 1960). 1993 Aug; [PubMed PMID: 7688950]
Level 1 (high-level) evidenceHayreh SS, Rojas P, Podhajsky P, Montague P, Woolson RF. Ocular neovascularization with retinal vascular occlusion-III. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology. 1983 May:90(5):488-506 [PubMed PMID: 6192376]
Armaly MF, Baloglou PJ. Diabetes mellitus and the eye. 1. Changes in the anterior segment. Archives of ophthalmology (Chicago, Ill. : 1960). 1967 Apr:77(4):485-92 [PubMed PMID: 6022720]
Luo J, Yan Z, Jia Y, Luo R. Clinical Analysis of 42 Cases of Ocular Ischemic Syndrome. Journal of ophthalmology. 2018:2018():2606147. doi: 10.1155/2018/2606147. Epub 2018 Mar 11 [PubMed PMID: 29713523]
Level 3 (low-level) evidenceLiao N,Li C,Jiang H,Fang A,Zhou S,Wang Q, Neovascular glaucoma: a retrospective review from a tertiary center in China. BMC ophthalmology. 2016 Jan 27; [PubMed PMID: 26818828]
Level 2 (mid-level) evidenceNarayanaswamy A, Baskaran M, Zheng Y, Lavanya R, Wu R, Wong WL, Saw SM, Cheng CY, Wong TY, Aung T. The prevalence and types of glaucoma in an urban Indian population: the Singapore Indian Eye Study. Investigative ophthalmology & visual science. 2013 Jul 10:54(7):4621-7. doi: 10.1167/iovs.13-11950. Epub 2013 Jul 10 [PubMed PMID: 23745009]
Level 2 (mid-level) evidencePaul C, Sengupta S, Choudhury S, Banerjee S, Sleath BL. Prevalence of glaucoma in Eastern India: The Hooghly River Glaucoma Study. Indian journal of ophthalmology. 2016 Aug:64(8):578-83. doi: 10.4103/0301-4738.191497. Epub [PubMed PMID: 27688279]
Hayreh SS. Neovascular glaucoma. Progress in retinal and eye research. 2007 Sep:26(5):470-85 [PubMed PMID: 17690002]
Terelak-Borys B, Zagajewska K, Jankowska-Lech I, Tesla P, Grabska-Liberek I. Combined treatment in punctate inner choroidopathy. Therapeutics and clinical risk management. 2016:12():1467-1471 [PubMed PMID: 27729795]
Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Survey of ophthalmology. 1998 Nov-Dec:43(3):245-69 [PubMed PMID: 9862312]
Level 3 (low-level) evidenceKim YH, Sung MS, Park SW. Clinical Features of Ocular Ischemic Syndrome and Risk Factors for Neovascular Glaucoma. Korean journal of ophthalmology : KJO. 2017 Aug:31(4):343-350. doi: 10.3341/kjo.2016.0067. Epub 2017 Jun 26 [PubMed PMID: 28682017]
. Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. Ophthalmology. 1995 Apr:102(4):647-61 [PubMed PMID: 7724182]
Level 2 (mid-level) evidenceTolentino MJ, Miller JW, Gragoudas ES, Chatzistefanou K, Ferrara N, Adamis AP. Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Archives of ophthalmology (Chicago, Ill. : 1960). 1996 Aug:114(8):964-70 [PubMed PMID: 8694732]
Level 3 (low-level) evidenceYu XB, Sun XH, Dahan E, Guo WY, Qian SH, Meng FR, Song YL, Simon GJ. Increased levels of transforming growth factor-betal and -beta2 in the aqueous humor of patients with neovascular glaucoma. Ophthalmic surgery, lasers & imaging : the official journal of the International Society for Imaging in the Eye. 2007 Jan-Feb:38(1):6-14. doi: 10.3928/15428877-20070101-01. Epub [PubMed PMID: 17278530]
Level 2 (mid-level) evidenceChen KH, Wu CC, Roy S, Lee SM, Liu JH. Increased interleukin-6 in aqueous humor of neovascular glaucoma. Investigative ophthalmology & visual science. 1999 Oct:40(11):2627-32 [PubMed PMID: 10509659]
