Introduction
Sarcocystis species are intracellular protozoan parasites. They were first reported by Miescher in 1843 as white threadlike cysts in the striated muscles of a house mouse, and they were referred to as Miescher tubules for the next 20 years. For many decades, it was unclear whether they were protozoa or fungi. Similar structures were found in 1865 in pig muscle. Subsequently, in 1967, these spindle or crescent-shaped bodies were studied under electron microscopy, and organelles were observed like those in apicomplexan protozoans of Toxoplasma and Eimeria.[1]
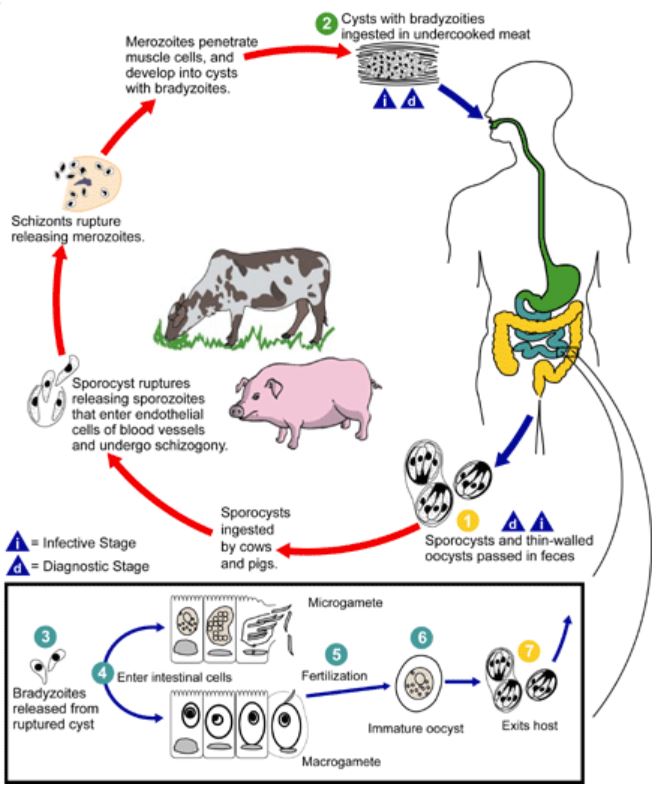
Sarcocystis species have a heteroxenous (ie, more than 1 obligatory host) life cycle based on a prey-predator host relationship of definitive and intermediate hosts, identified in 1972.[2] Over the years, about 150 symptomatic human cases have been reported, with more than 100 Sarcocystis spp known; most have been isolated from muscle tissues of various intermediate hosts, including mammals, birds, and reptiles.[3] See Image. Life Cycle.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
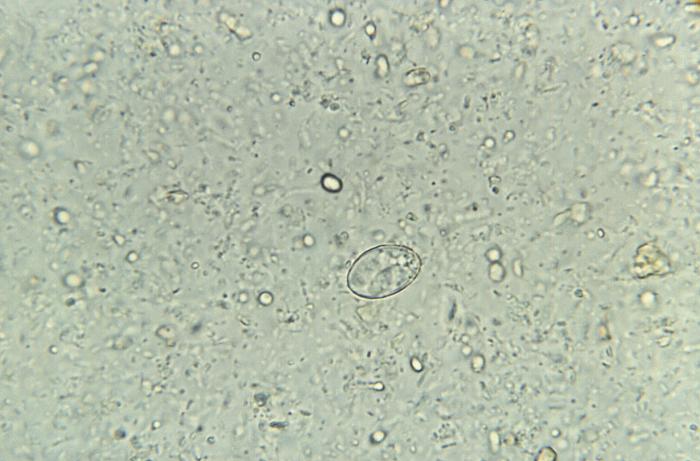
Sarcocystosis caused by Sarcocystis species can occur in 2 forms in humans: intestinal and muscular. Humans can be either definitive or intermediate hosts. Sarcocystis hominis (in cattle) and Sarcocystis suihominis (in pigs) use humans as definitive hosts and are known to cause intestinal Sarcocystosis. The definite host in some nonhuman species like Sarcocystis nesbitti is presumed to be reptiles, and humans acquire by ingestion of sporocysts through contaminated food and water. Undocumented species where humans can serve as a definitive host may be possible with the meat of domesticated animals, birds, wild animals, and birds being eaten worldwide.[1][4][5] See Image. Sporocyst of the Parasite, Sarcocystis Hominis.
