Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS), formerly called interstitial cystitis, is a chronic (>6 weeks duration) pelvic condition that affects or appears to affect the urinary bladder with symptoms of discomfort, pressure, or pain. The condition is characterized by chronic inflammation and lower urinary tract symptoms, not due to infection or any other clearly identifiable cause.[1][2] As the definition varies somewhat between different societies and organizations, we will use the American Urological Association (AUA) definition.
In many cases, because IC/BPS remains a diagnosis of exclusion, the condition is often identified late or misdiagnosed, particularly in men, as chronic prostatitis/chronic pelvic pain syndrome or overactive bladder.[3][4][5][6][7]
Patients often describe pain in the bladder or suprapubic region, with an intense sensation of urinary urgency. This sensation is worsened by bladder filling but is temporarily relieved by passing urine, which typically results in severe urinary frequency. This may be during the daytime and/or overnight. The urinary frequency is generally refractory to standard overactive bladder therapy, which should suggest considering a diagnosis of IC/BPS.
There may also be other symptoms, such as pain or burning when passing urine (dysuria) and discomfort during sexual intercourse, causing dyspareunia in women and ejaculatory pain in men. These chronic symptoms profoundly impact the patient's emotional, psychological, and social well-being as well as their quality of life.[8]
Anatomy
The urinary bladder is a hollow viscus organ located in the pelvis, anterior to the rectum in both sexes and the uterus in females. It is partially covered by the peritoneum on the superior surface. It is composed of 4 layers.[9]
- The mucosa, which contains the transitional epithelium, is the bladder lining and allows for the stretch of the urinary bladder. When stretched, the surface is smooth, but when relaxed, folds form in the mucosa, known as rugae. The mucosa also produces glycosaminoglycans (GAGs), making up the superficial coating that protects the mucosa from direct contact with potentially inflammatory urinary irritants.
- The submucosa is composed of elastic connective tissue and further assists with the stretch of the bladder.
- The muscularis layer (detrusor muscle) is composed of several layers (inner longitudinal, middle circular, and outer longitudinal) of smooth muscle oriented in multiple directions, which produce a uniform, simultaneous contraction that efficiently empties the bladder when a contraction is triggered.
- The muscles also form a small band that encircles the area between the opening of the bladder and the urethra. This is known as the internal urinary sphincter and is controlled by the autonomic nervous system. Another distal band around the urethra controls conscious voiding, called the external sphincter, composed of skeletal muscle and innervated by the somatic nervous system.[10]
- In women, the external urethral sphincter is distal and inferior to the bladder neck. The clitoris and pubic bone are anterior, while the anterior vaginal roof is immediately posterior. The external sphincter in women is also known as the urogenital sphincter.[10]
- The fibrous connective tissue layer is the outermost covering of the bladder, except for the superior surface, which is covered by the parietal peritoneum.[11]
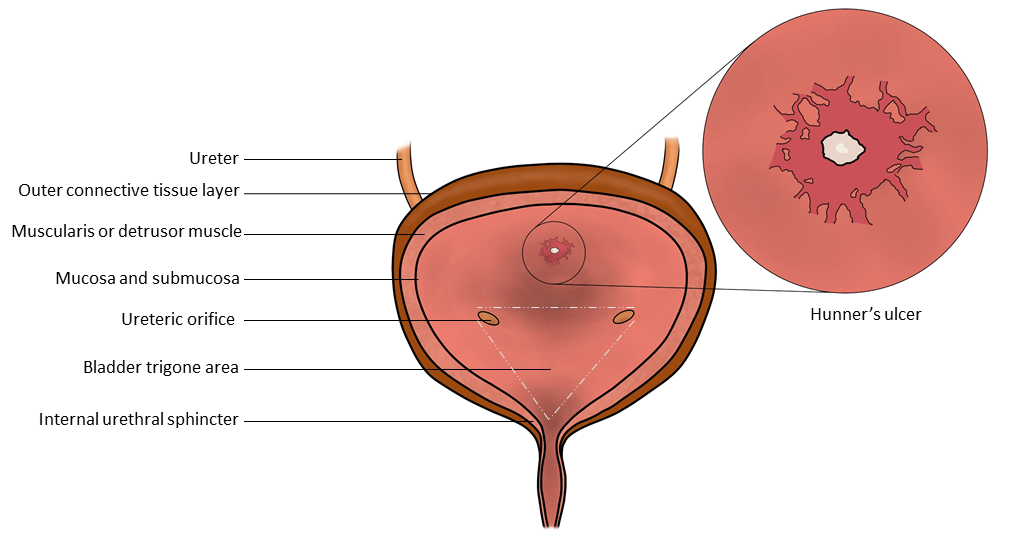
The bladder floor also contains a fixed, triangular area called the trigone. The openings to each ureter and the outlet to the urethra form the trigone. There are 3 apices, with a ureteral orifice in each lateral wing and the third arm entering the urethra.[11] The trigone is located at the posterior base, also known as the fundus of the urinary bladder.[12]
The distal intramural portion of each ureter follows a shallow submucosal course as it travels through the detrusor muscle into the bladder. This anatomy acts as an anti-reflux mechanism or valve to prevent urine reflux into the kidney.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of IC/BPS is not well understood, and the current thoughts around its pathogenesis remain multifactorial.
Current research strongly suggests an underlying inflammatory process, although the precise cause for this is not well-understood.[13]
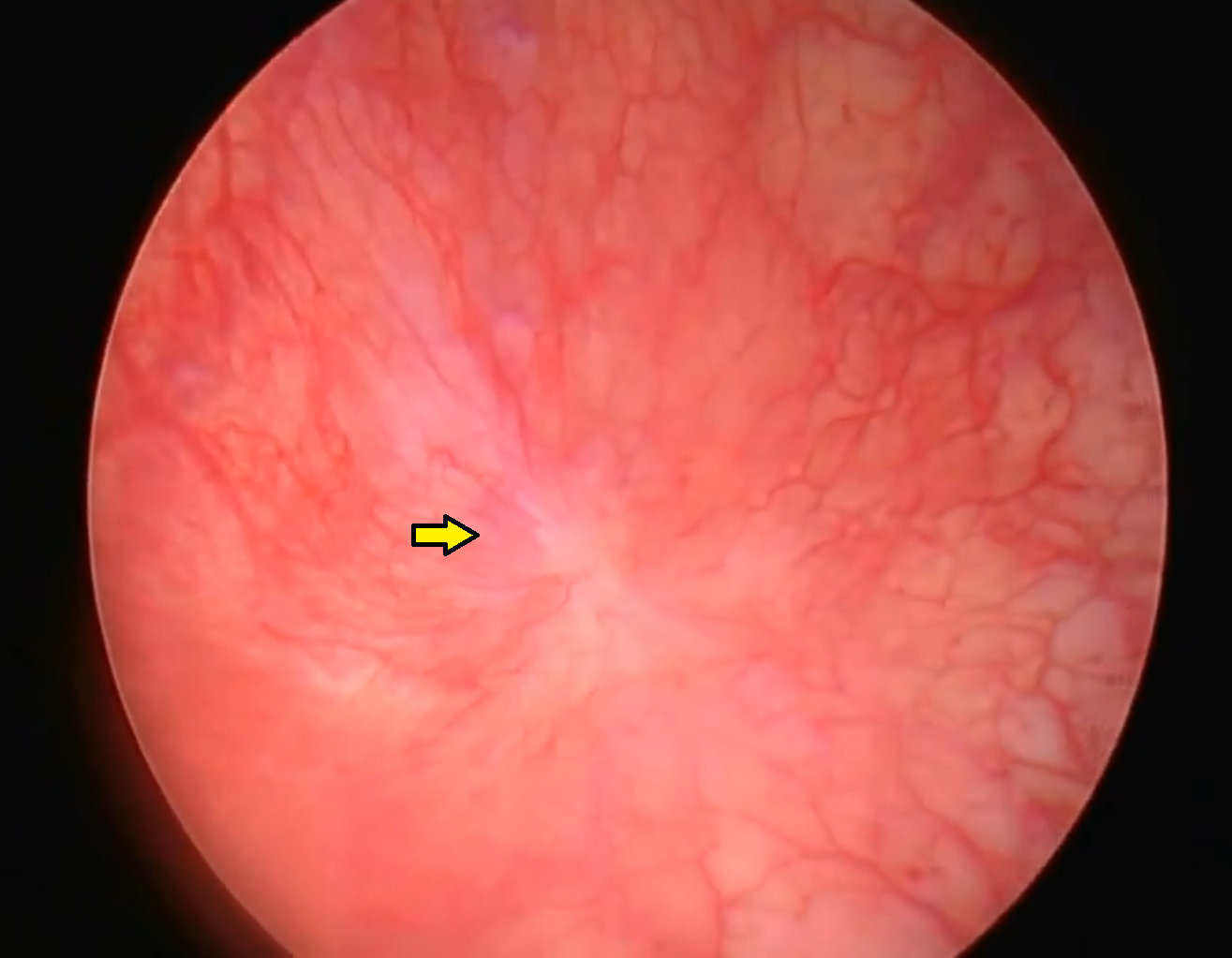
Cystoscopy findings in individuals with normal bladder function are characterized by a pale/pink mucosal appearance, a lack of erythema and swellings, and relatively straight capillaries (see Image. Normal Mucosa of the Bladder Wall). Cystoscopy in IC/BPS patients typically shows submucosal inflammation, which is seen as glomerulations (see Image. Abnormal Mucosa Wall). Large groups of mast cells are frequently found, further stimulating afferent sensory fibers. Increased urothelial permeability due to diminished GAG levels and ultrastructural abnormalities are seen on biopsies with a loss of tight junctions and adhesive junction proteins. This causes a loss of mucosal barrier protection, resulting in a "leaky urothelium." This is also thought to be the reason why immunoglobulin and immune mediators are detected at higher levels in the urine of affected individuals.[13][14][15]
Fibrosis also results from the chronic inflammatory process, evident by the upregulation of extracellular matrix proteins, increased myofibroblasts, and decreased capillary density, which reduce bladder capacity and lead to further stretching and stimulation of afferent sensory pain fibers.[16]
Increased grey matter volume has been found in some IC/BPS patients with the disorder in brain regions responsible for pain perception. This was observed using functional magnetic resonance imaging (fMRI).[17][18]
