Introduction
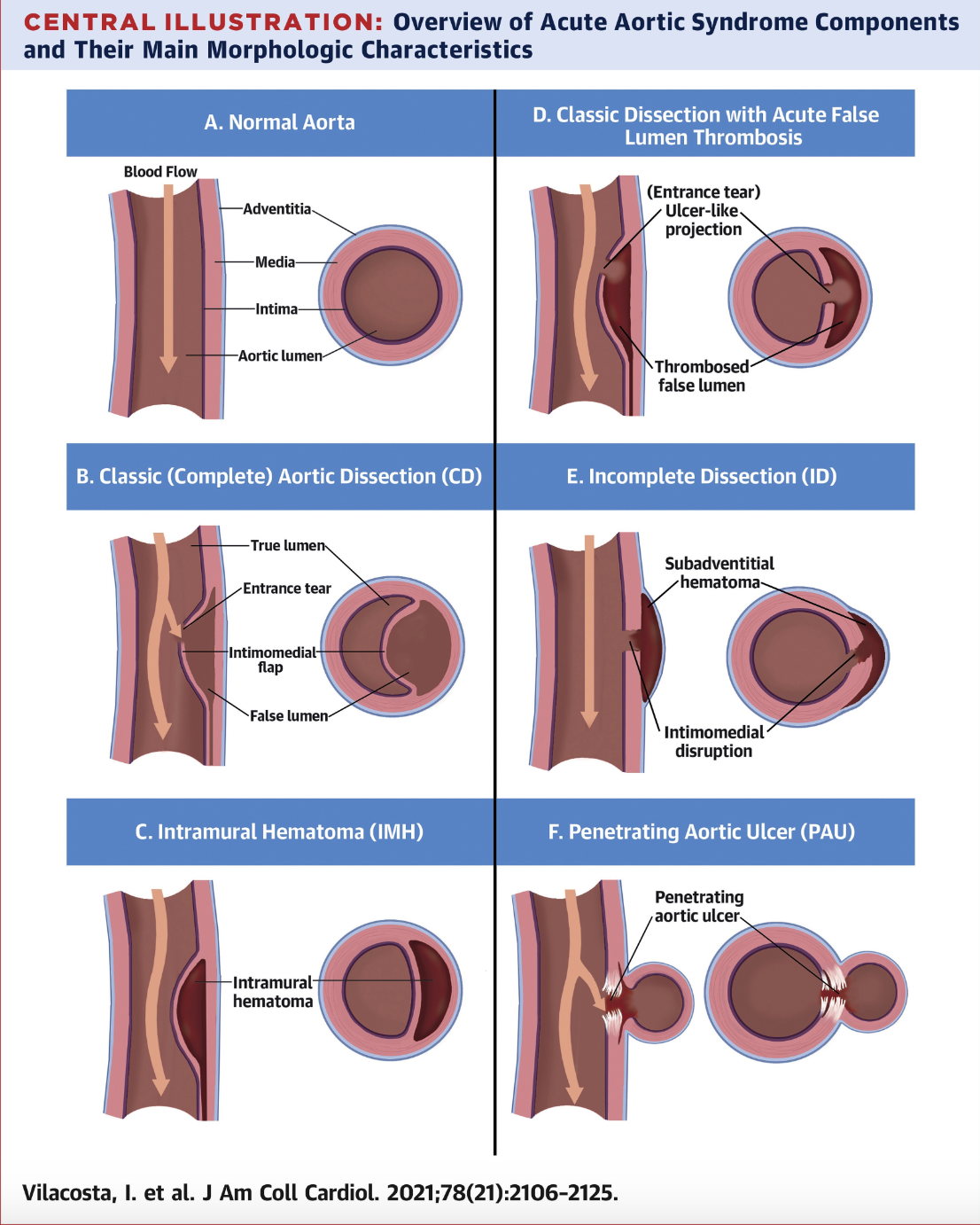
The term "acute aortic syndrome" (AAS) was first used in 1998 by Vilacosta et al. The term denoted a collection of painful and life-threatening aortic conditions, including acute aortic dissection (AAD), intramural hematoma (IMH), and penetrating aortic ulcers (PAU). (see Image. Acute Aortic Syndromes) Despite the overlapping signs and symptoms of these syndromes, AAD, IMH, and PAU are individual entities that can progress from one to the other or coexist at presentation, with approximately 12% of patients with AAD also having IMH or PAU.[1]
From a surgical and prognostic perspective, patients with AAS can be divided into 2 categories based on whether the ascending aorta is involved (Stanford type A) or not (Stanford type B).[2] Early AAS recognition and diagnosis are paramount due to the time-critical nature of the disease, with type A aortic dissection displaying up to a 2% per hour increase in mortality during the first 48 hours.[3] IMH is traditionally defined as a "dissection without an intimal tear," meaning it represents a noncommunicating form of dissection.[4] PAU is characterized by the ulceration of an atherosclerotic plaque that extends through the internal elastic lamina into the media layer of the aorta.[5] The clinical progression of patients with PAU varies. Many remain asymptomatic, but some develop AAS.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
AAS comprises 3 distinct pathologies that can present similarly, with overlapping etiologies and predisposing factors.[6] The etiologies of these conditions are discussed below.
Acute Aortic Dissection
AAD makes up 70% to 80% of all AAS. This condition arises from a tear in the innermost layer of the aorta and is commonly heralded by existing cystic medial necrosis or medial degeneration.[7] This pathology, combined with continuous exposure to raised blood pressure and shearing forces in a predisposed patient, can lead to a tear in the intimomedial layer, creating a flap. Blood enters this false lumen and, under pressure, causes dissection of the intimomedial layer from the outer wall of the aorta.
