Introduction
Sarcoidosis is a chronic multisystemic granulomatous disorder characterized by the accumulation of non-caseating granulomas in the involved tissues. Ocular sarcoidosis is an inflammatory condition that can involve any part of the eye leading to conjunctival granuloma, episcleritis/scleritis, uveitis, optic neuropathy, and/ or its adnexal tissues leading to lacrimal gland enlargement and orbital inflammation.[1]
The clinical course shows variability. Few patients may have acute self-limited disease, while others experience chronic, progressive deterioration. Corticosteroids remain the mainstay of treatment with the newer addition of immunosuppressants and biologics in the treatment armamentarium.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of sarcoidosis remains unknown, but evidence has pointed to various infective, non-infective, and genetic factors.
Infective etiology – The presence of bacterial DNA fragments in sarcoid granulomas and regional granulomatous response following inoculation of sarcoid material in experimental animals suggests infective etiology.[2] Few cases of transmission of cases of sarcoidosis post organ transplantation suggest the presence of a transmissible agent.[3] One hypothesis has suggested that tuberculosis and sarcoidosis may be the 'opposite ends of the same disease spectrum.'[4]
The similarities in clinical features, epidemiology, immunological, and molecular link between the two diseases, and similar immune response may support the hypothesis.[4] Molecular studies have suggested the role of Mycobacterium or Propionibacterium in the causation of sarcoidosis.[5]
Non-infective etiology – Sarcoidosis with pulmonary manifestation is known to occur as an occupational disease.[6][7] It is thought to occur secondary to air-borne organic and nonorganic dust exposure.[8][9]
Genetic factor – Association between sarcoidosis and certain human leukocyte antigen haplotypes support the role of genetic factors. HLA-DRB1*1101 allele is associated with sarcoidosis and has a population-attributable risk of 9% in whites and 16% in blacks.[10] Greater prevalence of the disease in certain sibling pairs or parent-offspring pairs suggests genetic factors to be as important as environmental factors.[11] Siblings have a five times higher chance of developing sarcoidosis.[12][13]
Epidemiology
The prevalence of sarcoidosis varies from region to region due to differences in case definition, asymptomatic nature of the disease, limited population screening, and ascertainment bias. In the United States, the age-adjusted incidence is reportedly three times higher in Blacks than Whites.[14]
In a recent study by Baughman et al., the incidence of sarcoidosis in the United States ranged from 8.1 per 100,000 in White race individuals to 17.8 in African Americans.[15] Northern European countries have the highest prevalence of sarcoidosis (40 in 100,000 population).[16] The prevalence of ocular features in cases of systemic sarcoidosis ranges from 12 to 76%. Uveitis is the most frequent ocular manifestation. In patients with systemic sarcoidosis, females (56%) were more commonly noted to develop ocular symptoms than men (23%) in patients with biopsy-proven sarcoidosis.[17]
The usual age at presentation of sarcoidosis is 20 to 50 years. However, sarcoidosis is an important cause of uveitis after 60 years of age. Sarcoidosis accounts for around 10% of all uveitides.[18] Late-onset sarcoidosis (diagnosis at a minimum age of 65 years) is more likely associated with uveitis, asthenia, and skin lesions. Such patients may have a lower likelihood of erythema nodosum and asymptomatic chest X-ray abnormalities, and salivary glands biopsy may be particularly helpful in the diagnosis.[19]
Children with age less than five years (early-onset sarcoidosis) have a triad of skin disease (rashes), uveitis, and arthritis and usually do not have lung disease. Older children have a multisystemic involvement similar to adults with bilateral hilar lymphadenopathy and lung involvement.[20]
Pathophysiology
Sarcoidosis is an inflammatory disease that, in sequence, involves the activation of antigen-driven CD4+ T lymphocytes, chemokine-induced migration of T cells to the disease site, local macrophage accumulation, and granuloma formation.[21]
In cases with active sarcoidosis, there is dominant expression of interferon γ (IFN γ), interleukin (IL)-2, IL-12, and tumor necrosis alpha (TNF α) at the disease site. TNFα is the key granuloma-promoting cytokine.[21] The CD4:CD8 ratio is increased (more than 3.5) in the bronchoalveolar lavage fluid in sarcoidosis.[22]
Histopathology
The formation of non-caseating (non-necrotizing) granuloma is the pathologic hallmark of sarcoidosis.[23] Granulomas are characterized by a collection of mononuclear cells and macrophages, which differentiate into epithelioid cells and multinucleated giant cells and are surrounded by an accumulation of lymphocytes, fibroblasts, collagen, and plasma cells. Asteroid bodies are star-shaped structures within the cytoplasm. Schaumann bodies (conchoidal bodies) are basophilic laminated calcified nodules seen within the multinucleated giant cells.[24] Histological evidence of infection or foreign body is usually not seen.
History and Physical
Sarcoidosis is a multisystem disease that predominantly involves lungs and thoracic lymph nodes. Patients may present with cough, dyspnea, and chest pain. Upper respiratory tract involvement leads to nonspecific rhinitis, recurrent sinusitis, and destructive lesions of the nasal septum and turbinates. Cutaneous manifestations include papules, nodules, plaques, ulcers, and erythema nodosum.[25]
Cardiac involvement leads to the development of arrhythmias and heart blocks.[26] Neurosarcoidosis occurs in about 5 to 10% of patients with sarcoidosis, with predominant involvement of basal leptomeninges and cranial nerves.[27]
Ocular involvement in sarcoidosis is characterized by granulomatous inflammation of the eye and its adnexal tissues. Ocular involvement is the presenting feature in 30 to 40% of cases.[28] The most common ocular features are uveitis (seen in 30 to 70%) and conjunctival nodules (seen in 40%).[29][1]
Orbital Disease
Involvement of orbital structures such as orbital fat, extraocular muscles, and optic nerve sheath leads to proptosis, globe displacement, pain, vision loss, and diplopia.[30] Most orbital lesions are located in the anterior orbit, especially the anteroinferior quadrant.[31] Orbital mass secondary to sarcoidosis can cause compression of the central retinal artery leading to central retinal artery occlusion (CRAO).[32]
Lacrimal System Disease
Keratoconjunctivitis sicca (KCS) is a common manifestation of ocular sarcoidosis.[33] Patients with keratoconjunctivitis sicca present with ocular irritation, epiphora, corneal infiltrate, corneal infection, and corneal scar. Inflammation of the lacrimal gland leads to enlargement of the gland with mass effects, proptosis, and dry eye syndrome (KCS) secondary to decreased aqueous production.[30] Inflammation of nasolacrimal sac and lacrimal drainage system leads to obstruction of tear outflow with epiphora, pain, and pus pointing secondary to dacryocystitis.[34]
Eyelid and Ocular Surface Disease
Eyelid - Erythematous eyelid swelling is seen in ocular sarcoidosis, which can vary in size from small papules to a large mass.[35] Granulomatous inflammation of eyelids can lead to ptosis, entropion, trichiasis, and madarosis.[36] Selective involvement of Müller's muscle may lead to eyelid retraction.[37]
Conjunctiva - Conjunctival nodules are seen as white discrete conjunctival deposits predominantly affecting bulbar conjunctiva but can also be seen in the perilimbal area and palpebral conjunctiva.[38] In some conditions, the conjunctival nodules mimic acute follicular conjunctivitis.[39] Complications include the development of symblepharon secondary to chronic cicatricial inflammation.[40]
Sclera – Scleritis is a rare manifestation of ocular sarcoidosis and can manifest as diffuse anterior, nodular anterior, or posterior scleritis.[41][42]
Cornea – The most common corneal manifestation of ocular sarcoidosis is superficial punctate keratitis secondary to dry eye (keratoconjunctivitis sicca). Other manifestations include band-shaped keratopathy, peripheral ulcerative keratitis, and interstitial keratitis.[43][44][45]
Uveitis
The mean age of presentation of uveitis is 42 years. Most sarcoid uveitis is bilateral, and approximately 90% are chronic.[46] Uveitis associated with sarcoidosis can be anterior, intermediate, posterior, or panuveitis.