Severe IC-like symptoms can develop after the use of illegally sourced ketamine. The precise etiology of this response remains unknown. The main theories involve urothelial damage, microvascular changes, autoimmunity, and infection either by ketamine or metabolites. The symptoms, cystoscopy, and biopsy findings have a great degree of crossover with IC, and the main difference would be the recreational abuse of ketamine. The risk of developing ketamine cystitis does not appear to be increased with the proper medical use of the drug.[19]
Leading concepts regarding the underlying etiology of IC/BPS include the following: [20][21][22][23]
- Autoimmune or immune-mediated processes
- Chronic inflammation
- Chronic stress
- Fibrosis
- Heightened pain sensitivity due to an increase in grey matter volume
- Mast cell dysfunction or hyperactivation
- Neurogenic inflammation/edema
- Pelvic floor hypertonicity or dysfunction
- Upregulation and proliferation of sensory afferent fibers
- Urothelial dysfunction and exposure, especially in the epithelial and glycosaminoglycans (GAGs) layer
- Vascular malformations that are seen as glomerulations on cystoscopy
Epidemiology
Due to the nature of IC/BPS, it is very difficult to formulate a clear early diagnosis, and no accepted screening tool exists. Therefore, the data surrounding its prevalence remains limited.
Some studies have specifically looked into the epidemiology of IC/BPS. There are 2 main study designs, one using physician/urologist-based diagnosis and the other using a patient questionnaire involving symptomatology. A lower prevalence is seen when physician-completed questionnaires and histological samples were employed to establish a diagnosis.
- Based on a large population, questionnaire-based prevalence study in the US, 2.7% of women and 1.9% of men met the specified criteria.[24][25]
- Other studies have estimated the prevalence at >6% with a higher incidence in women.[26]
- In terms of age groups, the most common prevalence was in women between 50 to 59 years of age and men between 56 to 74 years of age.[24][25]
- IC/BPS has an estimated prevalence of 3 million to 8 million women and 1 million to 4 million men in the US.[27]
- The number of men affected is probably underestimated, as many are misdiagnosed as having chronic prostatitis.[3][4][5][6][7][26]
- Investigations have noted a female predisposition, with 1 study in the US finding a 5 to 1 ratio.[28]
- A study in the Netherlands quoted a prevalence as low as 8 to 16 out of 100,000 individuals.[29]
- It is estimated that IC/BPS affects up to 400,000 patients in the UK, with almost 90% being women between 50 to 69 years of age.[30]
- The data for children affected by IC/BPS is poor, but the consensus is that the prevalence is very low in the pediatric population.
Pathophysiology
The underlying pathophysiology of IC/BPS is somewhat uncertain, contributing to the lack of a definitive, curative remedy.[13] Known involved bladder pathological findings include chronic inflammation, afferent sensory hyperactivity, nociceptor upregulation, mast cell overactivity, submucosal microvascular abnormalities, autoimmune disorders, lack of normal bladder epithelial cell growth, and urothelial thinning with a deficient or dysfunctional superficial mucosal GAGs layer.[13][14][22][31][32][33]
Other findings associated with chronic bladder inflammation include decreased mucosal protection from GAGs loss or dysfunction, lower adhesive protein E-cadherin, decreased basal cell growth, defective umbrella cell integrity, abnormal apical cell maturation, and increased urothelial cell apoptosis.[31][34] The increased serum levels of C-reactive protein and pro-inflammatory cytokines also point to inflammation as a primary underlying etiology of IC/BPS.[14][15] Tumor necrosis factor-α (TNF-α) and nerve growth factor (NGF) are also increased in IC/BPS patients.[35]
IC/BPS can appear as two separate entities: the IC entity clearly demonstrates an inflammatory component that is mostly missing in those with the predominantly BPS entity.[36]
IC/BPS patients are much more likely than the general population to have systemic lupus erythematosus.[37] There is also a close association with Hashimoto's thyroiditis, rheumatoid arthritis, ankylosing spondylitis, irritable bowel syndrome, fibromyalgia, chronic fatigue, and especially Sjogren syndrome.[38][39][40] This suggests an autoimmune component, possibly an autoantibody to M3 receptors in the bladder, at least in some patients.[41]
Chronic stress is found in >50% of IC/BPS patients, which is a factor in the development and exacerbation of symptoms.[36][42][43][44][45][46]
The chronic pain typically associated with IC/BPS is caused by sensory afferent nerve activity and central nervous system sensitization.[47]
Histopathology
Histologically, no specific pathognomonic finding is diagnostic for IC/BPS.[48] However, compared to a similar control group, IC/BPS patients demonstrated a higher frequency and severity of bladder mucosal ulcerations with denuded superficial urothelium, submucosal inflammation of the lamina propria, and lymphocytic inflammatory infiltrate with mast cell accumulations in the lamina propria and detrusor.[49][50] While interesting, these findings are insufficient to be of any diagnostic help except to exclude malignancy and other pathologies.[48][51]
Urothelial defects, as seen on electron microscopy, appear to correlate well with symptom severity in patients with IC, which suggests a causal relationship.[13]
History and Physical
Symptoms must last at least 6 weeks with negative urine cultures and no acceptable explanation or alternative diagnosis.[1] Typical symptoms involve urinary frequency, nocturia, and suprapubic pain, which persist even after voiding. There may be urgency, nocturia, or dysuria, but usually no incontinence.[1] IC/BPS pain is usually suprapubic but may also be perineal. The discomfort is typically improved but not totally relieved by voiding. Incontinence is not a typical symptom of IC/BPS.