Epidemiology
Sarcocystis species that infect humans are widely distributed across the world. Muscular sarcocystosis has been reported, with the highest incidence seen in Asia and Southeast Asia.[6] Sporadic cases from Europe, Africa, North America, Central America, and South America have also been reported.[1] A serology-based epidemiological survey in West Malaysia found Sarcocystis antibodies in 19.7% of the surveyed population.[7]
Intestinal sarcocystosis has been reported in all regions except Africa and the Middle East, with human cases being more common in Europe compared to other continents.[1] Underdetection and underreporting are more likely as many cases can be asymptomatic. Signs and symptoms are usually limited to acute and rarely chronic gastrointestinal symptoms, which can be easily misdiagnosed.[8] Prevalence of Sarcocystis spp was found in 10.4% and 7.3% of fecal samples in children in Poland and Germany, respectively. Cases have also been found in most countries, including Vietnam, Slovakia, Spain, Tibet, Cambodia, Iran, and Australia.[9][10][11][12][13]
Pathophysiology
In the infective form, sporocysts rupture to release sporozoites when ingested by the intermediate host (cattle and pigs). The sporozoites enter the endothelium of blood vessels to undergo schizogony and form first-generation schizonts. These further develop into merozoites and invade small capillaries and blood vessels to become second-generation schizonts. These merozoites invade the muscle cells to form sarcocysts made up of bradyzoites. After consuming undercooked meat containing these sarcocysts, bradyzoites are released in the small intestine of humans, invading the intestinal epithelium, where they differentiate into macro- and microgametocytes. Fusing these gametes results in oocyst formation, which is shed in the feces. Humans may also become dead-end or accidental hosts for many nonhuman Sarcocystis spp after the accidental ingestion of oocysts. The sporozoites ex-cyst and schizonts develop in the vascular endothelium of blood vessels, following which merozoites invade the muscle tissue, causing muscular sarcocystosis.[4][8]
Histopathology
Most sporocysts of various species measure 10 by 15 µm and contain 4 sporozoites and a residual body. The overall size, presence, or absence of septa and ultrastructural morphology of the wall are some of these features that help identify. However, it varies with the host cell type, the sarcocysts, and the fixation method. The intact oocysts appear as 2 adjacent sporocysts with a thin oocyst wall that often breaks to release the sporocysts, the most commonly found structures in the feces.[1]
The histopathological diagnosis is based on the part affected. Most sarcocysts in humans have been found in skeletal and cardiac muscle. Muscle biopsies show sarcocysts within the muscle fibers. Sarcocysts of Sarcocystis hominis in cattle are microscopic, whereas those of Sarcocystis suihominis in pigs are macroscopic. Hematoxylin-and-eosin stain can be used to detect sarcocysts in the muscles of the intermediate host. The walls of sarcocysts are positively stained by periodic acid-Schiff reactions.[1][8] Many case reports have shown no inflammatory reaction in the adjacent muscle tissue surrounding the sarcocysts with some eosinophilic infiltration. Most infections go undetected due to the disease's asymptomatic nature and the parasite's encysted nature.[14][15]
History and Physical
A history of consuming undercooked or raw pork and beef should raise suspicion of sarcocystosis. Other meat from domesticated or wild mammals, birds, or reptiles can potentially cause Sarcocystis infection if eaten undercooked.[4] Most Sarcocystis infections tend to be asymptomatic in humans.[4] In symptomatic disease, the infection can present as 2 distinct syndromes or a combination. Muscular sarcocystosis can present with fever, musculoskeletal pain, rashes, bronchospasms, cardiomyopathy, or subcutaneous swelling.[1] Intestinal sarcocystosis can present with nausea, anorexia, vomiting, abdominal pain, bloating, or diarrhea.[1] The physical examination can show lymphadenopathy, hepatomegaly, or tender muscles in case of disseminated disease or may be expected in localized muscular or intestinal disease.[3]
Evaluation
Blood investigations can show lymphocytosis or eosinophilia.[3][16] There have been reports of raised alanine aminotransferase, lactate dehydrogenase, slightly elevated C-reactive protein, and erythrocyte sedimentation rate.[8] In muscular disease, elevated creatine kinase or lactate dehydrogenase may be seen.[3][17] A definitive diagnosis may be made based on a history of gastroenteritis symptoms and epidemiological habits such as raw or undercooked meat consumption. Oocyst or sporocyst excretion happens during a small window. These pre-patent periods are 14 to 18 days for S hominis and 11 to 13 days for S superminis. Oocyst/sporocyst excretion may last for around a month.[18]
