Introduction
The insular cortex (i.e., insula, Latin for "island") is a still poorly understood and hidden structure located deep in the human brain. This telencephalic lobe makes up only about 2% of the complete cortical surface area but is part of complex neural circuitry involving the higher cortex, limbic structures, basal ganglia, and autonomic system. Further, the insula is located adjacent to several critical structures, and pathology originating from this area may risk significant neurological morbidity and even mortality. Care should include a coordinated effort between primary and specialty care alongside strong rehabilitation and social support. These factors make the insula an area of continued basic and clinical neuroscientific research.[1]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Structure
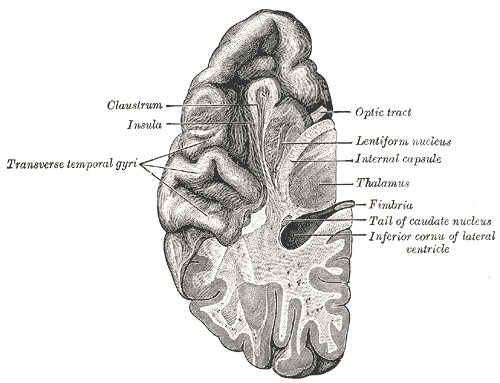
The insular cortex is a distinct lobe of the cerebral cortex and forms the floor of the lateral sulcus (i.e., Sylvian fissure) bilaterally. It can be grossly observed deep to the insular operculum, which is formed by the parietal, frontal, and temporal lobes. On axial magnetic resonance imaging (MRI) of the brain, the insula can be observed lateral-adjacent to the extreme capsule, with the claustrum, external capsule, then putamen appearing evermore medially.
The insula is divided into distinct anterior and posterior areas by the insular central sulcus and demarcated by the surrounding peri-insular (circular) sulcus. The anterior insula is composed of the three short gyri (anterior, middle, and posterior) separated by two pre-central sulci, while the posterior insula is composed of two long gyri (anterior and posterior) separated by the singular post-central sulcus. The accessory gyrus is located on the anterior face of the insula, and the transverse gyrus connects the insula to the orbital surface of the frontal lobe. Finally, the limen insulae in the anteroinferior apex of the insula is the point where the mesial surface of the temporal lobe, anterior perforator substance, and inferior insular point come together. This is also where the middle cerebral artery (MCA) generally bifurcates.
Function
The insula is important for gustatory and sensorimotor processing, risk-reward behavior, autonomics, pain pathways, and auditory and vestibular functioning. Translational work in animals and humans has demonstrated a link between the insula and a number of structures, including the neocortex (i.e., frontal, parietal, and temporal lobes), limbic cortex (i.e., olfactory bulb, anterior cingulum, amygdala, hippocampus, and thalamus), and basal ganglia. Injuries or lesions of the insula can thus manifest with symptoms corresponding to any of these structures. Further, functional magnetic resonance imaging (fMRI) has observed three major functional subdivisions with regard to neural circuitry (dorsal-anterior, ventral-anterior, and mid-posterior), although their specific connections are beyond the scope of this review. In general, the anterior part connects to more anterior structures related to emotional integration, and the posterior part connects to more posterior structures related to cognition.
Three cytoarchitectonic areas of the insula have been described in the literature: the central agranular zone, the transition dysgranular zone, and the surrounding granular zone. The dysgranular zone has been implicated in executive functioning such as attention and memory.[2][3][4][1]
Embryology
The insula is a paralimbic structure that lies between the neocortex and the paleocortex (sometimes referred to as the mesocortex). Cortical structures begin to form around the sixth week of fetal development, and the sulci fully appear in the second trimester. From an embryological standpoint, the insular cortex is the most primordial structure of the telencephalon, arising from the anterior prosencephalon (i.e., forebrain) during development, with the limen insulae forming as a result of the inferior cortical region folding on itself.