A baseline record of the patient's symptoms is useful to compare treatment efficacy. This may be a recommended pain scale index, a visual chart, or a patient-recorded 24-hour voiding diary.[1]
Pain can be evaluated and recorded using the IC symptoms index (ICSI), a visual analog chart, or the genitourinary pain index (GUPI).[52][53]
Voided volumes and a 24-hour timed voiding diary document the degree and severity of the patient's urinary frequency symptoms.[54][55][56]
Symptoms typically have periodic flares or exacerbations and remissions. As a complete remission is uncommon, over the long course of the disorder, patients often become anxious, depressed, and/or have difficulty sleeping.[57]
IC/BPS should be considered in all patients originally diagnosed with overactive bladder who do not improve on standard therapy.[1]
Unusually high voided volumes or infrequent voiding would suggest an alternative diagnosis.
Urinary and related symptoms to record and document include the following: [1]
- Number of voids per day
- Urine volume per voiding episode
- Episodes of incontinence
- Episodes of urgency
- Hematuria
- Details and characteristics of pain:
- Character
- Duration
- Dyspareunia (present or not) in women
- Dysuria (present or not)
- Ejaculatory dysfunction or pain (present or not) in men
- Location
- Pain relieved by voiding (present or not)
- Pressure or urge to void
- Relationship of pain to menstruation, food, or any other activity
- Severity (as well as any concomitant dysuria) should be recorded for at least 24 hours
- Time
- Unexplained fevers
- Vulvar pain
All patients suspected of having IC/BPS should have a neurological examination performed, including tone, reflexes, power, sensation, and cranial nerve testing. A post-void residual urine volume determination should be completed.[1][54]
A detailed gynecological history and pelvic examination should be performed on all female patients suspected of having IC/BPS.[1] Careful attention should be paid to cervical tenderness, masses, prolapse, and abnormalities found on adnexal palpation.
An appropriate hematuria evaluation should be performed in patients with unexplained or previously unevaluated urinary blood and those with symptoms of IC/BPS who also have a significant (≥10 pack-years) smoking history.[1][58]
A detailed history and careful examination are needed to exclude other causes, as there is considerable overlap between IC/BPS and other conditions.[1][8]
Interstitial Cystitis/Bladder Pain Syndrome and Chronic Prostatitis/Chronic Pelvic Pain Syndrome
Many experts believe that chronic prostatitis in men may often be misdiagnosed as IC, which may be the more common disorder even in males.[3][4][5][6][7] The primary differences are the lack of the urinary antiproliferative factor and histological bladder pathology in chronic prostatitis.[59] The 2 conditions have many similarities as both demonstrate the following:[3][4][6][7][60][61]
- Associated with psychosocial depression
- Characterized by chronic pelvic pain and urinary symptoms, usually urgency and frequency but not incontinence
- Diagnosis is primarily by exclusion
- Dietary changes often have a significant impact on the disorder
- Negative urine cultures
- Oral medications can offer symptomatic relief
- Pelvic floor dysfunction
- Physical therapy provides relief for the majority of patients
- Sensitivity to intravesical potassium instillation
- Symptoms are aggravated by the same foods and dietary irritants
- Symptoms may last for years
Men with IC/BPS or chronic prostatitis/chronic pelvic pain syndrome tend to have a higher incidence of erectile and ejaculatory dysfunction than unaffected males and are more likely to be associated with depression, pain, and stress.[62] Over 70% of men with IC/BPS report sexual dysfunction.[63]
Patients with IC/BPS who have not responded to standard therapy might have chronic prostatitis/chronic pelvic pain syndrome, and consideration should be given to a change in therapy. Likewise, patients with chronic prostatitis who have not responded to conservative treatment should be evaluated for possible IC/BPS.
Pudendal neuralgia can mimic IC/BPS in many ways. Common symptoms include chronic pelvic pain, sexual dysfunction, discomfort with sexual activity, and urinary dysfunction. Pudendal neuralgia patients often have very tight, dysfunctional pelvic floor musculature, but so can patients with IC/BPS.[64]
Patients with pudendal neuralgia tend to describe the pain as more intense and having a "burning" or "electrical shock" feeling. The pain is associated with sitting, bending, or squatting and is generally unrelated to bladder fullness or diet. They also tend not to have the degree of urinary frequency and urgency associated with IC/BPS. If in doubt, pudendal nerve blocks can be both diagnostic and potentially therapeutic, particularly in patients unresponsive to standard therapies for IC/BPS where pudendal neuralgia might be possible.[64]
Evaluation
The workup should include laboratory examinations and procedures to identify other disorders that can produce symptoms similar to IC/BPS. This typically includes standard blood tests (CBC, CMP, glucose, HbA1c), urinalyses (microscopic) and urine cultures, and appropriate sexual health screening, including testing for sexually transmitted infections as appropriate.
Urine cultures in patients with negative urinalysis are suggested to identify low or borderline bacteria levels that may still be clinically significant but escape detection on standard examinations.[1]
Neither cystoscopy nor urodynamics is required to diagnose IC/BPS according to the AUA guidelines. Neither is diagnostic, but they may be appropriate if the diagnosis is in doubt.[1]
IC/BPS patients with Hunner ulcers tend to be older with higher symptom scores, increased urinary frequency, more nocturia episodes, smaller voided volumes, and a reduced bladder capacity with hydrodistension than IC/BPS patients without Hunner lesions.[65] They were also more likely to have an autoimmune disorder comorbidity.
A number of urinary inflammatory biomarkers are increased in IC/BPS, such as TNF-α, PGE2, IL-2, IL-6, IL-8, IP10, TAC, and 8-OHdG, although the diagnostic and clinical utility of this finding still needs to be determined.[35][66] A recent study of urinary biomarkers in 191 pateints suggested that TNF-α, IP-10, TAC, 8-OHdG, and IL-2 could be useful in differentiating interstitial cystitis from other urinary disorders.[66] While promising, a definitive, clinically useful urinary cytokine or combination of biomarkers is still to be determined.[20][66]
Cystoscopy is appropriate if there is a suspicion of bladder cancer, intravesical foreign bodies, outlet obstruction, strictures, or vesical calculi. Glomerulations are often found but are considered non-specific. There are no specific cystoscopic findings diagnostic of IC/BPS other than a Hunner ulcer, which is more commonly found in patients over age 50 in whom a cystoscopy may, therefore, be justified.[67] Patients who fail conventional therapy may also be candidates for a cystoscopy, especially if they have not previously had the procedure, but the examination is generally discouraged in younger patients where Hunner ulcers are far less common, and it is likely to cause more complications and adverse effects.[1]
A Hunner ulcer is characteristic and diagnostic of classic IC. It is described as a central scar in an area of erythematous mucosa.[68] Small blood vessels can be seen radiating toward this scar, which will often have a small adherent blood clot. After hydrodistension, the surrounding mucosa ruptures, resulting in the bleeding typically associated with this pathological lesion.[68] Since many urologists rarely see a Hunner ulcer, reviewing a published atlas of these clinically important diagnostic lesions may be useful.[69]
Patients with Hunner ulcers generally respond well to treatment. Standard therapy for a Hunner ulcer includes immediate fulguration of the lesion and/or an injection of triamcinolone.[70] Symptom relief after Hunner ulcer treatment is reportedly as high as 97%.[71] If this fails, oral cyclosporine A is recommended.[72] Since the odds of identifying Hunner lesions are markedly reduced in patients younger than 50 years, routine cystoscopic examinations are not recommended or suggested for patients with IC/BPS symptoms in this age group.[1]
Bladder biopsies are not routinely necessary or recommended according to the AUA guidelines unless malignancy is suspected.[1] This is based on the relative rarity of bladder cancer in patients diagnosed with IC/BPS (1:600).[73]
However, guidelines from the European Association of Urology (EAU), the International Society for the Study of BPS (ESSIC), the International Continence Society (ICS), and the Canadian Urological Association (CUA) recommend hydrodistension with cystoscopic random biopsies due to the substantial overlap in visual appearance and symptoms between IC/BPS and bladder cancer.[74] In a recent study of 55 IC/BPS patients, 3 (5.5%) were found to have bladder cancer when random biopsies were performed.[74] It is unknown if urine cytology, FISH testing, or bladder washing would be sufficient to identify these cancers without requiring a more invasive biopsy.