Fenestrations can form distal to the initial tear, creating a communicating passage between true and false lumens. The dissection can progress proximally and distally from the site of the initial tear. AAS type A is any dissection involving the ascending aorta. Recently, the Society for Vascular Surgery and the Society of Thoracic Surgeons have defined type B AAS as any dissection where the entry tear occurs distal to the origin of the innominate artery.[8]
Intramural Hematoma
Discussion regarding the exact cause of IMH is ongoing. The most widely accepted theory is the spontaneous rupture of the vasa vasorum, the blood vessels that supply the outer layers of veins and arteries.[9] Another explanation is thrombosis between aortic wall layers that occurs due to stagnant blood after a dissection or microtear in the intima without a reentrant tear.[10] Both AAD and IMH can develop secondary to trauma from vascular catheter insertion.
Penetrating Aortic Ulcer
PAU involves atherosclerotic plaque formation, which leads to destruction and inflammation of the intima and penetrates outwardly through the layers of the aortic wall. PAU can either remain stable or progress to AAD or IMH. PAU may result in aortic rupture or visceral ischemia in severe cases.[11]
Common predisposing factors for AAS include:
- Hypertension
- Smoking
- Hyperlipidemia
- Cocaine use
- Connective tissue disorders such as Marfan syndrome, Ehlers-Danlos syndrome, and Turner syndrome
- Congenital cardiovascular anomalies such as bicuspid aortic valve and coarctation of the aorta
- Vascular inflammation from autoimmune causes, eg, giant cell arteritis, or infection, eg, syphilis
- Trauma, particularly from acceleration-deceleration injuries
- Iatrogenic damage, such as from catheter insertion and valvular or aortic surgery [12]
Epidemiology
Existing data sets suggest that the incidence of AAD is between 2.6 and 7.2 per 100,000 patient-years, with data mostly coming from Western countries. Up to 65% of patients are male, with modal presentation in the 7th decade of life.[13][14] Predisposing factors differ between adults older and younger than 70, with the older population more commonly presenting with hypertension, atherosclerosis, and iatrogenic causes such as cardiac catheterization. Younger patients are more likely to have a congenital pathology, including connective tissue disorders such as Marfan Syndrome, bicuspid aortic valves, and prenatal cocaine exposure.
Patients with IMH share the same risk factors as those with AAD, but the typical age at presentation is in the 80s. IMH constitutes 5% to 25% of AAS cases, with studies averaging 1.2 cases per 100,000 patient years. PAU comprises 2% to 7% of AAS cases, with figures averaging 2.1 cases per 100,000 person-years. PAU is most commonly associated with widespread, severe atherosclerosis and often presents multiple ulcerating lesions throughout the aorta. Most patients also suffer from hypertension and coronary artery disease, and some studies show up to 68% of patients also have chronic obstructive pulmonary disease. Between 42% and 61% of patients have concurrent aortic aneurysms.[15]
Pathophysiology
As mentioned, the most common AAS precursors are atherosclerotic disease and hypertension. Besides these conditions, trauma, infective processes, and any media pathology can also lead to AAS. Intimal tearing from blood flow's shearing forces or interference with the media separates the aortic wall layers.[16] This separation can create a true and a false lumen. The true lumen is lined by intima, connects undissected aortic segments, and is separated from the false lumen by an "intimal flap." Blood flowing through a false lumen over time can form an aortic aneurysm.[17]
IMH is traditionally thought to arise from the rupture of the vasa vasorum. However, recent pathological studies suggest the presence of microintimal tears in diagnosed IMH cases, implying that IMH and AAD may have common pathophysiological mechanisms. The difference is the lack of a large enough reentrant tear to preserve the false lumen's patency.
PAU develops as atherosclerotic plaque invades through the intimal layer toward the adventitia. PAU may progress to IMH, AAD, or a pseudoaneurysm.
Histopathology
The most common histopathological feature in AAD is the degeneration of the medial layer. Fundamental degenerative changes include fragmentation, thinning of elastic fibers, and accumulation of mucoid extracellular matrix. Additionally, a notably thin intimal layer and a dissection plane often found at the level of the vasa vasorum network in the outer media have been observed.[18] Patients with connective tissue disorders or aortic aneurysms tend to exhibit more severe medial degeneration. However, medial degeneration is considered the final common pathway for various forms of AAS.
The discussion surrounding the pathophysiology of IMH should not detract from its key feature: the restricted blood flow within the aortic wall, leading to its distinctive morphology and progression over time. IMH can resolve spontaneously or progress to AAD. Histologically, the hematoma extends within the media. Between AAD and IMH, the latter is more strongly associated with atherosclerotic lesions. While AAD typically results from an intimal tear, IMH is thought to arise from the rupture of the vasa vasorum, leading to intramural hemorrhage.[19] However, both conditions share a common mechanism in some cases, with IMH potentially representing an AAD with an acutely thrombosed false lumen. Studies showing a high incidence of tears or communicating points in presumed IMH cases support this shared mechanism theory.[20]
PAUs typically develop in the context of severe, widespread atherosclerotic disease and are most often found in the descending thoracic aorta. PAUs are frequently accompanied by localized intramedial bleeding, which may spread locally or, in rare cases, lead to an aortic dissection, with the ulcer's "crater" acting as the entry tear. Wall calcification and inflammation are believed to limit the extent of hemorrhage, resulting in a more localized form of AAD. PAUs penetrate the adventitia in some cases, causing either an aortic rupture or pseudoaneurysm formation, leading to aortic wall bulging.