After a series of complex rotations and folds, the insula is housed deep to the lateral sulcus with many connections to surrounding structures. Although isolated reports of insular malformations are rare, the insula's location and development process represent many opportunities for congenital cerebral disruption. Maldevelopment may be associated with lissencephaly, disorders of the adjacent basal ganglia, and others.[5][6][7][8][9]
Blood Supply and Lymphatics
Arterial Supply
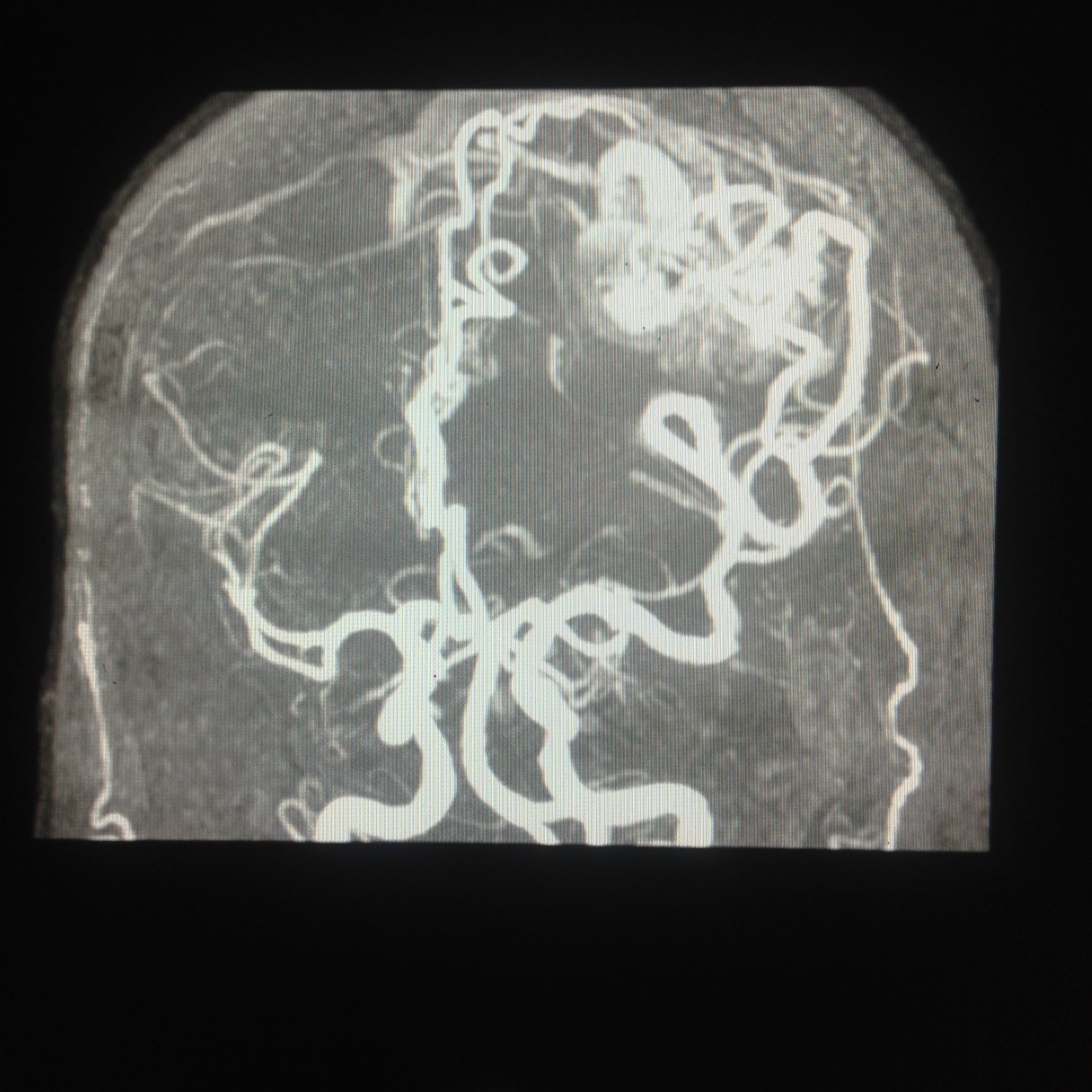
The vascular supply to the insular cortex comes from short perforator branches from the superior division of the middle cerebral artery (MCA). These most commonly arise from the M2 segment (i.e., "insular" or "Sylvian" segment), which courses over the lateral surface of the insula within the Sylvian fissure. Some vessels from M1 may supply the limen insulae, while occasionally, branches of the M3 segment supply the peri-insular sulcus. The M3 segment of the MCA travels primarily lateral to the insular cortex and loops around the operculum, supplying the cortex distal to the insula.