Cystoscopy primarily rules out malignancy, strictures, Hunner ulcers, and bladder outlet obstruction.
- The appearance of bladder lesions can be very similar to malignancy, particularly carcinoma-in-situ.
- The bladder walls may show scarring or petechial hemorrhages, known as glomerulations, which are considered non-specific.
- During visualization of the distended bladder, there may be Hunner ulcers, often described as pale central scars with or without a fibrin clot and surrounded by erythematous mucosa, with small vessels radiating towards the center (see Image. Abnormal Bladder Mucosa, Showing a Hunner Ulcer).
- Hunner ulcers are most commonly located at the posterior bladder mucosa (see Image. Illustration of the Bladder Anatomy, with an Illustration of a Classic Hunner's Ulcer).
- Hunner ulcers are considered diagnostic for IC, but they are uncommon and may only be present in 5% to 10% of cases.[75]
- Patients with Hunner ulcers tend to have the most inflammation and require a more aggressive therapeutic approach.[13]
- Reactive hemorrhages are also a sign of IC. This is when the mucosa appears normal when distended on an initial examination under cystoscopy, but when deflated and re-inspected, points of capillary bleeding are noted on a background of normal mucosa.[76]
- Further investigations or treatments may also be performed during a cystoscopy, such as hydrodistention, lidocaine or intravesical cocktail instillation, fulguration and/or triamcinolone injections for Hunner ulcers, or random bladder biopsies.
Urodynamics can be helpful when patients are refractory to standard medical treatments or there is evidence of bladder outlet obstruction, detrusor hypotonicity, neurogenic bladder, or other conditions that might otherwise explain the patient's symptoms. There are no specific urodynamic findings that are diagnostic of IC/BPS. However, a small maximum bladder capacity (<300 mL) is frequently found. Urodynamic studies are not recommended in routine cases.[1]
Treatment / Management
Before commencing treatment, the American Urological Association (AUA) Guidelines recommend educating the patient about the disease's complex nature and the need for multimodal therapy, as patient education about IC/BPS is a critical part of the treatment plan.[1] An individualized approach, including shared decision-making for every patient, is suggested.
For most patients, no single treatment exists to cure the condition or permanently relieve the symptoms. Patients should also be aware that multiple treatment options in combination may be needed for optimal symptom control and that the condition is chronic, often with periodic exacerbations and remissions.[1]
As the underlying pathophysiology is still largely unknown, treatment is based on managing the symptoms.
Treatment should be evaluated periodically, and ineffective therapies should be discarded. If simple pain management techniques are not adequate, additional therapy should be used, and consideration given to a pain management consultation as a multidisciplinary approach is recommended. Failure of multiple modalities of therapy suggests a possible misdiagnosis.
Conservative Measures
Initial treatment for IC/BPS should be based on dietary and lifestyle modifications. The determination of specific dietary adjustments is based on known common irritants. Elimination diets are used to identify additional exacerbating dietary factors. The final recommended diet should be individualized, with a diary to record which foods, beverages, additives, or substances cause symptoms to flare.
Generally, a number of common foods are known to irritate sensitive bladders, cause pain, and exacerbate urinary symptoms. A complete list can be found at the website for the Interstitial Cystitis Association at www.ichelp.org.The prevailing theory is many foods contain substances or chemicals that are inherently irritating to the bladder, such as a high acid content, potassium, or capsaicin. Indian, Mexican, and Thai food is not recommended. Specific foods known to cause bladder irritation include the following: [1]
- Alcohol
- Benzyl alcohol
- Caffeine
- Carbonated beverages
- Coffee
- Chili
- Chocolate
- Citric acid
- Citrus
- Cranberry juice
- Horseradish
- Hot peppers
- "Hot" sauces such as Tabasco
- Ketchup
- Pickles
- Pizza
- MSG
- Sauerkraut
- Spices
- Sweeteners (particularly artificial sweeteners such as saccharin)
- Tea
- Tomato juice
- Tomato sauce
- Tomatoes
- Vinegar
- Worcester sauce
Myofascial release, physiotherapy with relaxation exercises, and pelvic floor trigger point manual therapy provided by a skilled practitioner have shown significant symptom relief in 70% of patients.[77] Standard pelvic floor strengthening treatments, such as Kegel exercises, may cause IC/BPS symptoms to worsen and are not recommended.[1][78] (A1)
Specific behaviors and activities that can help mitigate symptoms include the following: [1]
- Avoidance of known dietary irritants (see list above)
- Bladder training with urge suppression
- Cognitive-behavioral therapy
- Dietary aids for bladder discomfort (calcium glycerophosphates, nutraceuticals, phenazopyridine)
- Increasing fluid intake to minimize urinary concentration
- Manual physical therapy for pelvic floor tenderness to relieve tender muscle points, contracture lengthening, and/or scars
- Meditation and imagery techniques for pain management
- Neuromodulation (can help urinary frequency/urgency but is relatively ineffective for pain control)
- Pelvic floor muscle relaxation training
- Stress management and reduction techniques
- Support groups
- Transcutaneous electrical nerve stimulation (TENS)
- Use of elimination diets to help determine which additional specific foods should be avoided
- Use of heat or cold to bladder and/or perineum for symptomatic relief
- Avoidance of behaviors and activities that are known to exacerbate bladder irritability, such as:
- Pelvic floor muscle strengthening exercises (Kegels)
- Prolonged or untreated constipation
- Sexual intercourse
- Tight clothing
Pain Management
Most patients do not respond to a single agent, and a complex multidisciplinary option with expert guidance under a pain specialist or clinic should be sought. Treatment should be introduced slowly, with the lowest possible dose and most minimal number of drugs used as possible, in line with the WHO pain ladder. Non-opioid treatments are much preferred over opioid therapy.[79]
- Acetaminophen
- Amitriptyline
- Gabapentin
- Hot sitz baths
- Intravesical "cocktail" therapy
- Meditation and imagery
- NSAIDs (ibuprofen, naproxen)
- Opioids
- Pregabalin
- Stress reduction
- Transcutaneous electrical nerve stimulation (TENS)
- Trigger point manual therapy
Pharmacological Therapy
Oral therapies have been used with moderate success for IC/BPS. Oral overactive bladder (OAB) medications, such as oxybutynin, have limited efficacy in controlling the urinary symptoms from IC/BPS, except possibly for some decrease in urinary frequency. Overactive bladder patients who fail to improve on OAB medications should be evaluated for possible IC/BPS.