History and Physical
A classic symptom of AAS is severe aortic pain, which is a chest or back pain commonly described as "tearing," "ripping," "migrating," or "pulsating."[21] The International Registry of Acute Aortic Dissection (IRAD), the largest single dataset on the subject, has sharp, severe pain as the most common presenting complaint. About 4.5% of patients deny pain, and AAS is incidentally detected through screening programs in these individuals.
Neurological signs such as syncope can copresent with pain, suggesting reduced blood flow to the central or peripheral nervous system. This cooccurrence results from the aortic wall pathology's disruption of spinal and aortic outflow, affecting both central and peripheral blood flow.[22] Dissection in the ascending aorta may be associated with myocardial ischemia, aortic regurgitation from the involvement of the aortic valve, or pericardial effusion.
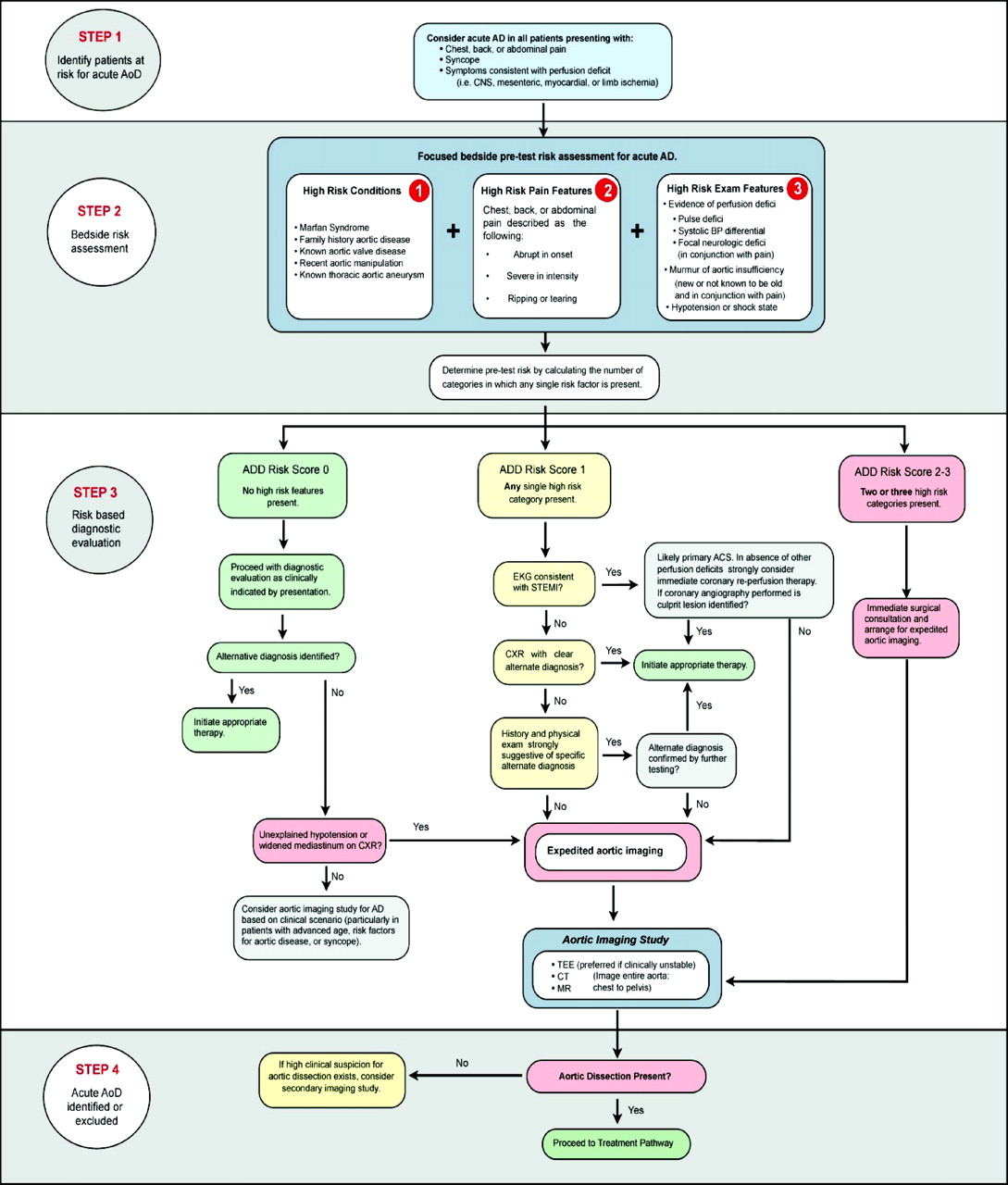
Chronic severe hypertension is the most common risk factor associated with AAS. In a large prospective study conducted by Landenhed et al, hypertension was observed in 86% of individuals who later developed aortic dissection and showed a strong correlation with its incidence.[23] However, hypertension is not highlighted as a significant predisposing factor in the Aortic Dissection Detection Risk Score based on 12 risk factors organized into 3 categories (see Image. Aortic Dissection Detection Risk Score).[24]
Evaluation
Besides taking the clinical history and performing a physical examination, patients must be further investigated with imaging and non-imaging-based examinations when AAS is suspected. The modalities that may be included in the workup are discussed below.