Venous Drainage
The venous drainage of the insula is complex and somewhat variable as a majority of cadaveric specimens demonstrate a mix of superficial and deep venous connections. The precentral insular vein is the most common drainage area of the superficial system, while the deep middle cerebral vein (DMCV) represents the predominant deep venous drainage conduit.[3][4][10]
Nerves
For surgery to the insula and Sylvian fissure, superficial nerves of the scalp should be preserved when possible during superficial dissection. For example, in a pterional (frontotemporal) craniotomy, the temporal branches of the facial nerve (CN VII) should be respected, given their supply to the frontalis muscle. The auriculotemporal (from CN V3) and zygomaticotemporal (from CN V2) nerves represent the somewhat variable sources of overlying superficial sensation of this area.[11][12]
Muscles
Similar to the superficial nerves, the superficial muscles overlying a chosen craniotomy site should be well known to the neurosurgeon. Interfascial dissection of the temporalis muscle for a pterional or subtemporal craniotomy is a common step required for a surgical approach to the insula.[13]
Surgical Considerations
The surgical management of insular lesions is particularly challenging, given the difficulty of accessing this region via the overlying eloquent operculae of the frontoparietal and temporal lobes and the intricate MCA vascular supply. Nevertheless, a better understanding of both gross and microsurgical insular anatomy, preoperative fMRI and diffusion tensor imaging (DTI) tractography, awake cortical mapping, and stereotactic and fluorescein guidance have collectively improved the ability of neurosurgeons to resect lesions in this area while respecting eloquent structures.
Speech and motor areas are of particular concern when attempting to gain access to the insula. In a patient's dominant hemisphere (typically the left), the major language areas overlie the insula. Care is also necessary regarding the arcuate, uncinate, and inferior frontal-occipital fasciculi. The basal ganglia lie medial to the insula. Finally, although short perforators of the M2-MCA can be sacrificed during surgery, long branches must be preserved, as these supply the adjacent corticospinal tract in the corona radiata. Disruption of these vessels may thus lead to hemiplegia.[14]
Berger-Sanai Classification of Insular Gliomas
In 2010, after a growing understanding of the challenges of surgery to the insula, the Berger-Sanai surgical classification system for insular gliomas was developed. This system, which bisects the insula along two perpendicular planes, the lateral sulcus and a line through the Foramen of Monro, divides the insula into four zones. It is useful both for strategizing the surgical approach to the insula and for a pragmatic functional and anatomic understanding of the area. It has been validated as a predictor of the extent of resection and postoperative morbidity in the surgical resection of insular gliomas. The zones are listed below.[15][16]
- Zone I: Anterior-Superior
- Zone II: Posterior-Superior
- Zone III: Posterior-Inferior
- Zone IV: Anterior-Inferior
Surgical Approaches to the Insula
An incision and craniotomy under general anesthesia are required to access the brain parenchyma. Choice of craniotomy (i.e., pterional, temporal) may follow the lesion's location and the preferred surgical approach. The two main surgical approaches to the insula are the transsylvian and transcortical techniques, and there are advantages and risks to both. The ultimate goal is to maximize the extent of resection while minimizing surgical risk to adjacent structures, and thus, the choice is ultimately surgeon-dependent. There remains debate over the preferred approach.[17][18]
Transsylvian Approach
The transsylvian approach to the insula, via a wide exposure through the lateral sulcus, was the first-described technique. It is of particular benefit when accessing lesions in the dominant hemisphere since the operator can better spare the eloquent language areas of the temporal and frontal lobes. Disadvantages include a need for extensive subarachnoid dissection, the manual distraction of the MCA, and opercular retraction for large lesions.