Fecal studies can include different flotation techniques, modified Kato thick smear, formalin-ether technique, or direct smear.[1][19] Both oocysts and sporocysts can be detected in fecal samples from definitive hosts using stains such as periodic acid-Schiff stain.[18] The mature sarcocysts in each species tend to vary in size, length, and circumference with varying thicknesses of the sarcocyst wall, but all contain numerous bradyzoites. Muscle biopsy light microscopy can show the sarcocyst in muscular sarcocystosis, which may or may not show bradyzoites.[1] Imaging, including magnetic resonance imaging, may help detect muscle cysts and direct biopsies.[20]
Accurate and sensitive diagnostic tests do not currently exist for Sarcocyst infections. Immunofluorescence assays, enzyme-linked immunosorbent assays, and serological methods such as antibodies to Sarcocystis have been tried but are not widely available.[18] Although it is known that human infection in susceptible individuals elicits a strong eosinophilic and T-cell–mediated response due to a limited number of cases and the complex host-parasite interplay, little is known about the immunology of the infection. Immunoglobulin G detection is not reliable in assessing any seroconversion.[8]
Treatment / Management
Intestinal Sarcocystosis
There are no effective prophylaxis or therapeutic options for intestinal sarcocystosis in either humans or animals.[4] Current recommendations are based on a handful of case reports and series highlighting the various treatment options that have been tried with varying degrees of success. Dithiazanine was tested in a patient, but Sarcocystis persisted.[21] Pyrimethamine with sulfisoxazole was also not effective.[22] Acetylspiramycin did not show adequate response either.[4] In some instances, there have been reports of resection surgery of the affected ileum.[23](B3)
Muscular Sarcocystosis
Albendazole therapy was trialed with steroid cover for muscular sarcocystosis, which was ineffective.[24] The role of cotrimoxazole remains unclear as there have been reports of improvement in symptoms and the absence of elevation of creatine kinase levels. Corticosteroids were demonstrated to ameliorate myositis.[17] There is uncertainty about whether immunosuppressive therapy for vasculitis or myositis reduces the severity of the inflammation or facilitates parasite proliferation.[1] In vitro study results have demonstrated that pyrimethamine and trimethoprim demonstrated activity against Sarcocystis, but no well-conducted clinical studies have been conducted.[25] Currently, no course of treatment can be recommended as superior or better than the other due to the lack of controlled studies and insufficient evidence of reported treatment protocols and clinical response.[1](B3)
Differential Diagnosis
Sarcocystis may occasionally be misidentified as other cyst-forming coccidian parasites such as Toxoplasma or Neospora.[26] Other conditions that can be differentials for muscular sarcocystosis include autoimmune myositis, infective myositis due to leptospirosis, toxocariasis, trichinellosis, rickettsial disease, and toxoplasmosis, drugs including alcohol, cocaine, antimalarials, penicillamine, statin, toxin-induced myositis, including Haff disease.[3]
Prognosis
Infections are usually self-limiting, although long-lasting.[26] Most cases are incidentally found where humans acted as intermediate hosts of unidentified Sarcocystis spp.[27] Occasionally, there can be a disseminated disease that can cause significant morbidity.[3]
Complications
Segmental eosinophilic enteritis or necrotizing enteritis may be seen in intestinal sarcocystosis.[23] Some cases of muscular sarcocystosis can develop into severe myositis.[17] Very rarely, there can be a disseminated disease that can affect multiple organs.[3]
Consultations
Consultations in cases of suspected sarcocystosis should involve the microbiologist or tropical medicine clinician trained in parasitology to identify the oocysts and sarcocysts in feces samples. In muscular sarcocystosis, a pathologist can determine the cyst with bradyzoites in the histopathology of a muscle biopsy. Treatment should be tailored and patient-specific without standardized treatment regimens, and infectious diseases or tropical medicine clinicians may be consulted. Gastroenterologists and rheumatologists should keep the possibility of sarcocystosis in mind when treating gastroenteritis and myositis in patients with an unclear etiology.