Non-Imaging Based Examinations
This set of tests consists of electrocardiography (ECG) and blood tests. An ECG allows for separating cardiac sources of chest pain, such as myocardial infarction. AAS and myocardial infarction can present concomitantly, though further investigation and careful management are required. Data from IRAD show that 31% in a group of 464 patients had type A dissection with normal ECG tracing, with the rest showing an array of ECG changes from nonspecific ST- and T-wave changes to features consistent with acute myocardial infarction.[25]
Blood tests that can help diagnose AAS or provide evidence for other differential diagnoses include:
- Complete blood count
- Blood urea nitrogen and electrolytes
- C-reactive protein
- Liver function tests
- D-dimer
- Troponin
- Creatinine kinase
- Arterial blood gas analysis, including lactate and glucose levels
The selection of non–imaging-based diagnostic tests should be guided by the clinical presentation, especially if patient or facility resources are limited.
Imaging
Imaging is the mainstay of the diagnostic process, and it may include chest radiography, computed tomography (CT), ultrasound, or magnetic resonance imaging (MRI). Simple chest radiography has shown a sensitivity of up to 64% and a specificity of 86% in identifying AAS. Chest radiographic features indicative of AAS include mediastinal or aortic notch widening, aortic kinking, tracheal shifting, and the aortic shadow demonstrating a double density.[26] The current gold standard in AAS imaging is CT angiography, which is available in most emergency departments. This modality is also noninvasive, less operator-dependent than ultrasound, and less time-consuming. The CT scan's average sensitivity in detecting AAS is upward of 95%. Recorded specificities are anywhere between 87% and 100%.[27][28]
Transesophageal echocardiography (TEE) and transthoracic echocardiography (TTE) may also be used in the diagnosis of AAS. However, the effectiveness of each varies greatly. TTE can help diagnose proximal dissection and its complications in an acute setting. However, views can be limited in many other regions along the aorta's entirety. TEE brings the ultrasound probe closer to the aorta than TTE. Thus, TEE's sensitivity for AAS is 99% and specificity is 89%.[29] However, TEE is operator-dependent and far less readily available in an emergency setting. Further, an investigation requiring intubation of the esophagus is much more invasive than CT or MRI.
MRI is seldom used as a primary mode of acute investigation due to the limited availability of this modality in emergency settings and the life-threatening nature of AAS. However, as the most sensitive and specific modality for identifying all AAS types, MRI may be used when primary investigations prove inconclusive.
Treatment / Management
AAS can be categorized based on whether the ascending or descending aorta is involved. Conditions affecting the ascending aorta are surgical emergencies. Static AAS involving the descending aorta are often managed conservatively, but cases that actively progress, cause organ or limb malperfusion and unmanageable pain, or pose a risk of rupture require urgent surgical management.[30] Immediate AAS management focuses on reducing systolic blood pressure below 120 mm Hg and slowing the rate of blood pressure change (dP/dt) to halt dissection or prevent rupture. Intravenous β-blockers, such as labetalol, are the mainstay of medical management, with nondihydropyridine calcium channel blockers as alternatives for patients intolerant of β-blockers. Vasodilators may be used alongside these treatments.
AAD involving the ascending aorta is a surgical emergency traditionally managed with an open approach. Treatment aims to eliminate the false lumen by closing or excising the initial intimal tear and any subsequent tears. Alternatively, synthetic grafts can reinforce the aortic wall. Proximal extension into the aortic valve may lead to aortic valve insufficiency and damage to the coronary arteries, which can be corrected by resuspending or replacing the valve. Endovascular repair has been attempted for type A dissections. However, literature on the topic is scarce, with only 92 published cases over the last 2 decades. A significant limitation of the endovascular approach is the difficulty in addressing dissection involving the aortic valve and root.
Acute type B dissection is divided into complicated and uncomplicated cases. Complicated dissection of the descending aorta affects 25% of patients with acute type B aortic dissection who are either hemodynamically unstable or present with malperfusion to organ systems or limbs. Other symptoms include unrelenting chest pain, uncontrollable hypertension, or imaging findings indicating progression.[31] Endovascular stenting with synthetic grafts and aggressive medical therapy yields the most favorable outcomes for this pathology.(A1)
Uncomplicated type B aortic dissection has been traditionally managed with medical therapy alone. However, a case can be made for prophylactic endovascular treatment to prevent disease progression. Data remains inconclusive due to the lack of large-scale, long-term, randomized controlled trials. IMH, despite having a lower mortality than AAD, receives similar treatment due to the risk of progression to dissection, aneurysm formation, or aortic rupture. Aneurysms affecting the ascending aorta are stented with an endovascular approach when possible. In contrast, lesions in the descending aorta may be managed by watch-and-wait therapy, optimal medical therapy, or surgery.[32]
IMH of the descending aorta can either progress or regress over time. Stable, regressive IMH can be managed with medical treatment, while unstable, progressive IMH requires endovascular management. The expansion and contraction of IMH complicate stent graft sizing and increase the risk of a type 1 endoleak. The hematoma expands during the acute phase, but over time, it thromboses and retracts, increasing the aortic lumen. Isolated asymptomatic PAU can be safely treated with medical therapy. Symptomatic PAU is more likely to progress to aneurysm, pseudoaneurysm, or aortic rupture, necessitating surgical management. Given the comorbidities and advanced age of patients with PAU, endovascular repair with stent grafts significantly reduces mortality.
Differential Diagnosis
The differential diagnoses for acute aortic syndrome include the following:
- AAD
- IMH
- PAU
- Thoracic aortic aneurysm
- Abdominal aortic aneurysm
- Traumatic aortic injury
- Myocardial infarction
- Pulmonary embolism
A thorough clinical examination and judicious diagnostic testing can help differentiate AAS from similarly presenting conditions, guiding management.