Anterior uveitis – The patient presents with eye pain, redness of eyes, blurred vision, and photophobia. Sarcoidosis is characterized by granulomatous uveitis, with mutton fat keratic precipitates [figure 1], iris, or trabecular meshwork nodules. Busacca nodules are noted in the iris stroma, and the Koeppe nodules involve the pupillary margin. Berlin nodules are present at the angle of the anterior chamber. Hypopyon is usually not seen in sarcoidosis.[47]
Without appropriate treatment, there is a development of peripheral anterior synechiae with raised intraocular pressure or posterior synechiae with complicated cataracts. Patients with ocular sarcoid uveitis tend to develop raised intraocular pressure due to outflow obstruction due to trabecular meshwork block by inflammatory cells, trabecular meshwork nodules, or development of peripheral anterior synechiae.[48]
Intermediate uveitis – Patients complain of either floaters or blurred vision. The most common cause of vision loss is the development of cystoid macular edema or vitreous inflammation. Inflammation of pars plana leads to the development of snowballs in the inferior vitreous and snow banking near ora serrata.[49] Neovascularization may develop in the peripheral retina leading to vitreous hemorrhage in a few patients.[50] The vitreous may show the string of pearls appearance.
Posterior uveitis – Choroidal inflammation leads to the development of choroidal granulomas, which may be unifocal or multifocal, and vary in size from small (like Dalen-Fuchs nodules) to significant (like choroidal tumors).[51] Inflammatory new vessels of the optic disc or the retina may cause vitreous hemorrhage.[52]
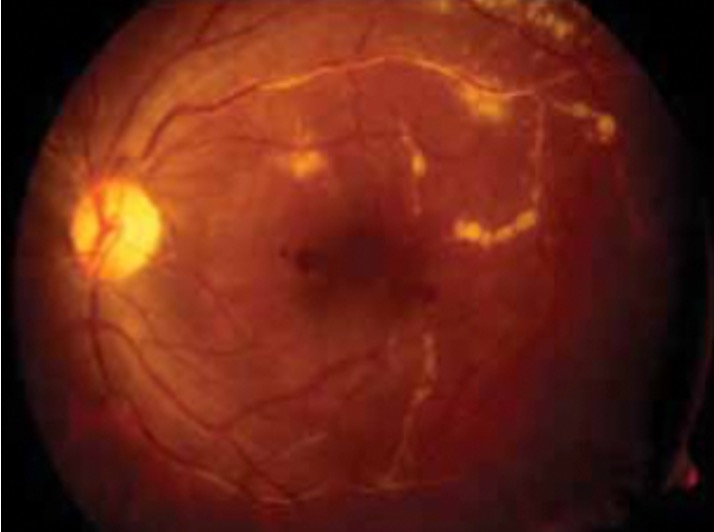
Choroidal neovascularization may develop from choroidal granulomas, which affect vision when present in the posterior pole. Mid peripheral periphlebitis is characteristic of ocular sarcoidosis. Occlusive vasculitis causing capillary nonperfusion and retinal new vessels may also occur. In severe cases, whitish-yellow perivascular exudates are seen along the retinal veins, classically described as "candle-wax drippings" or taches de bougie [Figure 2].[53]
Posterior uveitis is complicated with the development of cystoid macular edema, epiretinal membrane formation, intraretinal hemorrhage, and vitreous hemorrhage.
Neuro-ophthalmic Manifestations
The optic nerve and facial nerve are the most commonly affected nerves in ocular sarcoidosis.[54] Involvement of the optic nerve leads to the development of optic nerve granuloma [figure 3], optic disc edema, and optic atrophy. Optic disc edema is secondary to either posterior uveitis, direct involvement of optic nerve, optic nerve sheath infiltration by granulomatous tissue, or secondary to raised intracranial pressure due to neurosarcoidosis.[55][56]
Some patients may complain of visual hallucinations, nystagmus, and pupillary abnormalities such as relative afferent pupillary defect or light near dissociation due to the involvement of optic pathways.[57][58]
Facial nerve involvement leads to lower motor neuron facial paresis leading to epiphora, exposure keratopathy, and corneal ulcers. Facial nerve involvement occurs due to inflammation in the parotid gland or secondary to meningitic reaction or direct compression of the facial nerve.[59]
Syndromic Associations
Löfgren syndrome: It manifests as a triad of polyarthritis, erythema nodosum, and hilar lymphadenopathy. Other features include fever, malaise, uveitis, and parotitis.[60]
Heerfordt-Waldenström syndrome (uveoparotid fever): It manifests as swelling of the parotid gland, fever, uveitis, and facial nerve palsy.[61]
Blau syndrome: It is an autosomal dominant disorder seen in children below the age of 4 years. Clinical features include arthritis, rash, and uveitis.[62] Other names of the disease (OMIM #186580) include familial juvenile systemic granulomatosis, early-onset sarcoidosis, Jabs syndrome, granulomatous inflammatory arthritis, dermatitis, and uveitis. There is a mutation in the NOD2 (or CARD15) gene at chromosome 16q12.1.
Evaluation
For diagnosis of ocular sarcoidosis, three criteria must be met:
- The patient must have clinical findings consistent with sarcoidosis as per the international workshop on ocular sarcoidosis (IWOS) criteria [Figure 4]
- Evidence of non-caseating granuloma on tissue biopsy
- Other causes of granulomatous inflammation (such as tuberculosis syphilis) should be ruled out.[63]
The revised IWOS criteria for diagnosing ocular sarcoidosis [Figure 5] have removed the diagnosis of 'possible ocular sarcoidosis.'[22]
The gold standard investigation in the diagnosis of sarcoidosis is tissue biopsy. In ocular sarcoidosis, tissue biopsy can be done on tissues including the eyelids, lacrimal glands, conjunctival nodules, and orbital tissues. However, at certain sites where tissue biopsy is not possible, imaging techniques such as chest x-ray, chest CT scan, serum angiotensin-converting enzyme (ACE) levels, serum calcium levels, and serum lysozyme levels may aid in the diagnosis of a probable or presumed ocular sarcoidosis.[63]
Fundus photography - Helps document retinal findings and future objective comparisons of the lesions. Yellowish-white choroidal granulomas, periphlebitis, and other features of posterior uveitis can be documented as well as monitored on serial photographs.[64]
Fundus fluorescein angiography (FFA) – FFA helps in documenting retinal vasculitis and detection of peripheral retinal neovascularization.[65][66] FFA can reveal multiple retinal arterial macroaneurysms in an inflamed eye with or without optic disc granuloma, typical of ocular sarcoidosis.[67]
Fundus autofluorescence imaging (FAF) – FAF helps detect sub-clinical retinal pigment epithelial changes in chorioretinal pathologies and monitors treatment response in uveitis patients with chorioretinal lesions.[68]
Optical coherence tomography (OCT) – OCT helps assess and monitor macular edema and helps predict functional outcomes based on the structural integrity of various retinal layers.[69] Enhanced depth OCT imaging can be useful in the detection of choroidal granulomas.[70]
Anterior segment OCT – Anterior segment OCT helps identify and document peripheral anterior synechiae and the extent of angle closure.[71] In cases with granulomatous uveitis, it shows hyperreflective dots in the aqueous humor and on the posterior corneal surface.[72]
OCT of the optic nerve – OCT of the optic nerve head helps investigate an optic nerve head granuloma. It helps detect adjacent peripapillary retinal edema, monitor response to treatment, and evaluate the severity of optic nerve damage due to glaucoma.[73][74]
Ultrasonography of the eye – In patients with vitreous hemorrhage secondary to sarcoidosis, ultrasonography of the eye helps to understand the status of the retina. It helps as pre-operative aid in assessing the status of posterior vitreous detachment.