Deterrence and Patient Education
Prevention of infection remains the best option in the absence of effective prophylaxis and therapeutic options. Patient education should be carried out to ensure thorough cooking or freezing of meat to kill the bradyzoites in the sarcocysts. Complete cooking renders bradyzoites noninfectious.[4] Ensuring clean drinking water is important to avoid exposure to sporocysts. Boiling provides disinfection, and chemical disinfection using chlorine or other similar agents is ineffective in killing sporocysts.[28] Controlling the parasite among the animal population that patients frequently come in contact with is essential to preventing the infection.
Pearls and Other Issues
Key facts to keep in mind about Sarcocystosis are as follows:
- Sarcocystosis is considered a zoonotic disease with a complex interplay between animals and humans.
- Only 2 species, including S hominis and S. suihominis, have humans as definitive hosts in their life cycles.
- There may be multiple species that have humans as an intermediate host or as a dead-end host.
- Sarcocystosis is also considered an emerging disease, which, coupled with the zoonotic aspects of the disease, highlights the importance of a 1-health approach to treating, controlling, and preventing the disease.[29]
Enhancing Healthcare Team Outcomes
For early diagnosis, an interprofessional team approach is required for sarcocystosis to improve clinical outcomes. The interprofessional team can involve clinicians, pathologists, public health practitioners, and microbiologists. Given the global prevalence, a high index of suspicion is required in patients presenting with gastroenteritis or myositis. Increased education and awareness regarding disease prevention remain the most effective steps in managing this infection. Infection control should also involve attempts to eradicate the parasite from the animal population in domestic animals and other animal populations, such as pets that patients frequently encounter. Hence, a 1-health approach is paramount in combating this infection.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Fayer R. Sarcocystis spp. in human infections. Clinical microbiology reviews. 2004 Oct:17(4):894-902, table of contents [PubMed PMID: 15489353]
Level 3 (low-level) evidenceMarkus MB, Killick-Kendrick R, Garnham PC. The coccidial nature and life-cycle of Sarcocystis. The Journal of tropical medicine and hygiene. 1974 Nov:77(11):248-59 [PubMed PMID: 4219030]
Level 3 (low-level) evidenceMohammad N, Besari AM, Nair PK, Wan Ghazali WS. Muscular sarcocystosis: an index case in a native Malaysian. BMJ case reports. 2017 Jul 26:2017():. pii: bcr-2017-220490. doi: 10.1136/bcr-2017-220490. Epub 2017 Jul 26 [PubMed PMID: 28747414]
Level 3 (low-level) evidenceFayer R, Esposito DH, Dubey JP. Human infections with Sarcocystis species. Clinical microbiology reviews. 2015 Apr:28(2):295-311. doi: 10.1128/CMR.00113-14. Epub [PubMed PMID: 25715644]
Level 3 (low-level) evidenceBeaver PC, Gadgil K, Morera P. Sarcocystis in man: a review and report of five cases. The American journal of tropical medicine and hygiene. 1979 Sep:28(5):819-44 [PubMed PMID: 114067]