Staging
Classification
The 2 most widely used classification systems for AAD are the Debakey and Stanford Classifications. IMH is also classified in the same way.
DeBakey classification
- Type 1 – Starts in the ascending aorta and progresses to the arch and, sometimes, beyond this point
- Type 2 – Involves the ascending aorta only
- Type 3 – Involves the descending aorta only
Stanford classification
- Type A – Any dissection involving the ascending aorta
- Type B – Any dissection not involving the ascending aorta
Prognosis
AAD carries a high mortality rate that varies by dissection location. Ascending aorta dissections treated medically have an overall mortality of 24% within 24 hours, 44% at 7 days, and up to 49% at 14 days. Surgically managed type A dissections show lower mortality rates of 10% in the first 24 hours, 16% at 7 days, and up to 20% at 14 days. Type B dissections have a lower fatality rate, with 30-day mortality of up to 10%. In patients experiencing complications, mortality can exceed 25% by day 30.
Separating IMH from coexisting AAS is challenging, as studies indicate that up to 30% of patients present with both AAD and IMH at diagnosis.[33] IMH can either undergo reabsorption in up to 10% of cases or progress to aortic dissection in up to 47% of patients. Surgical management of type A IMH produces better outcomes, with mortality rates of 14% compared to 36% for medical management. Treatment approaches for type B IMH yield comparable mortality rates, with 14% for medical therapy and 20% for surgical intervention.[34]
Complications
The complications that can develop from AAS include:
- Aortic aneurysm formation
- Aortic rupture
- End organ ischemia
- Limb ischemia
- Aortic valve
- Haemoperidardium
- Pleural effusion
- Coronary artery dissection
- Death
- Stroke
- Myocardial infarction
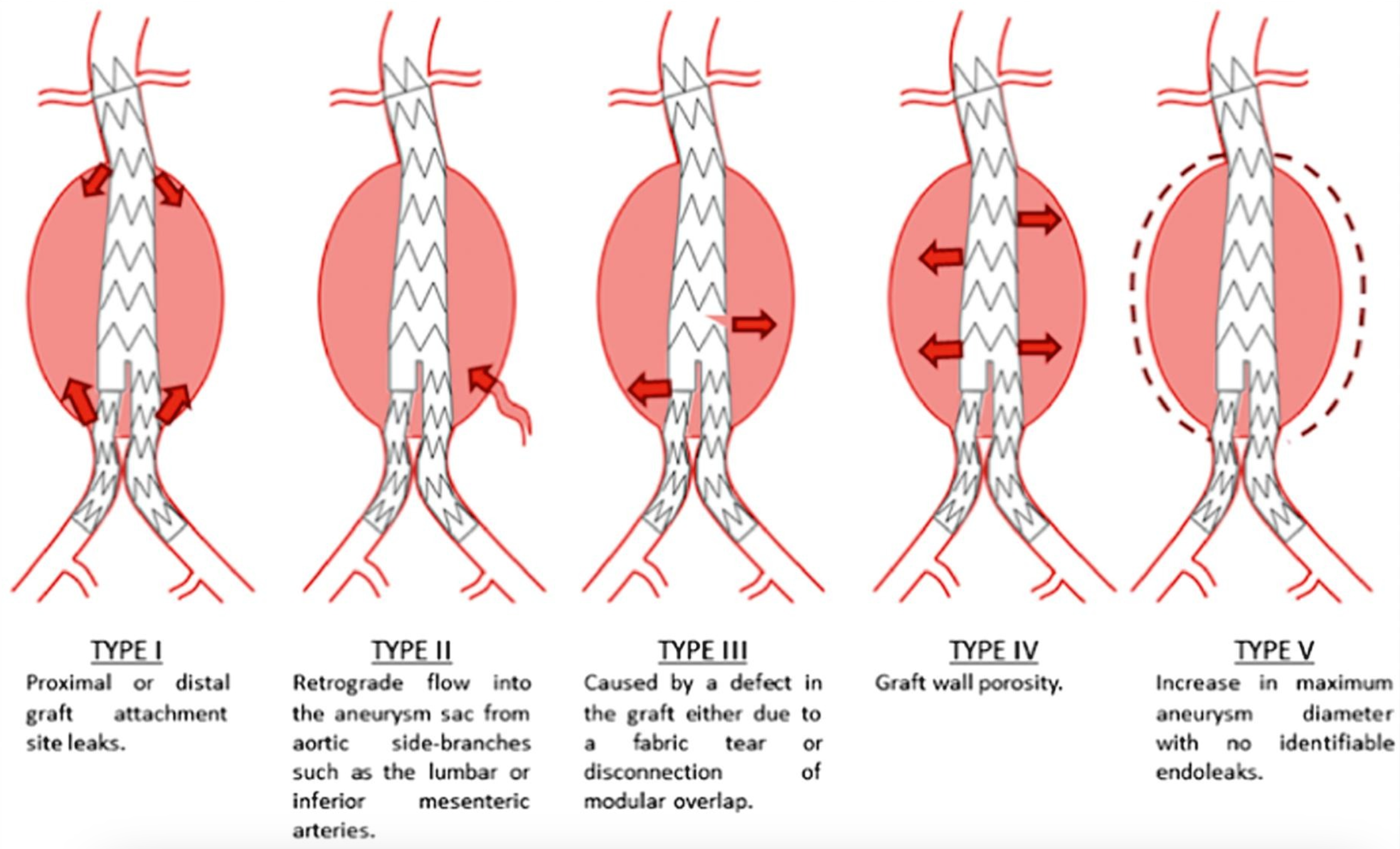
Endoleaks following endovascular stenting are classified into 5 types based on the source of leakage (see Image. Endoleak Types). These types are as follows:
- Type I: Leakage occurs at the attachment site due to insufficient sealing. This type is subdivided as:
- IA: Leakage at the proximal attachment
- IB: Leakage at the distal attachment
- IC: Involves aorto-mono-iliac grafts and femoro-femoral bypass from the contralateral nongrafted iliac artery.