Transcortical Approach
The transcortical approach is increasingly used for large lesions that may extend beyond the confines of the peri-insular sulcus, especially posteriorly. Cortical and subcortical mapping is important to identify eloquent cortex, whereby exposure windows through non-eloquent areas may be accessed. There appears to be a preference for the transcortical approach for large gliomas and those located in Berger-Sanai Zone III. The transcortical approach may also be associated with lower ischemic morbidity for Berger-Sanai Zone II lesions.[16][14][19][20][15][21]
Clinical Significance
Glioma
Glioma is the most common primary tumor of the central nervous system. The insula is prone to the formation of gliomas, including low- and high-grade astrocytomas and oligodendrogliomas, as well as glioblastoma (GBM). The incidence of intracranial insular gliomas ranges from 10% to 25%, and demographic epidemiology follows that of all intracranial gliomas. Prognosis remains dismal, especially for high-grade tumors, with optimally treated GBMs observing median overall survival around 1 year.
Insular gliomas are classified by the aforementioned Berger-Sinai system, with "giant" status given to those involving all four zones. Neuro-functional and survival outcomes are dependent on a host of factors. Survival is associated with tumor characteristics such as pathologic grade, molecular genetics, resection rate, and utilization of adjuvant chemotherapy and patient characteristics such as age and functional status. Resection typically occurs by either the transsylvian or transcortical approaches.
Complications of insular glioma resection include motor and executive dysfunction. Temporary and permanent motor deficits occur at rates of 11% and 4%, respectively, while temporary and permanent aphasia occurs at rates of 11% and 2%, respectively.[22][23][24][25][26][27]
Arteriovenous Malformation
Arteriovenous malformations (AVMs) are challenging to treat, and optimal therapy remains somewhat controversial depending on rupture status, location, and other factors. All AVMs can be classified by the Spetzler-Martin grading system (I-V) depending on the lesion's size, draining vein, and presence of regional eloquent cortex. Sylvian fissure AVMs near the insula represent a significant source of potential morbidity or mortality, especially for younger patients. Symptoms can present acutely with rupture or chronically due to mass effect. Sylvian AVMs can thus be further classified based on the location of their nidus by the Sugita system: medial, lateral, pure, and deep. The majority of Sylvian AVMs are either medial (40%) or lateral (40%). With improvements in radiosurgery technology and microsurgical techniques, the ability to manage Sylvian AVMs is becoming an increasingly interprofessional and successful venture.[28][29][30][31]
Stroke
Insular stroke can occur due to hemorrhagic (i.e., vessel rupture) or ischemic (i.e., thrombotic, embolic, or iatrogenic) insult. The MCA is the most commonly affected cerebral artery in acute stroke and presents with a wide range of symptoms, and thus it may be difficult to localize insular involvement based on the clinical exam alone. Further, strokes involving only the insular cortex are abundantly rare, with only 49 reports noted in the literature. Nevertheless, insular-confined strokes manifest as dysfunction of its anatomical-functional properties, including somatomotor deficits, dysarthria, aphasia, vestibulocochlear dysfunction, gustatory change, and behavioral-psychiatric disturbance. In general, insular stroke should be suspected in patients with paresis, speech deficit, and dizziness, with more proximal MCA strokes manifesting with the signs and symptoms of other areas accordingly. Diagnosis and treatment follow that of MCA strokes. [32][33][34]
Seizure and Epilepsy
Seizures arising from the aberrant and excessive neuronal activity may be provoked or unprovoked. Insular seizures may occur due to stroke or glioma but may also follow etiologically from genetic epileptogenic foci, metabolic derangements, or inflammation. With regard to insular epilepsy, manifestations are similar to that of temporal lobe epilepsy. Indeed, debate remains over the true epileptogenic origin of insular seizures (i.e., spread from temporal foci). This uncertainty is complicated by the deep location of the insula, making it difficult to observe seizure activity other than through invasive Phase II electroencephalography (EEG). There is some evidence of true insular epilpetogenic foci provided by video-EEG studies. Nevertheless, patients with insular seizures present with an ictal syndrome characterized by sensory and gustatory auras, dysautonomia, dysphagia, laryngeal constriction, aberrant movements (tonic-clonic or myoclonus), and/or vestibulopathy (i.e., nystagmus). Patients will tend to remain conscious of the event. Diagnosis and treatment follow that of other epileptic pathologies.[35][36]
Other Issues
As previously noted, the insula remains one of the least understood cortical structures of the human brain. Given its deep location and involvement in many neural circuits, there is an impetus to continue improving our understanding both through clinical and translational studies. New and improved diagnostic technologies such as EEG, fMRI, DTI, machine learning, and brain-computer interfaces (BCI) may elucidate the anatomic and functional characteristics of the insula to improve outcomes for patients.