The most effective oral agents for treating IC/BPS are amitriptyline, cyclosporine A, and pentosan polysulfate.[80][81](A1)
Amitriptyline is a tricyclic antidepressant that has shown activity in reducing chronic pelvic pain, including discomfort from IC/BPS.[82] Adverse effects (drowsiness, nausea, lethargy, sedation) limit usefulness.[83][84][85] Low-dose (10 mg) amitriptyline combined with intravesical instillation therapy is very effective, especially in improving the emotional status of patients with IC/BPS.[86] Standard dosing starts at 10 mg daily and gradually increases to a maximum of 100 mg as tolerated.[1][83](A1)
Cimetidine is an H2 blocker normally used for reducing gastric acid secretion, but it has also shown activity in reducing pain, frequency, and nocturia in approximately 60% to 70% of IC/BPS patients.[87][88][89][90] The mechanism for this is unclear and occurs regardless of the presence or absence of increased mast cells in the bladder tissue. No significant adverse effects are reported with its use, and it is very cost-effective as it is not only generic but available without a prescription.[87][88](A1)
Cyclosporine A, an immunosuppressive drug that blocks cell-mediated immune reactions, has shown superior efficacy compared to pentosan polysulfate, particularly in patients with Hunner ulcers who do not respond to triamcinolone injections or electrofulguration.[1][72][91][92] A response rate of 84% has been reported in IC/BPS patients with Hunner ulcers treated with cyclosporine A.[81] It has also been suggested that it be considered for use in otherwise intractable cases.[91](A1)
While cyclosporine A has shown good efficacy in controlling pelvic and bladder symptoms, it has only been used in relatively small numbers of IC/BPS patients. There are serious potential complications with regard to increased blood pressure, neurotoxicity, higher incidence of malignancies, greater infection risk due to immunosuppression, and decreased renal function.[1][93] It also has a relatively short duration of action. For these reasons, it is suggested to be used only by those familiar with this therapy and in patients with Hunner ulcers who fail other treatments.
Currently, cyclosporine A is the only immunosuppressive agent recommended by the AUA for clinical use. After a full and frank review of the risks and potential benefits, a mutual decision between the doctor and patient is needed. The patient must be fully aware of systemic immunosuppression risks, such as increased vulnerability to infection and the more rapid development of malignancies.
Experimentally, low-dose (1.5 mg/kg or less) cyclosporine A daily therapy has shown efficacy in providing long-term symptom relief when used immediately after Hunner ulcer fulguration. The lower dose limits adverse effects. Further studies are necessary until this approach's long-term safety and efficacy have been conclusively determined.[94](B3)
Gabapentin has shown some activity in reducing pain and other symptoms of IC/BPS.[95][96][97][98] Gabapentin was originally approved as an antiepileptic but has since found a role in managing various types of neuropathic pain. Dosage starts at 300 mg TID, which is then slowly titrated up to a maximum of 1,200 mg TID.[99](B3)
Hydroxyzine is an antihistamine and mast cell stabilizer that has shown effectiveness in providing significant symptom relief in some IC/BPS patients. Those IC/BPS patients with allergies may most likely benefit from this treatment.[100] Hydroxyzine prevents the degranulation of mast cells, reducing the release of toxic agents that would otherwise cause inflammation and IC/BPS symptoms in some patients. The degranulation of mast cells causes the release of neuroactive and vasoactive agents and is believed to be responsible for the symptoms of IC/BPS, at least in some patients.[101] Adverse effects tend to be minor and include mild sedation and weakness. The dosage is usually 50 mg to 100 mg QID.(B3)
Overactive bladder (OAB) medications (oxybutynin, mirabegron, etc) are usually insufficient when used alone in managing the urinary symptoms of IC/BPS. These medications may be beneficial in reducing frequency and urgency, but they generally do not help with pain where a multimodal approach is typically needed.[102][103] Patients with significant OAB symptoms who fail standard treatment should be considered for possible IC/BPS.
Pentosan polysulfate is the only FDA-approved oral medication approved for IC/BPS.[1] It acts by restoring a protective layer over the bladder mucosal lining. Studies on its efficacy are conflicting; some show a benefit while others do not.[100][104][105][106][107][108] Maximum symptomatic relief may take up to 5 or 6 months or longer.[109][110](A1)
A worrisome retinal pigmentary maculopathy has now been clearly associated with the long-term, cumulative use of pentosan polysulfate.[111][112][113][114][115] Symptoms include blurred vision, delayed adjustment to low-light situations, and difficulty reading. Patients should be warned about these effects, that they are related to the total cumulative amount of therapy, and that the visual damage does not appear to be reversible.[1][110][116] (B2)
For those who choose to use the medication, a retinal examination prior to initiating treatment is recommended, and a recheck at 6 months and periodically thereafter.[1][110] Recent evidence suggests that the retinal macular damage may continue even after cessation of the pentosan polysulfate, and the damage is irreversible.[116] This new data is very worrisome and makes pentosan polysulfate a far less attractive therapy as an oral agent.[117] For these reasons, pentosan polysulfate is now used infrequently for IC/BPS.
Sacral neuromodulation has been shown in most studies to be a reasonably effective treatment for controlling intractable bladder overactivity and chronic pelvic pain in some IC/BPS patients.[118][119] It is FDA-approved only for severe bladder overactivity and frequency, for which it seems more effective than its effect on pain control in IC/BPS.[1][120] Tibial nerve stimulation has also shown a benefit in treating IC/BPS.[121][122] A clinical trial of sacral neuromodulation or tibial nerve stimulation can be reasonably considered in selected IC/BPS patients who have failed other treatment modalities.[1][118][120][123](A1)
Intravesical Instillations
Various medications are effective when used as intravesical instillation therapy in IC/BPS when oral pharmaceuticals and dietary measures alone are insufficient.[124] No optimal or ideal cocktail has yet been agreed upon. The most commonly used medications include the following:
Dimethylsulfoxide (DMSO) is an effective agent for symptom control when used as an intravesical instillation in patients with IC/BPS.[125][126][127][128] It acts as an anti-inflammatory and a topical anesthetic, rapidly absorbed into the bladder mucosa and detrusor. It is a carrier, meaning it greatly enhances the absorption of other agents with which it may be combined. This should be considered when putting together a cocktail "recipe" to avoid potential toxicity. It is often mixed with bupivacaine or lidocaine, heparin, sodium bicarbonate, and hydrocortisone or triamcinolone.[1] (A1)
DMSO by itself is odorless, but when used in patients, it causes an extremely strong garlic odor that can last up to 72 hours following intravesical administration. This makes it unacceptable for some patients.
The results were similar when instillations of DMSO alone were compared to applications of a standard intravesical cocktail of bupivacaine, heparin, and triamcinolone.[129] However, bladder instillations of DMSO plus triamcinolone were found to be significantly (50%) superior in symptom relief compared to the same standard intravesical cocktail of bupivacaine, heparin, and triamcinolone in newly diagnosed women with IC/BPS.[126] There is also evidence that DMSO may be more effective in patients with Hunner ulcers.[130](A1)
DMSO becomes painful if held in the bladder longer than about 20 minutes. The typical dose is 50 mL of a 50% DMSO solution. Intravesical instillation of DMSO is FDA-approved and recommended by the AUA guidelines.[1]
Some experts have added gentamicin, an aminoglycoside antibiotic, into intravesical cocktail formulas in certain situations. Antibiotics are not routinely recommended for intravesical IC/BPS therapy, but adding gentamicin may be a reasonable precaution for patients who have had recent infections or where there is evidence of bacteriuria or pyuria. Patients with an active infection should not have IC/BPS intravesical instillations until the infection is cleared. There is no evidence that gentamicin alone has any significant efficacy in treating IC/BPS directly. The typical dose is 80 mg.