Simó R, Carrasco E, García-Ramírez M, Hernández C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Current diabetes reviews. 2006 Feb:2(1):71-98 [PubMed PMID: 18220619]
Senger DR, Perruzzi CA, Streit M, Koteliansky VE, de Fougerolles AR, Detmar M. The alpha(1)beta(1) and alpha(2)beta(1) integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis. The American journal of pathology. 2002 Jan:160(1):195-204 [PubMed PMID: 11786413]
Level 3 (low-level) evidenceChalam KV, Brar VS, Murthy RK. Human ciliary epithelium as a source of synthesis and secretion of vascular endothelial growth factor in neovascular glaucoma. JAMA ophthalmology. 2014 Nov:132(11):1350-4. doi: 10.1001/jamaophthalmol.2014.2356. Epub [PubMed PMID: 25079256]
Tripathi RC, Borisuth NS, Tripathi BJ. Detection, quantification, and significance of basic fibroblast growth factor in the aqueous humor of man, cat, dog and pig. Experimental eye research. 1992 Mar:54(3):447-54 [PubMed PMID: 1521572]
Level 3 (low-level) evidenceLeske MC. Ocular perfusion pressure and glaucoma: clinical trial and epidemiologic findings. Current opinion in ophthalmology. 2009 Mar:20(2):73-8. doi: 10.1097/ICU.0b013e32831eef82. Epub [PubMed PMID: 19240538]
Level 3 (low-level) evidenceGartner S, Henkind P. Neovascularization of the iris (rubeosis iridis). Survey of ophthalmology. 1978 Mar-Apr:22(5):291-312 [PubMed PMID: 349748]
Level 3 (low-level) evidenceJohn T, Sassani JW, Eagle RC Jr. The myofibroblastic component of rubeosis iridis. Ophthalmology. 1983 Jun:90(6):721-8 [PubMed PMID: 6193475]
Azad R, Arora T, Sihota R, Chandra P, Mahajan D, Sain S, Sharma Y. Retcam fluorescein gonioangiography: a new modality for early detection of angle neovascularization in diabetic retinopathy. Retina (Philadelphia, Pa.). 2013 Oct:33(9):1902-7. doi: 10.1097/IAE.0b013e318285cdd6. Epub [PubMed PMID: 23584699]
Kumar V, Surve A, Kumawat D, Takkar B, Azad S, Chawla R, Shroff D, Arora A, Singh R, Venkatesh P. Ultra-wide field retinal imaging: A wider clinical perspective. Indian journal of ophthalmology. 2021 Apr:69(4):824-835. doi: 10.4103/ijo.IJO_1403_20. Epub [PubMed PMID: 33727441]
Level 3 (low-level) evidenceTan B, Chua J, Lin E, Cheng J, Gan A, Yao X, Wong DWK, Sabanayagam C, Wong D, Chan CM, Wong TY, Schmetterer L, Tan GS. Quantitative Microvascular Analysis With Wide-Field Optical Coherence Tomography Angiography in Eyes With Diabetic Retinopathy. JAMA network open. 2020 Jan 3:3(1):e1919469. doi: 10.1001/jamanetworkopen.2019.19469. Epub 2020 Jan 3 [PubMed PMID: 31951275]
Roberts PK, Goldstein DA, Fawzi AA. Anterior Segment Optical Coherence Tomography Angiography for Identification of Iris Vasculature and Staging of Iris Neovascularization: A Pilot Study. Current eye research. 2017 Aug:42(8):1136-1142. doi: 10.1080/02713683.2017.1293113. Epub 2017 Apr 25 [PubMed PMID: 28441067]
Level 3 (low-level) evidenceTakihara Y, Inatani M, Fukushima M, Iwao K, Iwao M, Tanihara H. Trabeculectomy with mitomycin C for neovascular glaucoma: prognostic factors for surgical failure. American journal of ophthalmology. 2009 May:147(5):912-8, 918.e1. doi: 10.1016/j.ajo.2008.11.015. Epub 2009 Feb 5 [PubMed PMID: 19195639]