- Type II: Leakage occurs through collateral vessels, such as the lumbar, inferior mesenteric., and internal iliac arteries.
- Type III: Leakage results from defects in the graft, such as fractures or holes, indicating mechanical failure of the graft.
- Type IV: Leakage occurs without a specific source but is typically due to graft porosity.
- Type V: The aneurysm sac expands without visible leakage, a phenomenon known as endotension.
Endoleak management mainly depends on the type, as well as the presence of sac expansion. Types I and III require prompt intervention, while type II can be monitored and treated selectively if significant sac enlargement occurs. Since endoleaks can develop at any time after endovascular aneurysm repair, conducting a contrast-enhanced CT angiogram or duplex ultrasound every 5 years in a specialized laboratory is recommended. Types I and III pose the highest risk of rupture (7.5% within 2 years for type I and 8.9% within 1 year for type III) and should be addressed immediately.
Current guidelines indicate that intervention is warranted for other endoleak types if the aneurysm sac grows by more than 5 mm. Type II endoleaks are the most frequent, comprising 50% of cases. Up to 90% of type II endoleaks resolve spontaneously or do not cause sac expansion, requiring only observation. The risk of rupture is below 1%, but cases needing reintervention are complex, with a high recurrence rate and potential rupture even without sac growth. Types IV endoleaks and endotension are rare, usually benign, and best managed with observation.[35]
Deterrence and Patient Education
Patient education and deterrence should include the following:
- Smoking cessation advice
- Management of hypertension
- Avoiding the use of recreational drugs, especially cocaine
- Healthy lifestyle and diet
Comprehensive patient education and lifestyle modifications are essential for preventing aortic syndromes and their complications.
Enhancing Healthcare Team Outcomes
Rapid diagnosis is crucial for improving healthcare team outcomes for patients with AAS due to the high mortality rate, which increases the longer appropriate treatment is delayed. The emergency department team plays a vital role, as faster diagnosis and referral to a specialist enhance the chances of survival. The patient’s journey must be streamlined from triage nurse to emergency doctor to medical team if medical treatment is decided or vascular or cardiothoracic surgeon if surgical intervention is warranted. Initial investigations include vital signs monitoring, physical examination, and laboratory testing. Imaging depends on the patient's hemodynamic stability and the need for urgent surgical management.
Improving outcomes for patients with AAS involves consolidating interprofessional expertise in aortic centers, which have been shown to reduce mortality in this patient group.[36] Substantial evidence supports a volume-outcome relationship, underscoring the need to centralize the treatment of acute aortic pathology in specialized “aorta centers”—high-volume surgical facilities with dedicated expertise in aortic surgery. The goals include lowering early mortality rates, reducing the likelihood of reoperations, and enhancing long-term outcomes.[37]
To address delays in recognizing and managing AAS, implementing a streamlined emergency care pathway known as the “aorta code” is essential. This pathway should be available around the clock and activated from the emergency departments of smaller hospitals. Three critical reasons support establishing the aorta code. First, this code increases awareness and understanding of AAS among emergency care providers, leading to earlier diagnosis. Second, this code ensures rapid transfer of patients to an aorta center, reducing the time between diagnosis and treatment. Third, proper implementation of the aorta code enables timely, optimal treatment by engaging specialized aortic surgeons, thereby improving clinical outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Akin I, Kische S, Ince H, Nienaber C. Penetrating aortic ulcer, intramural hematoma, acute aortic syndrome: when to do what. The Journal of cardiovascular surgery. 2012 Feb:53(1 Suppl 1):83-90 [PubMed PMID: 22433727]
Vilacosta I, San Román JA, di Bartolomeo R, Eagle K, Estrera AL, Ferrera C, Kaji S, Nienaber CA, Riambau V, Schäfers HJ, Serrano FJ, Song JK, Maroto L. Acute Aortic Syndrome Revisited: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2021 Nov 23:78(21):2106-2125. doi: 10.1016/j.jacc.2021.09.022. Epub [PubMed PMID: 34794692]