Ultrasound biomicroscopy (UBM) - UBM helps objectively evaluate the inflammatory process in anterior chamber angle, iris, ciliary body, pars plana, and peripheral vitreous.[75] It helps in the evaluation of ocular hypotony in cases of uveitis.[76]
Visual fields – A wide range of visual field defects can be seen either due to glaucomatous optic neuropathy or ischemic optic neuropathy. Patients with ischemic optic neuropathy can present with central or centrocecal scotomas, hemianopic field defect, or altitudinal field defect.[77]
Diagnostic vitrectomy – In limited cases, the vitreous sample obtained by vitrectomy can be used for diagnostic analysis. High mobility group box -1 (HMGB1), a pro-inflammatory cytokine, is detected in high levels in the vitreous of patients with ocular sarcoidosis.[78]
Systemic Investigations
Chest X-ray – Bilateral hilar lymphadenopathy is the classical radiological feature of sarcoidosis.[79] Lung parenchymal infiltrates appear in around 20 to 50% of the patients.[80] Lung volume loss, hilar retraction, and linear opaque bands may be seen when lung fibrosis occurs.[81]
High resolution computed tomography (HRCT) chest – HRCT scans are superior to conventional chest X-rays in delineating hilar lymphadenopathy, understanding lung parenchymal damage, and differentiating inflammation from fibrosis.[82] Characteristic features of HRCT chest in sarcoidosis include mediastinal lymphadenopathy, lung parenchymal nodular opacities, nodular opacities at the bronchovascular bundles, predominant involvement of mid or upper lung zones, and ground-glass opacities.[83]
Pulmonary function tests – Pulmonary function tests typically reveal a restrictive pattern of ventilatory function. The tests show decreased lung volume and decreased carbon monoxide diffusing capacity.[84]
Serum angiotensin-converting enzyme (SACE) assay – SACE levels are elevated in about 30 to 80% of patients with sarcoidosis.[85] However, about 20% of patients show false positive elevated levels of SACE. SACE may also be normal in patients with active disease.
Bronchoalveolar lavage (BAL) – BAL fluid analysis shows an increased number of activated T lymphocytes, alveolar macrophages, and inflammatory cytokines. In 85% of patients with pulmonary sarcoidosis, lymphocytosis is seen with normal or low granulocyte count.[86] However, the BAL cell profile is not specific for sarcoidosis.[87]
Radionuclide techniques – Radionuclide techniques include Gallium (Ga) scans fluoro-2deoxy-glucose (FDG) positron emission tomography (PET) scans. Uptake of Gallium helps identify appropriate sites to take the biopsy.[88] The uptake of Gallium by intrathoracic lymph nodes resembles the Greek letter lambda (Lambda sign), and uptake in bilateral parotid and lacrimal gland resembles a panda face (Panda sign).[89] PET scan helps identify lung activity in pulmonary disease and sarcoidosis activity at non-pulmonary sites such as bone, cardiac, and the nervous system.[90][91][92][93]
Lung Biopsy – Transbronchial lung biopsy (TBLB) using a flexible fiberoptic bronchoscopy (FFB) is the diagnostic procedure of choice in patients with pulmonary sarcoidosis with a sensitivity range of 60 to 90% depending on the stage of the disease.[94] In the presence of lymphadenopathy, transbronchial needle aspiration (TBNA) biopsies are diagnostic in 63 to 90% of cases.[95][96] Biopsy shows the presence of non-caseating granulomas.
Kveim-Siltzbach test – This test involves intradermal injection of a suspension of granuloma containing spleen or lymph node from a patient with sarcoidosis.[97] A positive test shows the development of papule at the injection site within 4-6 weeks, which on biopsy shows non-caseating granuloma.[98] This test is now vaguely in use and is of historical interest.[99]
MRI (magnetic resonance imaging) of the brain – MRI brain in neurosarcoidosis reveals a wide spectrum of findings such as periventricular and white matter lesions, leptomeningeal enhancement, solitary or multiple supra- and infratentorial brain lesions, and involvement of cranial nerves.[100]
Treatment / Management
Corticosteroids
Corticosteroids are potent anti-inflammatory agents used in the management of uveitis. They can be used topically as eye drops to treat anterior uveitis. However, topical steroids are insufficient in the control of posterior uveitis. The most commonly used topical corticosteroids are prednisolone acetate 1% or difluprednate 0.05%.[101](A1)
Regional corticosteroids
These agents are used in cases of posterior uveitis or when the patient is poorly compliant to frequent dosages of topical corticosteroids. They can be given as posterior subtenon injection in a dose of 20 to 40 mg of triamcinolone acetonide or intravitreal injection of 1 to 4 mg of triamcinolone acetonide.[102][103][102](B2)
Long-acting sustained slow release steroid implants such as 0.7 mg of dexamethasone, 0.19 mg of fluocinolone acetonide, or 0.59 mg of fluocinolone acetonide provide longer periods of anti-inflammatory effect. The regional corticosteroids may cause glaucoma and cataracts, and thus the patients should be observed closely and promptly treated for the complications.[104][105][104](A1)
Systemic steroids
Systemically dosed steroids are used in cases of bilateral uveitis with systemic involvement or when there is poor disease control with local steroid use. Prednisolone in the dose of 1 to 1.5 mg/kg/day initially followed by slow tapering is the usual treatment protocol.
Cycloplegic Agents
These agents are used to relieve ciliary spasms and associated ocular pain. They help in breaking or preventing posterior synechiae. Commonly used agents include cyclopentolate 1%, homatropine 2%, and atropine 1%.
Systemic Immunosuppressive Agents
These agents are used in cases of corticosteroid-resistant cases or steroid-dependent cases where the side effects of the steroid regimen outweigh the anti-inflammatory effects. Most commonly used agents for the treatment of non-infectious uveitis are methotrexate (7.5 to 25 mg /week; oral, subcutaneous, or intramuscular route), mycophenolate mofetil (500–1500 mg bd; oral route), cyclosporine (2.5 to 10 mg/kg/day bd; oral route), and azathioprine (1 to 4 mg/kg/day; oral route).[106][107][108](B2)
Biologic Agents
These are used in cases of refractory non-infectious uveitis. The most commonly used biologic agents are tumor necrosis factor-alpha inhibitors (TNF α) such as infliximab, adalimumab, etanercept, and golimumab. These agents promise to offer a new option in resistant cases. The patient should be tested for latent tuberculosis and hepatitis B before starting biologic agents to prevent the reactivation of infection.[109] Interestingly, a 'sarcoid-like granulomatosis' has been reported following anti-TNF therapy, including etanercept, infliximab, and adalimumab for various indications, including rheumatoid arthritis and spondyloarthritis.[110](B3)
Treatment of Orbital Disease
Orbital inflammation is typically amenable to systemic steroids or immunosuppressive agents. In cases with chronic eyelid or lacrimal gland swelling, a biopsy is necessary to differentiate it from tumors.
Treatment of Ocular Surface Disease
Conjunctival lesions and KCS usually responds to topical cyclosporine eye drops.[111] For scleritis, initial treatment is with systemic non-steroidal anti-inflammatory drugs (NSAIDs), with steroids and immunosuppressants reserved for resistant cases.(B3)
Treatment of Ocular Complications
Glaucoma is one of the most common uveitis complications and a serious side effect of chronic corticosteroid use. Glaucoma needs to be monitored at each visit and requires early intervention either medically or surgically. Cataract surgery is usually planned after three months of disease inactivity. Uveitic cystoid macular edema is one of the common causes of ocular morbidity and requires intervention in the form of anti-inflammatory agents.
In certain cases, posterior subtenon triamcinolone, intravitreal injection of steroids (triamcinolone, dexamethasone implant, or fluocinolone implant), or anti-VEGF (vascular endothelial growth factor) agents like bevacizumab or ranibizumab are required to control cystoid macular edema. Development of epiretinal membrane at the macula, vitreomacular traction, and vitreous hemorrhage may warrant surgical intervention in the form of vitrectomy.[112][113]
Differential Diagnosis
Sarcoidosis is a disease that can simultaneously involve various parts of the eye with multiple presentations. Differential diagnoses should be considered for various ocular findings, including iris nodules, intermediate uveitis, chorioretinitis, retinitis, choroidal infiltrates, dacryoadenopathy, and peripheral retinal neovascularization.[114] The most common differential diagnoses to be considered include:
Iris nodules: Iris nodules may also be seen in inflammatory conditions such as tuberculosis, leprosy, and syphilis, tumors of the iris, and infiltration by retinoblastoma.