Depending on the pathologic entity, a patient may present to their primary care physician or the emergency department. Coordinated interprofessional care should subsequently involve neurology, neurosurgery, physiatry, occupational and physical therapy (OT/PT), and dedicated nursing care.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
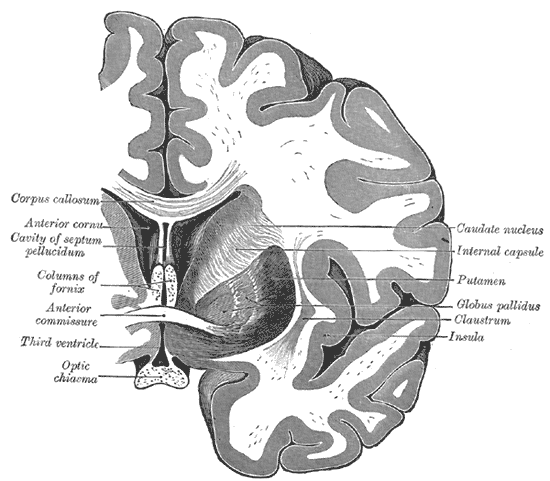
Coronal section of the Brain Through the Anterior Commissure. The caudate nucleus, internal capsule, putamen, globus pallidus, claustrum, insula, optic chiasma, third ventricle, anterior commissure, columns of fornix, cavity of septum pellucidum, anterior cornu, and the corpus callosum.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Nieuwenhuys R. The insular cortex: a review. Progress in brain research. 2012:195():123-63. doi: 10.1016/B978-0-444-53860-4.00007-6. Epub [PubMed PMID: 22230626]
Level 3 (low-level) evidenceUddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O. Structure and Function of the Human Insula. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2017 Jul:34(4):300-306. doi: 10.1097/WNP.0000000000000377. Epub [PubMed PMID: 28644199]
Varnavas GG, Grand W. The insular cortex: morphological and vascular anatomic characteristics. Neurosurgery. 1999 Jan:44(1):127-36; discussion 136-8 [PubMed PMID: 9894973]
Tanriover N, Rhoton AL Jr, Kawashima M, Ulm AJ, Yasuda A. Microsurgical anatomy of the insula and the sylvian fissure. Journal of neurosurgery. 2004 May:100(5):891-922 [PubMed PMID: 15137609]
Kalani MY, Kalani MA, Gwinn R, Keogh B, Tse VC. Embryological development of the human insula and its implications for the spread and resection of insular gliomas. Neurosurgical focus. 2009 Aug:27(2):E2. doi: 10.3171/2009.5.FOCUS0997. Epub [PubMed PMID: 19645558]
Elshazzly M, Lopez MJ, Reddy V, Caban O. Embryology, Central Nervous System. StatPearls. 2023 Jan:(): [PubMed PMID: 30252280]
Zhang Z, Hou Z, Lin X, Teng G, Meng H, Zang F, Fang F, Liu S. Development of the fetal cerebral cortex in the second trimester: assessment with 7T postmortem MR imaging. AJNR. American journal of neuroradiology. 2013 Jul:34(7):1462-7. doi: 10.3174/ajnr.A3406. Epub 2013 Feb 14 [PubMed PMID: 23413246]
Williams JP, Joslyn JN. Lissencephaly: computed tomographic diagnosis. The Journal of computed tomography. 1983 May:7(2):141-4 [PubMed PMID: 6872559]
Level 3 (low-level) evidenceDODGSON MC. A congenital malformation of insular cortex in man, involving the claustrum and certain subcortical centers. The Journal of comparative neurology. 1955 Apr:102(2):341-64 [PubMed PMID: 14381541]
Level 2 (mid-level) evidenceNavarro-Orozco D, Sánchez-Manso JC. Neuroanatomy, Middle Cerebral Artery. StatPearls. 2023 Jan:(): [PubMed PMID: 30252258]