Harris KM, Strauss CE, Eagle KA, Hirsch AT, Isselbacher EM, Tsai TT, Shiran H, Fattori R, Evangelista A, Cooper JV, Montgomery DG, Froehlich JB, Nienaber CA, International Registry of Acute Aortic Dissection (IRAD) Investigators. Correlates of delayed recognition and treatment of acute type A aortic dissection: the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2011 Nov 1:124(18):1911-8. doi: 10.1161/CIRCULATIONAHA.110.006320. Epub 2011 Oct 3 [PubMed PMID: 21969019]
Leone O, Pacini D, Foà A, Corsini A, Agostini V, Corti B, Di Marco L, Leone A, Lorenzini M, Reggiani LB, Di Bartolomeo R, Rapezzi C. Redefining the histopathologic profile of acute aortic syndromes: Clinical and prognostic implications. The Journal of thoracic and cardiovascular surgery. 2018 Nov:156(5):1776-1785.e6. doi: 10.1016/j.jtcvs.2018.04.086. Epub 2018 Apr 27 [PubMed PMID: 29803371]
Vilacosta I,San Román JA,Aragoncillo P,Ferreirós J,Mendez R,Graupner C,Batlle E,Serrano J,Pinto A,Oyonarte JM, Penetrating atherosclerotic aortic ulcer: documentation by transesophageal echocardiography. Journal of the American College of Cardiology. 1998 Jul; [PubMed PMID: 9669253]
Halushka MK, Angelini A, Bartoloni G, Basso C, Batoroeva L, Bruneval P, Buja LM, Butany J, d'Amati G, Fallon JT, Gallagher PJ, Gittenberger-de Groot AC, Gouveia RH, Kholova I, Kelly KL, Leone O, Litovsky SH, Maleszewski JJ, Miller DV, Mitchell RN, Preston SD, Pucci A, Radio SJ, Rodriguez ER, Sheppard MN, Stone JR, Suvarna SK, Tan CD, Thiene G, Veinot JP, van der Wal AC. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association For European Cardiovascular Pathology: II. Noninflammatory degenerative diseases - nomenclature and diagnostic criteria. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2016 May-Jun:25(3):247-257. doi: 10.1016/j.carpath.2016.03.002. Epub 2016 Mar 12 [PubMed PMID: 27031798]
Level 3 (low-level) evidenceNienaber CA, Clough RE. Management of acute aortic dissection. Lancet (London, England). 2015 Feb 28:385(9970):800-11. doi: 10.1016/S0140-6736(14)61005-9. Epub 2015 Feb 6 [PubMed PMID: 25662791]
Lombardi JV, Hughes GC, Appoo JJ, Bavaria JE, Beck AW, Cambria RP, Charlton-Ouw K, Eslami MH, Kim KM, Leshnower BG, Maldonado T, Reece TB, Wang GJ. Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) Reporting Standards for Type B Aortic Dissections. The Annals of thoracic surgery. 2020 Mar:109(3):959-981. doi: 10.1016/j.athoracsur.2019.10.005. Epub 2020 Jan 27 [PubMed PMID: 32000979]
Mulligan-Kehoe MJ. The vasa vasorum in diseased and nondiseased arteries. American journal of physiology. Heart and circulatory physiology. 2010 Feb:298(2):H295-305. doi: 10.1152/ajpheart.00884.2009. Epub 2009 Nov 25 [PubMed PMID: 19940078]
Level 3 (low-level) evidenceSong JK. Diagnosis of aortic intramural haematoma. Heart (British Cardiac Society). 2004 Apr:90(4):368-71 [PubMed PMID: 15020502]
Oderich GS, Kärkkäinen JM, Reed NR, Tenorio ER, Sandri GA. Penetrating Aortic Ulcer and Intramural Hematoma. Cardiovascular and interventional radiology. 2019 Mar:42(3):321-334. doi: 10.1007/s00270-018-2114-x. Epub 2018 Nov 9 [PubMed PMID: 30413917]
Akutsu K. Etiology of aortic dissection. General thoracic and cardiovascular surgery. 2019 Mar:67(3):271-276. doi: 10.1007/s11748-019-01066-x. Epub 2019 Jan 28 [PubMed PMID: 30689200]
Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000 Feb 16:283(7):897-903 [PubMed PMID: 10685714]
Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwöger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ, ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). European heart journal. 2014 Nov 1:35(41):2873-926. doi: 10.1093/eurheartj/ehu281. Epub 2014 Aug 29 [PubMed PMID: 25173340]
Level 1 (high-level) evidenceBossone E, Eagle KA. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nature reviews. Cardiology. 2021 May:18(5):331-348. doi: 10.1038/s41569-020-00472-6. Epub 2020 Dec 22 [PubMed PMID: 33353985]
Baliyan V, Parakh A, Prabhakar AM, Hedgire S. Acute aortic syndromes and aortic emergencies. Cardiovascular diagnosis and therapy. 2018 Apr:8(Suppl 1):S82-S96. doi: 10.21037/cdt.2018.03.02. Epub [PubMed PMID: 29850421]
Levy D, Goyal A, Grigorova Y, Farci F, Le JK. Aortic Dissection. StatPearls. 2024 Jan:(): [PubMed PMID: 28722992]
Grewal N, Velders BJJ, Gittenberger-de Groot AC, Poelmann R, Klautz RJM, Van Brakel TJ, Lindeman JHN. A Systematic Histopathologic Evaluation of Type-A Aortic Dissections Implies a Uniform Multiple-Hit Causation. Journal of cardiovascular development and disease. 2021 Jan 27:8(2):. doi: 10.3390/jcdd8020012. Epub 2021 Jan 27 [PubMed PMID: 33513898]
Level 1 (high-level) evidenceMacura KJ, Corl FM, Fishman EK, Bluemke DA. Pathogenesis in acute aortic syndromes: aortic dissection, intramural hematoma, and penetrating atherosclerotic aortic ulcer. AJR. American journal of roentgenology. 2003 Aug:181(2):309-16 [PubMed PMID: 12876003]