Intermediate uveitis: Intermediate uveitis may also be idiopathic or seen in Lyme disease, tuberculosis, and multiple sclerosis.
Chorioretinitis: Chorioretinitis may also be seen in tuberculosis, histoplasmosis, toxoplasmosis, and syphilis.
Choroidal infiltrates: Choroidal infiltrates may also be present in white dot syndromes such as acute posterior multifocal placoid pigment epitheliopathy, birdshot choroidopathy; Vogt-Koyanagi-Harada disease, amelanotic melanoma, and metastatic infiltrates.
Dacryoadenopathy: Dacryoadenopathy may also be seen in tuberculosis, brucellosis, Hodgkin's lymphoma, and parotid gland tumors.
Peripheral retinal neovascularization: Peripheral retinal neovascularization may also be present in diabetic retinopathy, vein occlusions, sickle cell retinopathy, and Eales disease.[115][116]
Staging
On chest X-ray posteroanterior (PA) view, the pulmonary disease is divided into five stages:
Prognosis
Outcomes in cases of ocular sarcoidosis depend on several variables such as the severity of disease, chronicity of disease, time of presentation to healthcare facilities, and secondary complications due to long-standing ocular inflammation.[46] The overall prognosis for ocular sarcoidosis is usually good, and most patients recover without significant functional damage.[119]
Irreversible loss of vision usually occurs due to glaucoma and chronic maculopathy. In a prospective study by Edelsten et al., it was noted that the prognosis of sarcoid uveitis is not affected by extraocular disease. The presence of the HLA DQB1*0201 allele is proven to be protective against severe sarcoidosis.[120]
Prognostic factors for poor visual outcomes include black race, delayed presentation to a subspecialist, glaucoma, intermediate or posterior uveitis, cystoid macular edema, and use of systemic steroids. The overall mortality from sarcoidosis may reach 5% or even 10% with neurosarcoidosis. Poor prognostic factors in sarcoidosis include female gender (compared to male), early-onset disease, African-American ethnicity, chronic sarcoidosis, Annexin A11 gene, HLA-DQB1*1501, HLA-DQB1*0602, pulmonary fibrosis, poor pulmonary function test, optic nerve involvement, panuveitis, lupus pernio, chronic arthritis, and stage 3 or 4 of the disease in radiography.[121]
Complications
Ocular Complications
- Glaucoma
- Cataract
- Central retinal artery occlusion
- Cystoid macular edema
- Epiretinal membrane
- Intraretinal hemorrhage
- Vitreous hemorrhage
- Phthisis[122]
Systemic Complications
- Neurosarcoidosis
- Pulmonary hypertension
- End-stage lung damage
Deterrence and Patient Education
Although ocular sarcoidosis can have an acute presentation with a limited course, some patients develop a chronic unrelenting form of the disease.[123] Patients with chronic disease should be made aware of complications due to chronic inflammation such as glaucoma, cataract, cystoid macular edema, and epiretinal membrane formation. Patients should also be made aware of the need for medical compliance and drug-related adverse effects. Patients with sarcoid uveitis are known to carry a risk of neurological involvement for at least the next 15 years.[124]
Enhancing Healthcare Team Outcomes
Sarcoidosis is a multisystem disease and requires effective coordination between an ophthalmologist, pulmonologist, infectious disease specialist, dermatologist, rheumatologist, radiologist, cardiologist, neurologist, and pharmacist. At each visit, an ophthalmic assistant should measure intraocular pressure in addition to vision, as both the disease and its treatment, i.e., steroids, can lead to raised intraocular pressure. The pharmacist plays a vital role in monitoring compliance to treatment and educating patients about the side effects of various drugs used in the treatment. Clinical overlap between sarcoidosis and tuberculosis is not uncommon, and an infectious disease specialist should be consulted in such cases. Nursing staff plays an important role in ensuring compliance with therapy. An optimal interprofessional collaboration can ensure the best outcomes in patients with ocular sarcoidosis.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Pasadhika S, Rosenbaum JT. Ocular Sarcoidosis. Clinics in chest medicine. 2015 Dec:36(4):669-83. doi: 10.1016/j.ccm.2015.08.009. Epub [PubMed PMID: 26593141]
Ikonomopoulos JA, Gorgoulis VG, Kastrinakis NG, Galanos AA, Karameris A, Kittas C. Experimental inoculation of laboratory animals with samples collected from sarcoidal patients and molecular diagnostic evaluation of the results. In vivo (Athens, Greece). 2000 Nov-Dec:14(6):761-5 [PubMed PMID: 11204496]
Level 3 (low-level) evidenceHeyll A, Meckenstock G, Aul C, Söhngen D, Borchard F, Hadding U, Mödder U, Leschke M, Schneider W. Possible transmission of sarcoidosis via allogeneic bone marrow transplantation. Bone marrow transplantation. 1994 Jul:14(1):161-4 [PubMed PMID: 7951107]
Level 3 (low-level) evidenceAgrawal R, Kee AR, Ang L, Tun Hang Y, Gupta V, Kon OM, Mitchell D, Zierhut M, Pavesio C. Tuberculosis or sarcoidosis: Opposite ends of the same disease spectrum? Tuberculosis (Edinburgh, Scotland). 2016 May:98():21-6. doi: 10.1016/j.tube.2016.01.003. Epub 2016 Jan 18 [PubMed PMID: 27156614]
Eishi Y. Etiologic link between sarcoidosis and Propionibacterium acnes. Respiratory investigation. 2013 Jun:51(2):56-68. doi: 10.1016/j.resinv.2013.01.001. Epub 2013 Mar 17 [PubMed PMID: 23790733]
Newman KL, Newman LS. Occupational causes of sarcoidosis. Current opinion in allergy and clinical immunology. 2012 Apr:12(2):145-50. doi: 10.1097/ACI.0b013e3283515173. Epub [PubMed PMID: 22314258]
Level 3 (low-level) evidenceKucera GP, Rybicki BA, Kirkey KL, Coon SW, Major ML, Maliarik MJ, Iannuzzi MC. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003 May:123(5):1527-35 [PubMed PMID: 12740270]
Kreider ME, Christie JD, Thompson B, Newman L, Rose C, Barnard J, Bresnitz E, Judson MA, Lackland DT, Rossman MD. Relationship of environmental exposures to the clinical phenotype of sarcoidosis. Chest. 2005 Jul:128(1):207-15 [PubMed PMID: 16002937]
Level 2 (mid-level) evidenceBarnard J, Rose C, Newman L, Canner M, Martyny J, McCammon C, Bresnitz E, Rossman M, Thompson B, Rybicki B, Weinberger SE, Moller DR, McLennan G, Hunninghake G, DePalo L, Baughman RP, Iannuzzi MC, Judson MA, Knatterud GL, Teirstein AS, Yeager H Jr, Johns CJ, Rabin DL, Cherniack R, ACCESS Research Group. Job and industry classifications associated with sarcoidosis in A Case-Control Etiologic Study of Sarcoidosis (ACCESS). Journal of occupational and environmental medicine. 2005 Mar:47(3):226-34 [PubMed PMID: 15761318]