Poblete T, Jiang X, Komune N, Matsushima K, Rhoton AL Jr. Preservation of the nerves to the frontalis muscle during pterional craniotomy. Journal of neurosurgery. 2015 Jun:122(6):1274-82. doi: 10.3171/2014.10.JNS142061. Epub 2015 Apr 3 [PubMed PMID: 25839922]
Kemp WJ 3rd,Tubbs RS,Cohen-Gadol AA, The innervation of the scalp: A comprehensive review including anatomy, pathology, and neurosurgical correlates. Surgical neurology international. 2011; [PubMed PMID: 22276233]
Wormald PJ, Alun-Jones T. Anatomy of the temporalis fascia. The Journal of laryngology and otology. 1991 Jul:105(7):522-4 [PubMed PMID: 1875131]
Przybylowski CJ, Hervey-Jumper SL, Sanai N. Surgical strategy for insular glioma. Journal of neuro-oncology. 2021 Feb:151(3):491-497. doi: 10.1007/s11060-020-03499-4. Epub 2021 Feb 21 [PubMed PMID: 33611715]
Hervey-Jumper SL, Li J, Osorio JA, Lau D, Molinaro AM, Benet A, Berger MS. Surgical assessment of the insula. Part 2: validation of the Berger-Sanai zone classification system for predicting extent of glioma resection. Journal of neurosurgery. 2016 Feb:124(2):482-8. doi: 10.3171/2015.4.JNS1521. Epub 2015 Sep 4 [PubMed PMID: 26339856]
Level 1 (high-level) evidenceSanai N, Polley MY, Berger MS. Insular glioma resection: assessment of patient morbidity, survival, and tumor progression. Journal of neurosurgery. 2010 Jan:112(1):1-9. doi: 10.3171/2009.6.JNS0952. Epub [PubMed PMID: 19612970]
Level 2 (mid-level) evidencePotts MB, Chang EF, Young WL, Lawton MT, UCSF Brain AVM Study Project. Transsylvian-transinsular approaches to the insula and basal ganglia: operative techniques and results with vascular lesions. Neurosurgery. 2012 Apr:70(4):824-34; discussion 834. doi: 10.1227/NEU.0b013e318236760d. Epub [PubMed PMID: 21937930]
Anderson BW, Kortz MW, Al Kharazi KA. Anatomy, Head and Neck, Skull. StatPearls. 2023 Jan:(): [PubMed PMID: 29763009]
Michaud K,Duffau H, Surgery of insular and paralimbic diffuse low-grade gliomas: technical considerations. Journal of neuro-oncology. 2016 Nov; [PubMed PMID: 27161250]
Hameed NUF, Qiu T, Zhuang D, Lu J, Yu Z, Wu S, Wu B, Zhu F, Song Y, Chen H, Wu J. Transcortical insular glioma resection: clinical outcome and predictors. Journal of neurosurgery. 2018 Oct 19:131(3):706-716. doi: 10.3171/2018.4.JNS18424. Epub [PubMed PMID: 30485243]
Level 2 (mid-level) evidencePanigrahi M, Chandrasekhar YB, Vooturi S, Ram GA, Rammohan VS. Surgical Resection of Insular Gliomas and Roles of Functional Magnetic Resonance Imaging and Diffusion Tensor Imaging Tractography-Single Surgeon Experience. World neurosurgery. 2017 Feb:98():587-593. doi: 10.1016/j.wneu.2016.11.001. Epub 2016 Nov 10 [PubMed PMID: 27838429]
Wesseling P, Capper D. WHO 2016 Classification of gliomas. Neuropathology and applied neurobiology. 2018 Feb:44(2):139-150. doi: 10.1111/nan.12432. Epub [PubMed PMID: 28815663]