Park KH, Lim C, Choi JH, Sung K, Kim K, Lee YT, Park PW. Prevalence of aortic intimal defect in surgically treated acute type A intramural hematoma. The Annals of thoracic surgery. 2008 Nov:86(5):1494-500. doi: 10.1016/j.athoracsur.2008.06.061. Epub [PubMed PMID: 19049737]
Vilacosta I, San Román JA. Acute aortic syndrome. Heart (British Cardiac Society). 2001 Apr:85(4):365-8 [PubMed PMID: 11250953]
Gaul C, Dietrich W, Erbguth FJ. Neurological symptoms in aortic dissection: a challenge for neurologists. Cerebrovascular diseases (Basel, Switzerland). 2008:26(1):1-8. doi: 10.1159/000135646. Epub 2008 May 30 [PubMed PMID: 18511865]
Rogers AM, Hermann LK, Booher AM, Nienaber CA, Williams DM, Kazerooni EA, Froehlich JB, O'Gara PT, Montgomery DG, Cooper JV, Harris KM, Hutchison S, Evangelista A, Isselbacher EM, Eagle KA, IRAD Investigators. Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation: results from the international registry of acute aortic dissection. Circulation. 2011 May 24:123(20):2213-8. doi: 10.1161/CIRCULATIONAHA.110.988568. Epub 2011 May 9 [PubMed PMID: 21555704]
Bima P, Pivetta E, Nazerian P, Toyofuku M, Gorla R, Bossone E, Erbel R, Lupia E, Morello F. Systematic Review of Aortic Dissection Detection Risk Score Plus D-dimer for Diagnostic Rule-out Of Suspected Acute Aortic Syndromes. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2020 Oct:27(10):1013-1027. doi: 10.1111/acem.13969. Epub 2020 Apr 21 [PubMed PMID: 32187432]
Level 1 (high-level) evidenceTsai TT, Nienaber CA, Eagle KA. Acute aortic syndromes. Circulation. 2005 Dec 13:112(24):3802-13 [PubMed PMID: 16344407]
von Kodolitsch Y, Nienaber CA, Dieckmann C, Schwartz AG, Hofmann T, Brekenfeld C, Nicolas V, Berger J, Meinertz T. Chest radiography for the diagnosis of acute aortic syndrome. The American journal of medicine. 2004 Jan 15:116(2):73-7 [PubMed PMID: 14715319]
Sommer T, Fehske W, Holzknecht N, Smekal AV, Keller E, Lutterbey G, Kreft B, Kuhl C, Gieseke J, Abu-Ramadan D, Schild H. Aortic dissection: a comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography, and MR imaging. Radiology. 1996 May:199(2):347-52 [PubMed PMID: 8668776]
Level 2 (mid-level) evidenceNienaber CA, von Kodolitsch Y. [Diagnostic imaging of aortic diseases]. Der Radiologe. 1997 May:37(5):402-9 [PubMed PMID: 9312783]
Erbel R, Engberding R, Daniel W, Roelandt J, Visser C, Rennollet H. Echocardiography in diagnosis of aortic dissection. Lancet (London, England). 1989 Mar 4:1(8636):457-61 [PubMed PMID: 2563839]
Level 1 (high-level) evidenceNienaber CA, Powell JT. Management of acute aortic syndromes. European heart journal. 2012 Jan:33(1):26-35b. doi: 10.1093/eurheartj/ehr186. Epub 2011 Aug 2 [PubMed PMID: 21810861]
Moulakakis KG, Mylonas SN, Dalainas I, Kakisis J, Kotsis T, Liapis CD. Management of complicated and uncomplicated acute type B dissection. A systematic review and meta-analysis. Annals of cardiothoracic surgery. 2014 May:3(3):234-46. doi: 10.3978/j.issn.2225-319X.2014.05.08. Epub [PubMed PMID: 24967162]
Level 1 (high-level) evidenceHouben IB, van Bakel TMJ, Patel HJ. Type B intramural hematoma: thoracic endovascular aortic repair (TEVAR) or conservative approach? Annals of cardiothoracic surgery. 2019 Jul:8(4):483-487. doi: 10.21037/acs.2019.05.18. Epub [PubMed PMID: 31463210]
Alomari IB, Hamirani YS, Madera G, Tabe C, Akhtar N, Raizada V. Aortic intramural hematoma and its complications. Circulation. 2014 Feb 11:129(6):711-6. doi: 10.1161/CIRCULATIONAHA.113.001809. Epub [PubMed PMID: 24515957]
Level 3 (low-level) evidenceMaraj R, Rerkpattanapipat P, Jacobs LE, Makornwattana P, Kotler MN. Meta-analysis of 143 reported cases of aortic intramural hematoma. The American journal of cardiology. 2000 Sep 15:86(6):664-8 [PubMed PMID: 10980220]
Level 2 (mid-level) evidenceCifuentes S, Mendes BC, Tabiei A, Scali ST, Oderich GS, DeMartino RR. Management of Endoleaks After Elective Infrarenal Aortic Endovascular Aneurysm Repair: A Review. JAMA surgery. 2023 Sep 1:158(9):965-973. doi: 10.1001/jamasurg.2023.2934. Epub [PubMed PMID: 37494030]
Mariscalco G, Maselli D, Zanobini M, Ahmed A, Bruno VD, Benedetto U, Gherli R, Gherli T, Nicolini F. Aortic centres should represent the standard of care for acute aortic syndrome. European journal of preventive cardiology. 2018 Jun:25(1_suppl):3-14. doi: 10.1177/2047487318764963. Epub [PubMed PMID: 29708034]
Czerny M, Schmidli J, Adler S, van den Berg JC, Bertoglio L, Carrel T, Chiesa R, Clough RE, Eberle B, Etz C, Grabenwöger M, Haulon S, Jakob H, Kari FA, Mestres CA, Pacini D, Resch T, Rylski B, Schoenhoff F, Shrestha M, von Tengg-Kobligk H, Tsagakis K, Wyss TR, EACTS/ESVS scientific document group. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2019 Jan 1:55(1):133-162. doi: 10.1093/ejcts/ezy313. Epub [PubMed PMID: 30312382]
Level 3 (low-level) evidence