Heparin acts as a synthetic GAG layer to help protect the bladder mucosa from urinary irritants when used for intravesical therapy in IC/BPS patients. Initial studies with heparin used only 10,000 IU 3 times a week for 3 months and reported significant improvement in 56% of patients.[131] Heparin is more effective than lidocaine alone for relieving IC/BPS symptoms.[132][133] Most intravesical instillation cocktails include heparin, which the AUA guidelines recommend.[1][134](A1)
The typical dose is 10,000 IU to 20,000 IU, but dosages up to 50,000 IU have been used as the optimal dose has not been determined.[134]
Hyaluronic acid alone or with chondroitin sulfate has shown some efficacy in treating patients with IC/BPS.[135][136][137][138] Both of these chemicals are decreased in chronically inflamed bladders. Hyaluronic acid is a mucopolysaccharide non-sulfated component of the normal bladder GAG layer. There is no standard dosing schedule or good data on this medication, but the most recent review used 40 mg starting at several treatments a week and then extending the time interval based on the response. The Spanish Association of Urology conducted the review through the Functional, Female, and Urodynamic Urology Group, which found that dosage and schedule to be safe and effective.[135] A study from Taiwan used a schedule of 4 weekly intravesical treatments followed by monthly therapy for 5 months. They reported improved urinary symptoms in 73% of patients.[139] Another study of intravesical hyaluronic acid found it to be more effective for relieving bladder pain than alleviating urinary storage symptoms.[140](A1)
Lidocaine is a local anesthetic that has demonstrated usefulness in reducing short-term (<2 weeks) bladder pain and symptoms when used for intravesical therapy. Lidocaine reduces histamine release from mast cells, exerts an anti-inflammatory effect on eosinophils, decreases leukocyte adherence, and is bactericidal.[141] Alkalinization with sodium bicarbonate improves bladder tissue penetration, potentially leading to toxicity.[132][133] (A1)
The AUA guidelines recommend lidocaine as an effective agent for IC/BPS when used in an intravesical cocktail.[1] A typical intravesical dose is 25 mL to 50 mL of a 2% lidocaine solution or viscous gel. Bupivacaine (a longer-acting lidocaine analog) 0.5% is usually dosed at 10 mL to 20 mL for intravesical use. Sodium bicarbonate (alkalinizer) is typically given as an 8.4% solution in 5 mL to 50 mL volumes.
Misoprostol is a prostaglandin E1 analog used orally to help prevent gastric ulcers from heavy NSAID use by interfering with nuclear factor kappa B (NF-kappa B) signaling activity. This interference affects cytokine production, DNA transcription, immune response, and cell survival.[142] This same pathway is activated in patients with IC/BPS bladders.[143] Additionally, misoprostol acts synergistically when used with cyclosporine and enhances GAG synthesis.[144] (B3)
It was theorized by Dr. Ray Rackley at the Cleveland Clinic that misoprostol might be helpful when used intravesically for IC/BPS. It is typically added to intravesical cocktails when they are ineffective before advancing to more invasive therapeutic options.[145] There are isolated anecdotal reports and small studies of misoprostol helping IC/BPS patients both as an oral agent (200 mcg TID) and as a supplement to an intravesical cocktail (200 mcg to 400 mcg).[146][147](B3)
Triamcinolone is a frequent ingredient in IC/BPS intravesical cocktails as an anti-inflammatory agent, but its effectiveness is unclear. A randomized trial of 2 identical cocktails, except for adding triamcinolone to one, demonstrated no detectable difference in efficacy as both groups improved similarly.[148] The studied cocktails did not include DMSO, which has proven efficacy when combined with triamcinolone.[126] Taken together, this suggests some efficacy, particularly when combined with DMSO. Triamcinolone is a frequent ingredient in many intravesical cocktails used for IC/BPS. The typical dosage used is 40 mg or 80 mg.(A1)
Intravesical triamcinolone injections for patients with Hunner ulcers have clearly shown effectiveness in IC/BPS and are recommended by the AUA guidelines when these pathognomonic lesions are found.[1][149]
Electromotive Drug Administration (EMDA)
EMDA is a method of improving bladder tissue penetration of intravesical drug instillations. A low-voltage gradient is created using electrical probes in the bladder and abdomen, creating a voltage differential that enhances drug delivery and improves tissue penetration.
A small randomized prospective trial demonstrated improved short-term efficacy.[150] The main issue with the study is the small sample size (n=31). Additional extensive, randomized, long-term studies are needed to compare EMDA to current passive diffusion to determine its ultimate efficacy, cost-effectiveness, safety, and utility.[151](A1)
Botulinum Toxin Type A Detrusor Injections
Botulinum toxin A has been widely used for neurogenic detrusor overactivity, overactive bladder, and detrusor-sphincter dyssynergia to help control urinary symptoms in patients with IC/BPS. Botulinum toxin A can inhibit the release of sensory nerve terminal vesicles, which can help reduce inflammation in addition to its effect on bladder motor activity.[31] It is effective for individuals with IC/BPS when used alone or with hydrodistension and can also reduce pain.[152][153][154][155][156][157][158] The overall success rate with botulinum toxin type A bladder injections is reported to be over 60%.[159](A1)
Retreatments with botulinum toxin A remained effective when the initial therapeutic benefit faded.[160]
The European Association of Urology recommends hydrodistension followed by botulinum toxin detrusor injections for intravesical instillation therapy failures.
Postvoid residual volumes tend to increase, and even successfully treated patients may notice some dysuria or difficulty in voiding. The therapy will eventually wear off after some months, so retreatments are to be expected. There is also a risk of urinary retention after botulinum toxin A bladder injections, which is why it is most useful in patients who can do intermittent self-catheterization if necessary.
A 2020 meta-analysis reviewed treatment efficacy in IC/BPS symptom control from all available treatments as reported in 81 randomized, controlled trials. The review focused on antidepressants, neuromuscular blockade (botulinum toxin A), and pentosan polysulfate therapies. Intravesical instillations were not included due to inadequate numbers meeting reporting and statistical criteria. The study found improved outcomes only from botulinum toxin A therapy.[161](A1)
Intravesical detrusor injections of botulinum toxin A are recommended in the AUA guidelines for use when other oral and intravesical options have failed, but only for patients willing and able to perform intermittent self-catheterization after treatment if necessary.[1] Alternate delivery systems that do not require detrusor injections are being studied.
Cystoscopy Guided Treatment
- Cystoscopy is performed to rule out other diagnoses and to look for Hunner ulcers.
- If Hunner lesions are present, they can be treated using direct fulguration with electrocautery or laser and/or injection of triamcinolone.
- Oral cyclosporine A is recommended if this fails.
- If no Hunner ulcers are found at cystoscopy, short-duration, low-pressure hydrodistension under anesthesia can be performed.