Level 2 (mid-level) evidenceRossman MD, Thompson B, Frederick M, Maliarik M, Iannuzzi MC, Rybicki BA, Pandey JP, Newman LS, Magira E, Beznik-Cizman B, Monos D, ACCESS Group. HLA-DRB1*1101: a significant risk factor for sarcoidosis in blacks and whites. American journal of human genetics. 2003 Oct:73(4):720-35 [PubMed PMID: 14508706]
Mehra NK, Bovornkitti S. HLA and sarcoidosis. Sarcoidosis. 1988 Sep:5(2):87-9 [PubMed PMID: 3227192]
Calender A, Weichhart T, Valeyre D, Pacheco Y. Current Insights in Genetics of Sarcoidosis: Functional and Clinical Impacts. Journal of clinical medicine. 2020 Aug 13:9(8):. doi: 10.3390/jcm9082633. Epub 2020 Aug 13 [PubMed PMID: 32823753]
Iannuzzi MC. Genetics of sarcoidosis. Seminars in respiratory and critical care medicine. 2007 Feb:28(1):15-21 [PubMed PMID: 17330189]
Rybicki BA, Major M, Popovich J Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. American journal of epidemiology. 1997 Feb 1:145(3):234-41 [PubMed PMID: 9012596]
Level 2 (mid-level) evidenceBaughman RP, Field S, Costabel U, Crystal RG, Culver DA, Drent M, Judson MA, Wolff G. Sarcoidosis in America. Analysis Based on Health Care Use. Annals of the American Thoracic Society. 2016 Aug:13(8):1244-52. doi: 10.1513/AnnalsATS.201511-760OC. Epub [PubMed PMID: 27509154]
Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. The New England journal of medicine. 2007 Nov 22:357(21):2153-65 [PubMed PMID: 18032765]
Rothova A, Alberts C, Glasius E, Kijlstra A, Buitenhuis HJ, Breebaart AC. Risk factors for ocular sarcoidosis. Documenta ophthalmologica. Advances in ophthalmology. 1989 Aug:72(3-4):287-96 [PubMed PMID: 2625091]
Level 3 (low-level) evidenceYemm RW, Pecen PE, Fliney GD, Palestine AG. Chest X-ray and Uveitis Evaluation in a Population with Low Incidence of Sarcoidosis. Ophthalmology and therapy. 2020 Sep:9(3):577-584. doi: 10.1007/s40123-020-00274-6. Epub 2020 Jul 1 [PubMed PMID: 32613593]
Varron L, Cottin V, Schott AM, Broussolle C, Sève P. Late-onset sarcoidosis: a comparative study. Medicine. 2012 May:91(3):137-143. doi: 10.1097/MD.0b013e3182569f91. Epub [PubMed PMID: 22543629]
Level 2 (mid-level) evidenceShetty AK, Gedalia A. Childhood sarcoidosis: A rare but fascinating disorder. Pediatric rheumatology online journal. 2008 Sep 23:6():16. doi: 10.1186/1546-0096-6-16. Epub 2008 Sep 23 [PubMed PMID: 18811966]
Patterson KC, Chen ES. The Pathogenesis of Pulmonary Sarcoidosis and Implications for Treatment. Chest. 2018 Jun:153(6):1432-1442. doi: 10.1016/j.chest.2017.11.030. Epub 2017 Dec 7 [PubMed PMID: 29224832]
Mochizuki M, Smith JR, Takase H, Kaburaki T, Acharya NR, Rao NA, International Workshop on Ocular Sarcoidosis Study Group. Revised criteria of International Workshop on Ocular Sarcoidosis (IWOS) for the diagnosis of ocular sarcoidosis. The British journal of ophthalmology. 2019 Oct:103(10):1418-1422. doi: 10.1136/bjophthalmol-2018-313356. Epub 2019 Feb 23 [PubMed PMID: 30798264]
Polverino F, Balestro E, Spagnolo P. Clinical Presentations, Pathogenesis, and Therapy of Sarcoidosis: State of the Art. Journal of clinical medicine. 2020 Jul 24:9(8):. doi: 10.3390/jcm9082363. Epub 2020 Jul 24 [PubMed PMID: 32722050]
Gupta N, Rajwanshi A, Gupta D. Schaumann body in a case of sarcoidosis diagnosed on transbronchial FNAC. Journal of cytology. 2011 Apr:28(2):88-9. doi: 10.4103/0970-9371.80753. Epub [PubMed PMID: 21713156]
Level 3 (low-level) evidenceMañá J, Marcoval J. Skin manifestations of sarcoidosis. Presse medicale (Paris, France : 1983). 2012 Jun:41(6 Pt 2):e355-74. doi: 10.1016/j.lpm.2012.02.046. Epub 2012 May 9 [PubMed PMID: 22579238]
Lynch JP 3rd, Hwang J, Bradfield J, Fishbein M, Shivkumar K, Tung R. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Seminars in respiratory and critical care medicine. 2014 Jun:35(3):372-90. doi: 10.1055/s-0034-1376889. Epub 2014 Jul 9 [PubMed PMID: 25007089]
Chapelon C, Ziza JM, Piette JC, Levy Y, Raguin G, Wechsler B, Bitker MO, Blétry O, Laplane D, Bousser MG. Neurosarcoidosis: signs, course and treatment in 35 confirmed cases. Medicine. 1990 Sep:69(5):261-76 [PubMed PMID: 2205782]
Level 3 (low-level) evidenceUngprasert P, Tooley AA, Crowson CS, Matteson EL, Smith WM. Clinical Characteristics of Ocular Sarcoidosis: A Population-Based Study 1976-2013. Ocular immunology and inflammation. 2019:27(3):389-395. doi: 10.1080/09273948.2017.1386791. Epub 2017 Oct 12 [PubMed PMID: 29023165]
Rothova A. Ocular involvement in sarcoidosis. The British journal of ophthalmology. 2000 Jan:84(1):110-6 [PubMed PMID: 10611110]
Mavrikakis I, Rootman J. Diverse clinical presentations of orbital sarcoid. American journal of ophthalmology. 2007 Nov:144(5):769-775 [PubMed PMID: 17869205]
Level 2 (mid-level) evidencePrabhakaran VC, Saeed P, Esmaeli B, Sullivan TJ, McNab A, Davis G, Valenzuela A, Leibovitch I, Kesler A, Sivak-Callcott J, Hoyama E, Selva D. Orbital and adnexal sarcoidosis. Archives of ophthalmology (Chicago, Ill. : 1960). 2007 Dec:125(12):1657-62 [PubMed PMID: 18071118]
Level 2 (mid-level) evidenceKim DS, Korgavkar K, Zahid S, De Lott L, Prabhakar A, Foerster BR, Besirli CG. Vision Loss After Central Retinal Artery Occlusion Secondary to Orbital Sarcoid Mass. Ophthalmic plastic and reconstructive surgery. 2016 Mar-Apr:32(2):e37-40. doi: 10.1097/IOP.0000000000000223. Epub [PubMed PMID: 25072221]
Level 3 (low-level) evidenceJONES BR, STEVENSON CJ. Keratoconjunctivitis sicca due to sarcoidosis. The British journal of ophthalmology. 1957 Mar:41(3):153-60 [PubMed PMID: 13404198]
Kay DJ, Saffra N, Har-El G. Isolated sarcoidosis of the lacrimal sac without systemic manifestations. American journal of otolaryngology. 2002 Jan-Feb:23(1):53-5 [PubMed PMID: 11791250]
Level 3 (low-level) evidenceHall JG, Cohen KL. Sarcoidosis of the eyelid skin. American journal of ophthalmology. 1995 Jan:119(1):100-1 [PubMed PMID: 7825675]
Level 3 (low-level) evidenceMoin M, Kersten RC, Bernardini F, Kulwin DR. Destructive eyelid lesions in sarcoidosis. Ophthalmic plastic and reconstructive surgery. 2001 Mar:17(2):123-5 [PubMed PMID: 11281585]
Level 3 (low-level) evidenceBehbehani R, Nipper KS, Eagle RC Jr, Bilyk JR. Systemic sarcoidosis manifested as unilateral eyelid retraction. Archives of ophthalmology (Chicago, Ill. : 1960). 2006 Apr:124(4):599-600 [PubMed PMID: 16606895]