Level 3 (low-level) evidenceWong KT, Leggett PF, Heatley M. Apparent absence of Sarcocystis infection in human tongue and diaphragm in Northern Ireland. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1993 Jul-Aug:87(4):496 [PubMed PMID: 8249098]
Level 3 (low-level) evidenceThomas V, Dissanaike AS. Antibodies to Sarcocystis in Malaysians. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1978:72(3):303-6 [PubMed PMID: 97821]
Harris VC, van Vugt M, Aronica E, de Bree GJ, Stijnis C, Goorhuis A, Grobusch MP. Human Extraintestinal Sarcocystosis: What We Know, and What We don't Know. Current infectious disease reports. 2015 Aug:17(8):495. doi: 10.1007/s11908-015-0495-4. Epub [PubMed PMID: 26115699]
Clavel A, Doiz O, Varea M, Morales S, Castillo FJ, Rubio MC, Gómez-Lus R. [Abdominal discomfort and soft stools in a habitual consumer of rare beef]. Enfermedades infecciosas y microbiologia clinica. 2001 Jan:19(1):29-30 [PubMed PMID: 11256244]
Level 3 (low-level) evidenceYu S. [Field survey of sarcocystis infection in the Tibet autonomous region]. Zhongguo yi xue ke xue yuan xue bao. Acta Academiae Medicinae Sinicae. 1991 Feb:13(1):29-32 [PubMed PMID: 1831698]
Level 3 (low-level) evidenceKhieu V, Marti H, Chhay S, Char MC, Muth S, Odermatt P. First report of human intestinal sarcocystosis in Cambodia. Parasitology international. 2017 Oct:66(5):560-562. doi: 10.1016/j.parint.2017.04.010. Epub 2017 May 3 [PubMed PMID: 28476340]
Agholi M, Taghadosi Z, Mehrabani D, Zahabiun F, Sharafi Z, Motazedian MH, Hatam GR, Naderi Shahabadi S. Human intestinal sarcocystosis in Iran: there but not seen. Parasitology research. 2016 Dec:115(12):4527-4533 [PubMed PMID: 27637226]
Meloni BP, Thompson RC, Hopkins RM, Reynoldson JA, Gracey M. The prevalence of Giardia and other intestinal parasites in children, dogs and cats from aboriginal communities in the Kimberley. The Medical journal of Australia. 1993 Feb 1:158(3):157-9 [PubMed PMID: 8450779]
Level 3 (low-level) evidenceMakhija M. Histological identification of muscular sarcocystis: a report of two cases. Indian journal of pathology & microbiology. 2012 Oct-Dec:55(4):552-4. doi: 10.4103/0377-4929.107813. Epub [PubMed PMID: 23455804]
Level 3 (low-level) evidenceAgarwal PK, Srivastava AN. Sarcocystosis in man: a report of two cases. Histopathology. 1983 Sep:7(5):783-7 [PubMed PMID: 6414925]
Level 3 (low-level) evidenceChen X, Zuo Y, Zuo W. [Observation on the clinical symptoms and sporocyst excretion in human volunteers experimentally infected with Sarcocystis hominis]. Zhongguo ji sheng chong xue yu ji sheng chong bing za zhi = Chinese journal of parasitology & parasitic diseases. 1999:17(1):25-7 [PubMed PMID: 12563811]
Tappe D, Stich A, Langeheinecke A, von Sonnenburg F, Muntau B, Schäfer J, Slesak G. Suspected new wave of muscular sarcocystosis in travellers returning from Tioman Island, Malaysia, May 2014. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2014 May 29:19(21):. pii: 20816. Epub 2014 May 29 [PubMed PMID: 24906376]
Poulsen CS, Stensvold CR. Current status of epidemiology and diagnosis of human sarcocystosis. Journal of clinical microbiology. 2014 Oct:52(10):3524-30. doi: 10.1128/JCM.00955-14. Epub 2014 Apr 23 [PubMed PMID: 24759707]