- Low-pressure, short-duration hydrodistension under anesthesia offers substantial pain relief to some patients but risks a temporary symptom flair.[162]
- High-pressure hydrodistension under anesthesia is not recommended due to inconsistent symptom improvement and an increased risk of sepsis and bladder rupture.[163] (B2)
Neuromodulation
Neuromodulation has been shown in most studies to be a reasonably effective treatment for controlling intractable bladder overactivity and chronic pelvic pain in some patients who have not achieved adequate symptom control with other modalities.[119] Neuromodulation therapy is FDA-approved only for intractable bladder overactivity and frequency, which seems more effective than its effect on pain control in IC/BPS.[1][118][120][164][165][166](A1)
Sacral neuromodulation has been shown in most studies to be a reasonably effective treatment for controlling intractable bladder overactivity and chronic pelvic pain in some IC/BPS patients.[118][119] It is FDA-approved only for severe bladder overactivity and frequency, for which it seems more effective than its effect on pain control in IC/BPS.[1][120] (A1)
The treatment is relatively invasive, expensive, frequently requires reprogramming, may need a reoperation, and lacks long-term data on efficacy. Complications include infection or pain at the implantation site, lead migration, and loss of efficacy over time. Tibial nerve stimulation has also been beneficial in treating patients with IC/BPS.[121][122] (B3)
A clinical trial of sacral neuromodulation or tibial nerve stimulation can be reasonably considered in selected IC/BPS patients who have failed other treatments.[1][118][120][121][122][123](A1)
Treatments Specifically Not Recommended
Treatments that are specifically not recommended by multiple sources (the American Urological Association, the National Institute for Clinical Excellence, and the European Association of Urology) include the following:
- Intravesical BCG
- Intravesical clorpactin (oxychlorosene)
- Long-term oral antibiotics
- Prolonged high-pressure bladder hydrodistention
- Prolonged oral steroids
Surgical Treatment
Surgery has recently seen a fall in popularity, especially after the introduction of intra-detrusor injections of Botulinum toxin A. However, surgery remains the last resort option for patients with intractable disease as a final resolution of symptoms. About 75% of patients can expect pain relief with open surgery. A urethrectomy is usually not necessary and adds considerable time and morbidity to the procedure.[167]
A supra-trigonal bladder resection with augmentation ileocystoplasty has been suggested as it appears to be a successful surgical therapy for most of these patients and avoids the need for a urostomy.[167][168][169][170] Good results have been reported with over 14 years of follow-up using this procedure.[170][171] Concern about inadequate symptom control, if the trigone is not removed appears unfounded.[170][171] A combined robot-assisted laparoscopic and mini-laparotomy approach for this procedure has been described and used successfully.[167][170](B2)
A summary of possible last-resort surgical options includes the following: [172]
- Cystoplasty only
- Cystoplasty with supra-trigonal resection
- Cystoplasty with sub-trigonal resection
- Urinary diversion, with or without cystectomy, usually with an ileal conduit
Investigational Therapies
Monoclonal antibody therapy has shown some promise, but additional research needs to be done to find the ideal antibody mix.[173]
Certolizumab pegol is an investigational pharmacologic therapy under development for treating IC/BPS.[82]
Chondroitin sulfate (2% solution) is approved for intravesical use in Europe and Canada. Chondroitin appears to repair, restore, or replace the mucosal GAG layer and is often used with hyaluronic acid.[174][175][176][177](B3)
Hyperbaric oxygen therapy appears beneficial in treating IC/BPS patients, but it requires a special chamber, is not very cost-effective, and is quite time-consuming.[178][179] There is limited data on long-term results.(B3)
Intravesical liposomes have shown activity in reducing symptoms of IC/BPS.[180][181] Liposomes can be used as biocompatible lipid-based drug carriers for intravesical therapy. They tend to stick to the bladder walls, and their lipid-based walls reduce mucosal inflammation while promoting and facilitating drug penetration (such as Botulinum toxin type A) into bladder tissue.[181] While promising, only limited data is currently available, and long-term, randomized prospective trials are needed.
Mesenchymal stem cells may be valuable in treating patients with IC/BPS as they have been shown to minimize bladder tissue injury and inflammation. After migrating into the detrusor tissue, they differentiate into bladder cells, inhibit mast cell buildup, decrease cellular apoptosis, reduce inflammation, mitigate oxidative stress, diminish collagen deposition, and promote normal regenerative functions. Mesenchymal stem cells also secrete exosomes and other factors that protect against tissue injury.[182]
Platelet-rich plasma is widely used to promote wound healing.[183] It contains abundant cytokines and growth factors that affect the inflammatory process to promote healing and reduce neuropathic pain.[13][184][185] Direct platelet-rich plasma detrusor injections have been shown to increase urothelial cell growth, decrease inflammation, improve mucosal lining barrier function, repair ultrastructural damage, and relieve symptoms.[13][178][186][187][188][189][190](B2)
Low-energy shock wave therapy has experimentally shown a benefit in reducing bladder pain, decreasing tumor necrosis factor-α (TNF-α), and lowering nerve growth factor (NGF) in IC/BPS patients.[191] It suppresses bladder overactivity, reduces the inflammatory response, and increases urothelial permeability, making it potentially valuable for enhancing the penetration of therapeutic drug instillations of bladder tissue.[192] (B3)
A preliminary study has shown that low-energy shock wave therapy can allow for bladder muscle absorption of intravesical instillation of botulinum toxin A with a corresponding improvement in IC/BPS symptoms.[192] The benefit of this approach is that it does not require invasive detrusor injections or anesthesia, appears to be safe and effective with no reported adverse effects, and combines the benefits of 2 different therapeutic modalities. These results are very preliminary, and substantial study is needed before such treatments can be recommended for clinical use.
The chronic inflammatory bladder changes and negative bacterial cultures associated with IC/BPS could be explained by a viral infection such as Epstein-Barr virus (EBV), found in up to 87.5% of patients.[193] This suggests the possible use of valacyclovir as an antiviral treatment in infected individuals. Early unpublished data suggest this may be a promising treatment, particularly for patients unresponsive to other therapies who are shown to have EBV exposure.[13] (B3)
Treatment Summary
- Conservative treatment of IC/BPS involves dietary adjustments, pelvic floor relaxation therapies, myofascial release and trigger point therapy, and stress reduction.
- Initial oral pharmacotherapy includes amitriptyline, cimetidine, and hydroxyzine.
- Oral pentosan polysulfate may be effective in some patients but is associated with potentially significant and permanent retinal damage.
- OAB medications can help with urinary symptoms but are generally insufficient when used alone.
- Gabapentin may be useful for pain control.
- Cyclosporine A can be used in patients with Hunner ulcers who have failed fulguration and/or triamcinolone injection therapy.
- Intravesical instillations are very effective non-surgical therapies. Recommended drug cocktail options include DMSO, lidocaine (or bupivacaine) with sodium bicarbonate, and heparin.
- Additional intravesical drugs include chondroitin sulfate, hyaluronic acid, misoprostol, and triamcinolone.
- Cystoscopy with low-pressure, short-duration hydrodistension and possible direct Hunner ulcer therapy should be considered for patients who continue to have symptoms.
- Patients who do not respond adequately to the above treatments may be candidates for the following:
- Botulinum toxin A detrusor injections
- Cyclosporine A
- Neuromodulation
- Misoprostol (oral or intravesical)
- Tibial nerve stimulation
- Definitive surgical therapy is reserved for patients who continue to have significant symptoms that have not been adequately controlled with any of the listed alternative treatments.
Differential Diagnosis
The possible list of differential diagnoses is extensive, and this list is not exhaustive, but the causes can be broken down into categories as follows: [194]
Infections
- Sexually transmitted infections such as chlamydia and gonorrhoea
- Tuberculous infections of the bladder
- Urinary tract infections
Urological and Urogynecological Conditions
- Bladder stones
- Chronic urethral syndrome
- Distal ureteral calculi
- Pelvic organ prolapse
- Prior pelvic surgery, especially involving mesh
- Prostate pathology, including but not limited to benign prostatic hyperplasia, prostate cancer, and chronic prostatitis
- Neoplasms of the urinary bladder
- Neurogenic bladder
- Overactive bladder
- Stress incontinence
- Urge incontinence/detrusor instability
Gynecological Conditions
- Endometriosis
- Pelvic inflammatory disease
- Pregnancy
- Vulvodynia
Neurological
- Cauda equina/cord compression
- Demyelinating diseases
- Diabetic neuropathy
- Pudendal neuralgia
- Stroke
Other Causes
- Congenital bladder malformations
- Diabetes
- Diverticular disease
- Inflammatory bowel disease
- Radiation-induced effects
- Rheumatoid arthritis
- Trauma
- Urinary diverticula
Prognosis
The majority of patients suffer from chronic symptoms with periodic exacerbations and remissions of varying intervals, but there is a wide variation in prognosis, course, and resolution. Patterns of the disease have been observed and described. These include the following:
- Complete resolution
- Relapsing-remitting
- Intermittent disease flares
- Chronic progression
The patient may also experience spontaneous resolution and unexpected recurrences regardless of treatment.[195]
IC/BPS appears to be an extremely stable condition, as urinary symptoms and discomfort tend to remain unchanged in severity for up to 9 years.[196]
Complications
If left untreated and without spontaneous resolution, the bladder may undergo more fibrosis, further reducing the bladder volume and further compounding the symptoms experienced. The patient would experience psychological and social health deterioration through sleep disturbance, sexual dysfunction, anxiety, depression, and social embarrassment.