Level 3 (low-level) evidenceDithmar S, Waring GO 3rd, Goldblum TA, Grossniklaus HE. Conjunctival deposits as an initial manifestation of sarcoidosis. American journal of ophthalmology. 1999 Sep:128(3):361-2 [PubMed PMID: 10511034]
Level 3 (low-level) evidenceManrique Lipa RK, de los Bueis AB, De los Ríos JJ, Manrique Lipa RD. Sarcoidosis presenting as acute bulbar follicular conjunctivitis. Clinical & experimental optometry. 2010 Sep:93(5):363-5. doi: 10.1111/j.1444-0938.2010.00508.x. Epub 2010 Aug 16 [PubMed PMID: 20718787]
Level 3 (low-level) evidenceGeggel HS, Mensher JH. Cicatricial conjunctivitis in sarcoidosis: recognition and treatment. Annals of ophthalmology. 1989 Mar:21(3):92-4 [PubMed PMID: 2735697]
Level 3 (low-level) evidenceHeiligenhaus A, Michel D, Koch JM. Nodular scleritis in a patient with sarcoidosis. The British journal of ophthalmology. 2003 Apr:87(4):507-8 [PubMed PMID: 12642326]
Level 3 (low-level) evidenceDodds EM, Lowder CY, Barnhorst DA, Lavertu P, Caravella LP, White DE. Posterior scleritis with annular ciliochoroidal detachment. American journal of ophthalmology. 1995 Nov:120(5):677-9 [PubMed PMID: 7485375]
Level 3 (low-level) evidenceCrick RP, Hoyle C, Smellie H. THE EYES IN SARCOIDOSIS. The British journal of ophthalmology. 1961 Jul:45(7):461-81 [PubMed PMID: 18170695]
Siracuse-Lee D, Saffra N. Peripheral ulcerative keratitis in sarcoidosis: a case report. Cornea. 2006 Jun:25(5):618-20 [PubMed PMID: 16783154]
Level 3 (low-level) evidenceLennarson P, Barney NP. Interstitial keratitis as presenting ophthalmic sign of sarcoidosis in a child. Journal of pediatric ophthalmology and strabismus. 1995 May-Jun:32(3):194-6 [PubMed PMID: 7636703]
Level 3 (low-level) evidenceDana MR, Merayo-Lloves J, Schaumberg DA, Foster CS. Prognosticators for visual outcome in sarcoid uveitis. Ophthalmology. 1996 Nov:103(11):1846-53 [PubMed PMID: 8942880]
Level 2 (mid-level) evidenceZaidi AA, Ying GS, Daniel E, Gangaputra S, Rosenbaum JT, Suhler EB, Thorne JE, Foster CS, Jabs DA, Levy-Clarke GA, Nussenblatt RB, Kempen JH, Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study. Hypopyon in patients with uveitis. Ophthalmology. 2010 Feb:117(2):366-72. doi: 10.1016/j.ophtha.2009.07.025. Epub 2009 Dec 14 [PubMed PMID: 20006905]
Level 2 (mid-level) evidenceShimizu A, Maruyama K, Yokoyama Y, Tsuda S, Ryu M, Nakazawa T. Characteristics of uveitic glaucoma and evaluation of its surgical treatment. Clinical ophthalmology (Auckland, N.Z.). 2014:8():2383-9. doi: 10.2147/OPTH.S72383. Epub 2014 Nov 26 [PubMed PMID: 25473265]
Chauhan K, Tripathy K. Pars Planitis. StatPearls. 2024 Jan:(): [PubMed PMID: 28613790]
Spalton DJ, Sanders MD. Fundus changes in histologically confirmed sarcoidosis. The British journal of ophthalmology. 1981 May:65(5):348-58 [PubMed PMID: 6166318]
Letocha CE, Shields JA, Goldberg RE. Retinal changes in sarcoidosis. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 1975 Apr:10(2):184-92 [PubMed PMID: 1125843]
Kumawat BL, Chawla R, Venkatesh P, Tripathy K. Inflammatory optic disc neovascularisation managed with oral steroids/immunosuppressants and intravitreal ranibizumab. BMJ case reports. 2017 Nov 3:2017():. pii: bcr-2017-222262. doi: 10.1136/bcr-2017-222262. Epub 2017 Nov 3 [PubMed PMID: 29102974]
Level 3 (low-level) evidenceGOULD H, KAUFMAN HE. Sarcoid of the fundus. Archives of ophthalmology (Chicago, Ill. : 1960). 1961 Mar:65():453-6 [PubMed PMID: 13707501]
Phillips YL, Eggenberger ER. Neuro-ophthalmic sarcoidosis. Current opinion in ophthalmology. 2010 Nov:21(6):423-9. doi: 10.1097/ICU.0b013e32833eae4d. Epub [PubMed PMID: 20736834]
Level 3 (low-level) evidenceGass JD, Olson CL. Sarcoidosis with optic nerve and retinal involvement. Archives of ophthalmology (Chicago, Ill. : 1960). 1976 Jun:94(6):945-50 [PubMed PMID: 938285]
Level 3 (low-level) evidencePirau L, Lui F. Neurosarcoidosis. StatPearls. 2022 Jan:(): [PubMed PMID: 30521189]
Poole CJ. Argyll Robertson pupils due to neurosarcoidosis: evidence for site of lesion. British medical journal (Clinical research ed.). 1984 Aug 11:289(6441):356 [PubMed PMID: 6432097]
Level 3 (low-level) evidenceSimakurthy S, Tripathy K. Marcus Gunn Pupil. StatPearls. 2025 Jan:(): [PubMed PMID: 32491607]
Zajicek JP, Scolding NJ, Foster O, Rovaris M, Evanson J, Moseley IF, Scadding JW, Thompson EJ, Chamoun V, Miller DH, McDonald WI, Mitchell D. Central nervous system sarcoidosis--diagnosis and management. QJM : monthly journal of the Association of Physicians. 1999 Feb:92(2):103-17 [PubMed PMID: 10209662]
Level 3 (low-level) evidenceChauhan A,Jandial A,Mishra K,Sandal R, Acute arthritis, skin rash and Lofgren's syndrome. BMJ case reports. 2021 Jun 7; [PubMed PMID: 34099443]
Level 3 (low-level) evidenceDenny MC, Fotino AD. The Heerfordt-Waldenström syndrome as an initial presentation of sarcoidosis. Proceedings (Baylor University. Medical Center). 2013 Oct:26(4):390-2 [PubMed PMID: 24082416]
Sfriso P, Caso F, Tognon S, Galozzi P, Gava A, Punzi L. Blau syndrome, clinical and genetic aspects. Autoimmunity reviews. 2012 Nov:12(1):44-51. doi: 10.1016/j.autrev.2012.07.028. Epub 2012 Aug 2 [PubMed PMID: 22884558]
Level 3 (low-level) evidenceHerbort CP, Rao NA, Mochizuki M, members of Scientific Committee of First International Workshop on Ocular Sarcoidosis. International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop On Ocular Sarcoidosis (IWOS). Ocular immunology and inflammation. 2009 May-Jun:17(3):160-9. doi: 10.1080/09273940902818861. Epub [PubMed PMID: 19585358]
Dickson D, Agarwal A, Sadiq MA, Hassan M, High R, Nguyen QD, Sepah YJ. Assessment of vitreous haze using ultra-wide field retinal imaging. Journal of ophthalmic inflammation and infection. 2016 Dec:6(1):35. doi: 10.1186/s12348-016-0105-0. Epub 2016 Sep 29 [PubMed PMID: 27687961]
Nicholson BP, Nigam D, Miller D, Agrón E, Dalal M, Jacobs-El N, da Rocha Lima B, Cunningham D, Nussenblatt R, Sen HN. Comparison of wide-field fluorescein angiography and 9-field montage angiography in uveitis. American journal of ophthalmology. 2014 Mar:157(3):673-7. doi: 10.1016/j.ajo.2013.12.005. Epub 2013 Dec 7 [PubMed PMID: 24321475]
Level 2 (mid-level) evidenceRuia S, Tripathy K. Fluorescein Angiography. StatPearls. 2024 Jan:(): [PubMed PMID: 35015403]
Singh D, Tripathy K. Retinal Macroaneurysm. StatPearls. 2024 Jan:(): [PubMed PMID: 35015432]
Reznicek L, Seidensticker F, Stumpf C, Kampik A, Thurau S, Kernt M, Neubauer A. Systematic analysis of wide-field fundus autofluorescence (FAF) imaging in posterior uveitis. Current eye research. 2014 Feb:39(2):164-71. doi: 10.3109/02713683.2013.834938. Epub 2013 Oct 21 [PubMed PMID: 24144279]