Tungtrongchitr A, Chiworaporn C, Praewanich R, Radomyos P, Boitano JJ. The potential usefulness of the modified Kato thick smear technique in the detection of intestinal sarcocystosis during field surveys. The Southeast Asian journal of tropical medicine and public health. 2007 Mar:38(2):232-8 [PubMed PMID: 17539271]
Level 3 (low-level) evidenceItaliano CM, Wong KT, AbuBakar S, Lau YL, Ramli N, Syed Omar SF, Kahar Bador M, Tan CT. Sarcocystis nesbitti causes acute, relapsing febrile myositis with a high attack rate: description of a large outbreak of muscular sarcocystosis in Pangkor Island, Malaysia, 2012. PLoS neglected tropical diseases. 2014 May:8(5):e2876. doi: 10.1371/journal.pntd.0002876. Epub 2014 May 22 [PubMed PMID: 24854350]
LAARMAN JJ. Isospora hominis (Railliet and Lucet 1891) in the Netherlands. Acta Leidensia. 1962:31():111-6 [PubMed PMID: 13927789]
Giboda M, Rakár J. First record of "Isospora hominis" in Czechoslovakia. Folia parasitologica. 1978:25(1):16 [PubMed PMID: 640517]
Level 3 (low-level) evidenceBunyaratvej S, Bunyawongwiroj P, Nitiyanant P. Human intestinal sarcosporidiosis: report of six cases. The American journal of tropical medicine and hygiene. 1982 Jan:31(1):36-41 [PubMed PMID: 6800273]
Level 3 (low-level) evidenceEsposito DH, Stich A, Epelboin L, Malvy D, Han PV, Bottieau E, da Silva A, Zanger P, Slesak G, van Genderen PJ, Rosenthal BM, Cramer JP, Visser LG, Muñoz J, Drew CP, Goldsmith CS, Steiner F, Wagner N, Grobusch MP, Plier DA, Tappe D, Sotir MJ, Brown C, Brunette GW, Fayer R, von Sonnenburg F, Neumayr A, Kozarsky PE, Tioman Island Sarcocystosis Investigation Team. Acute muscular sarcocystosis: an international investigation among ill travelers returning from Tioman Island, Malaysia, 2011-2012. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014 Nov 15:59(10):1401-10. doi: 10.1093/cid/ciu622. Epub 2014 Aug 4 [PubMed PMID: 25091309]
Lindsay DS, Dubey JP. Determination of the activity of pyrimethamine, trimethoprim, sulfonamides, and combinations of pyrimethamine and sulfonamides against Sarcocystis neurona in cell cultures. Veterinary parasitology. 1999 Apr 12:82(3):205-10 [PubMed PMID: 10348099]
Level 3 (low-level) evidenceGottstein B. [Cyst-forming Coccidia: Toxoplasma, Neospora, Sarcocystis]. Schweizerische medizinische Wochenschrift. 1995 May 6:125(18):890-8 [PubMed PMID: 7770750]
Level 3 (low-level) evidencePathmanathan R, Kan SP. Three cases of human Sarcocystis infection with a review of human muscular sarcocystosis in Malaysia. Tropical and geographical medicine. 1992 Jan:44(1-2):102-8 [PubMed PMID: 1496700]
Level 3 (low-level) evidenceDubey JP, Saville WJ, Sreekumar C, Shen SK, Lindsay OS, Pena HF, Vianna MC, Gennari SM, Reed SM. Effects of high temperature and disinfectants on the viability of Sarcocystis neurona sporocysts. The Journal of parasitology. 2002 Dec:88(6):1252-4 [PubMed PMID: 12537123]
Level 3 (low-level) evidenceChhabra MB, Samantaray S. Sarcocystis and sarcocystosis in India: status and emerging perspectives. Journal of parasitic diseases : official organ of the Indian Society for Parasitology. 2013 Apr:37(1):1-10. doi: 10.1007/s12639-012-0135-y. Epub 2012 Aug 17 [PubMed PMID: 24431532]
Level 3 (low-level) evidence