Level 2 (mid-level) evidenceSaito Y, Higashide T, Takeda H, Ohkubo S, Sugiyama K. Beneficial effects of preoperative intravitreal bevacizumab on trabeculectomy outcomes in neovascular glaucoma. Acta ophthalmologica. 2010 Feb:88(1):96-102. doi: 10.1111/j.1755-3768.2009.01648.x. Epub 2009 Sep 23 [PubMed PMID: 19775309]
Level 2 (mid-level) evidenceGnanavelu S, Puthuran GV, Chiranjeevi KP, Wijesinghe HK, Reddy V, Mani I, Krishnadas SR, Gedde SJ. Intermediate-term outcomes of the Aurolab aqueous drainage implant in neovascular glaucoma. The British journal of ophthalmology. 2023 Mar:107(3):355-360. doi: 10.1136/bjophthalmol-2021-319999. Epub 2021 Sep 29 [PubMed PMID: 34588180]
Noor NA, Mustafa S, Artini W. Glaucoma drainage device implantation with adjunctive intravitreal bevacizumab in neovascular glaucoma: 3-year experience. Clinical ophthalmology (Auckland, N.Z.). 2017:11():1417-1422. doi: 10.2147/OPTH.S137470. Epub 2017 Aug 7 [PubMed PMID: 28848323]
Vinod K, Gedde SJ, Feuer WJ, Panarelli JF, Chang TC, Chen PP, Parrish RK 2nd. Practice Preferences for Glaucoma Surgery: A Survey of the American Glaucoma Society. Journal of glaucoma. 2017 Aug:26(8):687-693. doi: 10.1097/IJG.0000000000000720. Epub [PubMed PMID: 28692597]
Level 3 (low-level) evidenceArcieri ES, Paula JS, Jorge R, Barella KA, Arcieri RS, Secches DJ, Costa VP. Efficacy and safety of intravitreal bevacizumab in eyes with neovascular glaucoma undergoing Ahmed glaucoma valve implantation: 2-year follow-up. Acta ophthalmologica. 2015 Feb:93(1):e1-6. doi: 10.1111/aos.12493. Epub 2014 Jul 2 [PubMed PMID: 24989855]
Level 1 (high-level) evidenceGil-Carrasco F, Jiménez-Román J, Turati-Acosta M, Bello-López Portillo H, Isida-Llerandi CG. Comparative study of the safety and efficacy of the Ahmed glaucoma valve model M4 (high density porous polyethylene) and the model S2 (polypropylene) in patients with neovascular glaucoma. Archivos de la Sociedad Espanola de Oftalmologia. 2016 Sep:91(9):409-14. doi: 10.1016/j.oftal.2016.02.009. Epub 2016 Apr 8 [PubMed PMID: 27068138]
Level 2 (mid-level) evidenceZhou MW, Wang W, Huang WB, Chen SD, Li XY, Gao XB, Zhang XL. Adjunctive with versus without intravitreal bevacizumab injection before Ahmed glaucoma valve implantation in the treatment of neovascular glaucoma. Chinese medical journal. 2013:126(8):1412-7 [PubMed PMID: 23595369]
Level 2 (mid-level) evidenceMahdy RA, Nada WM, Fawzy KM, Alnashar HY, Almosalamy SM. Efficacy of intravitreal bevacizumab with panretinal photocoagulation followed by Ahmed valve implantation in neovascular glaucoma. Journal of glaucoma. 2013 Dec:22(9):768-72. doi: 10.1097/IJG.0b013e318259aec4. Epub [PubMed PMID: 22790513]
Level 1 (high-level) evidenceSevim MS, Buttanri IB, Kugu S, Serin D, Sevim S. Effect of intravitreal bevacizumab injection before Ahmed glaucoma valve implantation in neovascular glaucoma. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2013:229(2):94-100. doi: 10.1159/000345490. Epub 2012 Dec 14 [PubMed PMID: 23364308]
Level 2 (mid-level) evidenceMa KT, Yang JY, Kim JH, Kim NR, Hong S, Lee ES, Seong GJ, Kim CY. Surgical results of Ahmed valve implantation with intraoperative bevacizumab injection in patients with neovascular glaucoma. Journal of glaucoma. 2012 Jun-Jul:21(5):331-6. doi: 10.1097/IJG.0b013e31820e2fd0. Epub [PubMed PMID: 21673594]
Level 2 (mid-level) evidence