Level 1 (high-level) evidencePakzad-Vaezi K, Or C, Yeh S, Forooghian F. Optical coherence tomography in the diagnosis and management of uveitis. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2014 Feb:49(1):18-29. doi: 10.1016/j.jcjo.2013.10.005. Epub 2013 Dec 22 [PubMed PMID: 24513352]
Invernizzi A, Mapelli C, Viola F, Cigada M, Cimino L, Ratiglia R, Staurenghi G, Gupta A. Choroidal granulomas visualized by enhanced depth imaging optical coherence tomography. Retina (Philadelphia, Pa.). 2015 Mar:35(3):525-31. doi: 10.1097/IAE.0000000000000312. Epub [PubMed PMID: 25105317]
Level 2 (mid-level) evidenceSu DH, Friedman DS, See JL, Chew PT, Chan YH, Nolan WP, Smith SD, Huang D, Zheng C, Li Y, Foster PJ, Aung T. Degree of angle closure and extent of peripheral anterior synechiae: an anterior segment OCT study. The British journal of ophthalmology. 2008 Jan:92(1):103-7 [PubMed PMID: 17584995]
Concilio M, Cennamo G, Giordano M, Fossataro F, D'Andrea L, Ciampa N, Naddei R, Orlando F, Tranfa F, Alessio M. Anterior Segment-Optical Coherence Tomography features in Blau syndrome. Photodiagnosis and photodynamic therapy. 2021 Jun:34():102278. doi: 10.1016/j.pdpdt.2021.102278. Epub 2021 Apr 1 [PubMed PMID: 33813016]
Hickman SJ,Quhill F,Pepper IM, The Evolution of an Optic Nerve Head Granuloma Due to Sarcoidosis. Neuro-ophthalmology (Aeolus Press). 2016 Apr; [PubMed PMID: 27928387]
Sathyan P, Shilpa S, Anitha A. Optical Coherence Tomography in Glaucoma. Journal of current glaucoma practice. 2012 Jan-Apr:6(1):1-5 [PubMed PMID: 27990063]
Tran VT, LeHoang P, Herbort CP. Value of high-frequency ultrasound biomicroscopy in uveitis. Eye (London, England). 2001 Feb:15(Pt 1):23-30 [PubMed PMID: 11318288]
Roters S, Szurman P, Engels BF, Bartz-Schmidt KU, Krieglstein GK. Ultrasound biomicroscopy in chronic ocular hypotony: its impact on diagnosis and management. Retina (Philadelphia, Pa.). 2002 Oct:22(5):581-8 [PubMed PMID: 12441723]
Level 2 (mid-level) evidenceKidd DP, Burton BJ, Graham EM, Plant GT. Optic neuropathy associated with systemic sarcoidosis. Neurology(R) neuroimmunology & neuroinflammation. 2016 Oct:3(5):e270. doi: 10.1212/NXI.0000000000000270. Epub 2016 Aug 2 [PubMed PMID: 27536707]
Takeuchi M,Taguchi M,Sato T,Karasawa K,Sakurai Y,Harimoto K,Ito M, Association of High-Mobility Group Box-1 With Th Cell-Related Cytokines in the Vitreous of Ocular Sarcoidosis Patients. Investigative ophthalmology [PubMed PMID: 28125840]
Lynch JP 3rd, Kazerooni EA, Gay SE. Pulmonary sarcoidosis. Clinics in chest medicine. 1997 Dec:18(4):755-85 [PubMed PMID: 9413657]
Hillerdal G,Nöu E,Osterman K,Schmekel B, Sarcoidosis: epidemiology and prognosis. A 15-year European study. The American review of respiratory disease. 1984 Jul; [PubMed PMID: 6742607]
Lynch JP 3rd, Ma YL, Koss MN, White ES. Pulmonary sarcoidosis. Seminars in respiratory and critical care medicine. 2007 Feb:28(1):53-74 [PubMed PMID: 17330192]
Lynch JP 3rd, Computed tomographic scanning in sarcoidosis. Seminars in respiratory and critical care medicine. 2003 Aug; [PubMed PMID: 16088560]
Hennebicque AS,Nunes H,Brillet PY,Moulahi H,Valeyre D,Brauner MW, CT findings in severe thoracic sarcoidosis. European radiology. 2005 Jan; [PubMed PMID: 15449010]
Soto-Gomez N, Peters JI, Nambiar AM. Diagnosis and Management of Sarcoidosis. American family physician. 2016 May 15:93(10):840-8 [PubMed PMID: 27175719]
Lieberman J, Schleissner LA, Nosal A, Sastre A, Mishkin FS. Clinical correlations of serum angiotensin-converting enzyme (ACE) in sarcoidosis. A longitudinal study of serum ACE, 67gallium scans, chest roentgenograms, and pulmonary function. Chest. 1983 Nov:84(5):522-8 [PubMed PMID: 6313303]
Level 3 (low-level) evidenceWelker L, Jörres RA, Costabel U, Magnussen H. Predictive value of BAL cell differentials in the diagnosis of interstitial lung diseases. The European respiratory journal. 2004 Dec:24(6):1000-6 [PubMed PMID: 15572545]
Drent M, Mulder PG, Wagenaar SS, Hoogsteden HC, van Velzen-Blad H, van den Bosch JM. Differences in BAL fluid variables in interstitial lung diseases evaluated by discriminant analysis. The European respiratory journal. 1993 Jun:6(6):803-10 [PubMed PMID: 8339798]
Ismail S, Embong Z, Hitam WH. Gallium scan in diagnosing ocular sarcoidosis. The Malaysian journal of medical sciences : MJMS. 2005 Jan:12(1):64-7 [PubMed PMID: 22605949]
Level 3 (low-level) evidenceYoshimizu T, Suga K, Orihashi N, Soejima K, Kaneko T, Kawamura M, Nakanishi T, Utsumi H, Yamada N. [The appearance of "lambda" and "panda" sign on Ga-67 scintigraphy in sarcoidosis]. Kaku igaku. The Japanese journal of nuclear medicine. 1991 Oct:28(10):1151-7 [PubMed PMID: 1800775]
Brudin LH, Valind SO, Rhodes CG, Pantin CF, Sweatman M, Jones T, Hughes JM. Fluorine-18 deoxyglucose uptake in sarcoidosis measured with positron emission tomography. European journal of nuclear medicine. 1994 Apr:21(4):297-305 [PubMed PMID: 8005153]
Aberg C, Ponzo F, Raphael B, Amorosi E, Moran V, Kramer E. FDG positron emission tomography of bone involvement in sarcoidosis. AJR. American journal of roentgenology. 2004 Apr:182(4):975-7 [PubMed PMID: 15039174]
Level 3 (low-level) evidenceTakeda N, Yokoyama I, Hiroi Y, Sakata M, Harada T, Nakamura F, Murakawa Y, Nagai R. Positron emission tomography predicted recovery of complete A-V nodal dysfunction in a patient with cardiac sarcoidosis. Circulation. 2002 Mar 5:105(9):1144-5 [PubMed PMID: 11877369]
Level 3 (low-level) evidenceDubey N, Miletich RS, Wasay M, Mechtler LL, Bakshi R. Role of fluorodeoxyglucose positron emission tomography in the diagnosis of neurosarcoidosis. Journal of the neurological sciences. 2002 Dec 15:205(1):77-81 [PubMed PMID: 12409188]
Level 3 (low-level) evidenceGilman MJ, Wang KP. Transbronchial lung biopsy in sarcoidosis. An approach to determine the optimal number of biopsies. The American review of respiratory disease. 1980 Nov:122(5):721-4 [PubMed PMID: 7447156]
Trisolini R, Lazzari Agli L, Cancellieri A, Poletti V, Candoli P, Paioli D, Alifano M, Tinelli C, Patelli M. Transbronchial needle aspiration improves the diagnostic yield of bronchoscopy in sarcoidosis. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2004 Jun:21(2):147-51 [PubMed PMID: 15281436]
Level 2 (mid-level) evidenceTrisolini R, Lazzari Agli L, Cancellieri A, Poletti V, Tinelli C, Baruzzi G, Patelli M. The value of flexible transbronchial needle aspiration in the diagnosis of stage I sarcoidosis. Chest. 2003 Dec:124(6):2126-30 [PubMed PMID: 14665490]