Duffau H, Capelle L. Preferential brain locations of low-grade gliomas. Cancer. 2004 Jun 15:100(12):2622-6 [PubMed PMID: 15197805]
Lu VM, Goyal A, Quinones-Hinojosa A, Chaichana KL. Updated incidence of neurological deficits following insular glioma resection: A systematic review and meta-analysis. Clinical neurology and neurosurgery. 2019 Feb:177():20-26. doi: 10.1016/j.clineuro.2018.12.013. Epub 2018 Dec 17 [PubMed PMID: 30580067]
Level 1 (high-level) evidenceStupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005 Mar 10:352(10):987-96 [PubMed PMID: 15758009]
Level 1 (high-level) evidenceMcGirt MJ,Chaichana KL,Attenello FJ,Weingart JD,Than K,Burger PC,Olivi A,Brem H,Quinoñes-Hinojosa A, Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008 Oct; [PubMed PMID: 18981880]
Level 2 (mid-level) evidenceWalid MS. Prognostic factors for long-term survival after glioblastoma. The Permanente journal. 2008 Fall:12(4):45-8 [PubMed PMID: 21339920]
Sugita K, Takemae T, Kobayashi S. Sylvian fissure arteriovenous malformations. Neurosurgery. 1987 Jul:21(1):7-14 [PubMed PMID: 3614608]
Level 3 (low-level) evidenceBokhari MR, Bokhari SRA. Arteriovenous Malformation Of The Brain. StatPearls. 2023 Jan:(): [PubMed PMID: 28613495]
Chiluwal AK,Klironomos G,Dehdashti AR, Surgical Resection of a Complex Spetzler-Martin Grade IV Medial Sylvian Arteriovenous Malformation: 3-Dimensional Operative Video. Operative neurosurgery (Hagerstown, Md.). 2020 Jul 1; [PubMed PMID: 31742361]
Pabaney AH, Reinard KA, Massie LW, Naidu PK, Mohan YS, Marin H, Malik GM. Management of perisylvian arteriovenous malformations: a retrospective institutional case series and review of the literature. Neurosurgical focus. 2014 Sep:37(3):E13. doi: 10.3171/2014.7.FOCUS14246. Epub [PubMed PMID: 25175432]
Level 2 (mid-level) evidenceCereda C, Ghika J, Maeder P, Bogousslavsky J. Strokes restricted to the insular cortex. Neurology. 2002 Dec 24:59(12):1950-5 [PubMed PMID: 12499489]
Nogles TE, Galuska MA. Middle Cerebral Artery Stroke. StatPearls. 2025 Jan:(): [PubMed PMID: 32310592]
Di Stefano V, De Angelis MV, Montemitro C, Russo M, Carrarini C, di Giannantonio M, Brighina F, Onofrj M, Werring DJ, Simister R. Clinical presentation of strokes confined to the insula: a systematic review of literature. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2021 May:42(5):1697-1704. doi: 10.1007/s10072-021-05109-1. Epub 2021 Feb 11 [PubMed PMID: 33575921]
Level 1 (high-level) evidenceHuff JS, Murr N. Seizure. StatPearls. 2023 Jan:(): [PubMed PMID: 28613516]
Isnard J, Guénot M, Sindou M, Mauguière F. Clinical manifestations of insular lobe seizures: a stereo-electroencephalographic study. Epilepsia. 2004 Sep:45(9):1079-90 [PubMed PMID: 15329073]