James DG, Williams WJ. Kveim-Siltzbach test revisited. Sarcoidosis. 1991 Mar:8(1):6-9 [PubMed PMID: 1669943]
Mikhail JR, Mitchell DN. The Kveim test in sarcoidosis. Postgraduate medical journal. 1970 Aug:46(538):484-5 [PubMed PMID: 5481096]
Newman LS, Rose CS, Maier LA. Sarcoidosis. The New England journal of medicine. 1997 Apr 24:336(17):1224-34 [PubMed PMID: 9110911]
Fels C, Riegel A, Javaheripour-Otto K, Obenauer S. Neurosarcoidosis: findings in MRI. Clinical imaging. 2004 May-Jun:28(3):166-9 [PubMed PMID: 15158219]
Level 2 (mid-level) evidenceSheppard JD, Toyos MM, Kempen JH, Kaur P, Foster CS. Difluprednate 0.05% versus prednisolone acetate 1% for endogenous anterior uveitis: a phase III, multicenter, randomized study. Investigative ophthalmology & visual science. 2014 May 6:55(5):2993-3002. doi: 10.1167/iovs.13-12660. Epub 2014 May 6 [PubMed PMID: 24677110]
Level 1 (high-level) evidenceKok H, Lau C, Maycock N, McCluskey P, Lightman S. Outcome of intravitreal triamcinolone in uveitis. Ophthalmology. 2005 Nov:112(11):1916.e1-7 [PubMed PMID: 16171868]
Level 2 (mid-level) evidenceKhurana RN, Porco TC. EFFICACY AND SAFETY OF DEXAMETHASONE INTRAVITREAL IMPLANT FOR PERSISTENT UVEITIC CYSTOID MACULAR EDEMA. Retina (Philadelphia, Pa.). 2015 Aug:35(8):1640-6. doi: 10.1097/IAE.0000000000000515. Epub [PubMed PMID: 25741813]
Level 2 (mid-level) evidenceJaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T, Fluocinolone Acetonide Uveitis Study Group. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006 Jun:113(6):1020-7 [PubMed PMID: 16690128]
Level 1 (high-level) evidencePavesio C, Zierhut M, Bairi K, Comstock TL, Usner DW, Fluocinolone Acetonide Study Group. Evaluation of an intravitreal fluocinolone acetonide implant versus standard systemic therapy in noninfectious posterior uveitis. Ophthalmology. 2010 Mar:117(3):567-75, 575.e1. doi: 10.1016/j.ophtha.2009.11.027. Epub 2010 Jan 15 [PubMed PMID: 20079922]
Level 1 (high-level) evidenceGangaputra S, Newcomb CW, Liesegang TL, Kaçmaz RO, Jabs DA, Levy-Clarke GA, Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE, Foster CS, Kempen JH, Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study. Methotrexate for ocular inflammatory diseases. Ophthalmology. 2009 Nov:116(11):2188-98.e1. doi: 10.1016/j.ophtha.2009.04.020. Epub 2009 Sep 12 [PubMed PMID: 19748676]
Level 2 (mid-level) evidenceKaçmaz RO, Kempen JH, Newcomb C, Daniel E, Gangaputra S, Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE, Jabs DA, Levy-Clarke GA, Foster CS. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010 Mar:117(3):576-84. doi: 10.1016/j.ophtha.2009.08.010. Epub 2010 Jan 19 [PubMed PMID: 20031223]
Level 2 (mid-level) evidencePasadhika S, Kempen JH, Newcomb CW, Liesegang TL, Pujari SS, Rosenbaum JT, Thorne JE, Foster CS, Jabs DA, Levy-Clarke GA, Nussenblatt RB, Suhler EB. Azathioprine for ocular inflammatory diseases. American journal of ophthalmology. 2009 Oct:148(4):500-509.e2. doi: 10.1016/j.ajo.2009.05.008. Epub 2009 Jul 1 [PubMed PMID: 19570522]
Level 2 (mid-level) evidenceKane SV. Preparing for biologic or immunosuppressant therapy. Gastroenterology & hepatology. 2011 Aug:7(8):544-6 [PubMed PMID: 22298992]
Daïen CI, Monnier A, Claudepierre P, Constantin A, Eschard JP, Houvenagel E, Samimi M, Pavy S, Pertuiset E, Toussirot E, Combe B, Morel J, Club Rhumatismes et Inflammation (CRI). Sarcoid-like granulomatosis in patients treated with tumor necrosis factor blockers: 10 cases. Rheumatology (Oxford, England). 2009 Aug:48(8):883-6. doi: 10.1093/rheumatology/kep046. Epub 2009 May 7 [PubMed PMID: 19423648]
Level 3 (low-level) evidenceAkpek EK, Ilhan-Sarac O, Green WR. Topical cyclosporin in the treatment of chronic sarcoidosis of the conjunctiva. Archives of ophthalmology (Chicago, Ill. : 1960). 2003 Sep:121(9):1333-5 [PubMed PMID: 12963621]
Level 3 (low-level) evidenceCordero Coma M, Sobrin L, Onal S, Christen W, Foster CS. Intravitreal bevacizumab for treatment of uveitic macular edema. Ophthalmology. 2007 Aug:114(8):1574-1579.e1 [PubMed PMID: 17363060]
Acharya NR, Hong KC, Lee SM. Ranibizumab for refractory uveitis-related macular edema. American journal of ophthalmology. 2009 Aug:148(2):303-309.e2. doi: 10.1016/j.ajo.2009.03.028. Epub 2009 May 9 [PubMed PMID: 19427988]
Geetha R, Tripathy K. Chorioretinitis. StatPearls. 2024 Jan:(): [PubMed PMID: 31869169]
Shukla UV, Tripathy K. Diabetic Retinopathy. StatPearls. 2024 Jan:(): [PubMed PMID: 32809640]
Raizada K, Tripathy K. Eales Disease. StatPearls. 2022 Jan:(): [PubMed PMID: 32644547]
Levy A, Hamzeh N, Maier LA. Is it time to scrap Scadding and adopt computed tomography for initial evaluation of sarcoidosis? F1000Research. 2018:7():. pii: F1000 Faculty Rev-600. doi: 10.12688/f1000research.11068.1. Epub 2018 May 16 [PubMed PMID: 29946423]
Miller BH, Rosado-de-Christenson ML, McAdams HP, Fishback NF. Thoracic sarcoidosis: radiologic-pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 1995 Mar:15(2):421-37 [PubMed PMID: 7761646]
Karma A, Huhti E, Poukkula A. Course and outcome of ocular sarcoidosis. American journal of ophthalmology. 1988 Oct 15:106(4):467-72 [PubMed PMID: 3177566]
Sato H, Grutters JC, Pantelidis P, Mizzon AN, Ahmad T, Van Houte AJ, Lammers JW, Van Den Bosch JM, Welsh KI, Du Bois RM. HLA-DQB1*0201: a marker for good prognosis in British and Dutch patients with sarcoidosis. American journal of respiratory cell and molecular biology. 2002 Oct:27(4):406-12 [PubMed PMID: 12356573]
Kobak S. Catch the rainbow: Prognostic factor of sarcoidosis. Lung India : official organ of Indian Chest Society. 2020 Sep-Oct:37(5):425-432. doi: 10.4103/lungindia.lungindia_380_19. Epub [PubMed PMID: 32883904]
Tripathy K, Chawla R, Temkar S, Sagar P, Kashyap S, Pushker N, Sharma YR. Phthisis Bulbi-a Clinicopathological Perspective. Seminars in ophthalmology. 2018:33(6):788-803. doi: 10.1080/08820538.2018.1477966. Epub 2018 Jun 14 [PubMed PMID: 29902388]
Level 3 (low-level) evidenceMatsou A, Tsaousis KT. Management of chronic ocular sarcoidosis: challenges and solutions. Clinical ophthalmology (Auckland, N.Z.). 2018:12():519-532. doi: 10.2147/OPTH.S128949. Epub 2018 Mar 19 [PubMed PMID: 29593377]
Edelsten C, Pearson A, Joynes E, Stanford MR, Graham EM. The ocular and systemic prognosis of patients presenting with sarcoid uveitis. Eye (London, England). 1999 Dec:13 ( Pt 6)():748-53 [PubMed PMID: 10707138]