Treatment complications depend on various factors, such as the pharmacological agent, cystoscopy, or surgical complications. Proper counseling and consent before treatment are paramount.[197] Complications can be broadly categorized as:
- Bleeding
- COVID-19 [198]
- Death (from procedure or anesthesia)
- Infection (introduced through intravesical routes or surgery)
- Perforation of the bladder during high-pressure hydrodistention
- Trauma
- Anesthetic complications are predominantly due to human factors and have an estimated mortality of 0.4 per 100,000 patients.[199] Anesthetic complications include the following: [200]
- Anaphylaxis
- Aspiration
- Cardiovascular collapse
- Medication errors
- Nausea and vomiting
- Respiratory depression
There is also a theoretical risk of urinary tract infections. The symptoms may change and worsen during this time, and tests for infection should always be checked in those circumstances. This is due to the damage and breach of the urothelium, which increases the probability of bacterial invasion, bladder damage, and symptoms. A short course of therapeutic antibiotics could be started in selected cases like these, but prolonged treatment is not recommended for therapy or prophylaxis.
Deterrence and Patient Education
Once the diagnosis is made, the patient should be counseled on what that means, why they experience the symptoms, and that the etiology is not well-understood at present.
The patient should be directed and encouraged to engage with self-help and local support groups, such as those offered by the Interstitial Cystitis Association (https://www.ichelp.org/) in the United States and Bladder Health UK or PainUK in the United Kingdom. Some patients who have used self-help resources found it a very effective treatment option.[201]
There are many treatments for the condition, with variable results. It can take up to months before the patient notices an improvement, and the effects may be only marginal. Patients must understand that the condition may not be cured, and symptoms may return anytime. However, with proper treatment, there can be significant improvements in symptoms and quality of life.
Pearls and Other Issues
Recent evidence suggests that many men diagnosed with chronic prostatitis/chronic pelvic pain syndrome may have IC/BPS as the symptoms are very similar, and each is a diagnosis of exclusion.[3][4][5][6][7]
Men diagnosed with chronic prostatitis/chronic pelvic pain syndrome who fail to respond to the standard therapy for prostatitis and continue to have symptoms, including frequency, painful bladder filling, suprapubic discomfort, dysuria, urgency, or pelvic discomfort, should be considered for treatment of IC/BPS.[3][4][5][6][7]
Pudendal neuralgia and nerve compression should also be considered when dealing with patients with IC/BPS symptoms, especially those unresponsive to conservative, oral, and intravesical therapies.[202]
Pain management should be involved in the care of patients with IC/BPS who have ongoing chronic pain.
Before progressing to invasive therapies when oral and intravesical instillations have failed, consider a trial of cyclosporine A, misoprostol (oral or intravesical), botulinum toxin A, or neuromodulation therapy.
Failure of all non-invasive therapies should suggest a reconsideration of the underlying diagnosis.
There is no need to wait for oral therapies to fail before starting intravesical instillations in appropriate cases.
A number of urinary inflammatory biomarkers are increased in IC/BPS, such as TNF-α, PGE2, IL-6, and IL-8, although the diagnostic and clinical utility of this finding still needs to be determined.[35] A clinically useful biomarker for IC/BPS is still awaiting discovery.[20]
Supplements
For most dietary supplements used for pelvic pain or IC/BPS, there is only very limited scientific data or proof of efficacy, but there is some interesting, if limited, favorable evidence:
- L-arginine at a dose of 1,500 mg/day has been shown to improve symptoms of IC/BPS by increasing urinary nitric oxide synthase activity in a preliminary randomized, double-blinded, placebo-controlled trial. About half of the patients treated noted symptomatic improvement.[203]
- Omega-3 fatty acids and alpha lipoic acid are effective anti-oxidants and have shown some anti-inflammatory effects and pelvic pain relief.[204][205]
- Quercetin is a bioflavonoid that may also help reduce inflammation by scavenging free radicals. It has shown some clinical activity in improving symptoms in IC/BPS in a limited study that was not placebo-controlled or randomized.[206]
- Calcium glycerophosphate should be taken before meals, especially before eating foods known to trigger an IC reaction. A survey by the Interstitial Cystitis Association reported that 75% of patients who tried the supplement thought it provided some symptomatic benefit. A 2001 prospective nonrandomized study of over 200 IC patients reported a benefit in >40% of participants.[207]
- There are some anecdotal reports of a benefit from oral hyaluronic acid therapy, but no formal published studies exist.
Bladder Cocktails for Intravesical Instillation
Home bladder cocktail instillations done by the patient are preferred over office/clinic administration whenever possible.
A typical schedule might be weekly instillations until symptom control is reached. Then, the interval between instillations is slowly extended as tolerated.
Some experts recommend having 2 intravesical cocktail formulas available: one with DMSO and the other without. If the original cocktail selected appears to be losing effectiveness or is unacceptable for some reason, the second formula can be substituted. In this way, intravesical therapy is maintained as it remains the single most effective overall non-surgical treatment.
While there is no accepted standard intravesical cocktail, several noted experts have published their own personal recipes:
Alkalinized Lidocaine Cocktail
- Gentamicin 80 mg in 5 mL
- Heparin 10,000 IU in 10 mL
- Lidocaine 2% solution: 25 mL
- Sodium Bicarbonate 8.4%: 5 mL–10 mL
- Schedule:
- Weekly as needed in office/clinic.
- Should be mixed immediately before intravesical instillation to avoid precipitation.
- Increase the interval between instillations as symptoms improve.
Dimethylsulfoxide (DMSO) Cocktail
- Gentamicin 80 mg in 5 mL
- Heparin 10,000 IU–20,000 IU
- Rimso-50 (DMSO) 50 mL
- Sodium Bicarbonate 8.4%: 10 mL
- Triamcinolone 40 mg
- Schedule:
- Weekly as needed in the office/clinic.
- Increase the interval between instillations as symptoms improve.
Robert Moldwin, MD—Smith Institute for Urology & Long Island Jewish Medical Center
- Bupivacaine (Marcaine) 0.5% and Lidocaine jelly 2% in a 1:1 mixture: Total of 30 mL–40 mL
- Heparin 10,000 IU–20,000 IU
- Gentamicin 80 mg
- Triamcinolone 40 mg (Increase to 80 mg in patients with documented Hunner ulcer)
- Schedule:
- Weekly instillations in the office/clinic
- May also be self-administered by patients at home up to 3 times weekly.
- Increase the interval between instillations as symptoms improve.
- Maximum symptom improvement is expected by 12 weeks.
- If no longer effective, switch to “DMSO Cocktail.” May resume the above bupivacaine combination cocktail later if symptoms return.
Christopher Payne, MD “Payne Cocktail 1”
- Bupivacaine (Marcaine) 0.5%: 10 mL
- Heparin 10,000 IU
- Schedule:
- Weekly instillations in the office/clinic.
- May also be self-administered by patients at home as often as daily.
- Increase the interval between instillations as symptoms improve.
Christopher Payne, MD “Payne Cocktail 2”
- Bupivacaine (Marcaine) 0.5%: 10 mL
- Heparin 10,000 IU (Optional, may be reserved as separate instillation treatment)
- Hydrocortisone (Solu-Cortef) 100 mg
- Rimso-50 (DMSO): 50 mL
- Sodium Bicarbonate 8.4%: 5 mL
- Schedule:
- Weekly instillations of 30 to 50 mL for 6 to 8 weeks.
- Increase the interval between instillations as symptoms improve.
- Use B&O suppositories if patients cannot hold the solution for at least 30 minutes.
Kristene Whitmore, MD—Drexel University College of Medicine
- Bupivacaine (Marcaine) 0.5%: 20 mL
- Gentamicin 80 mg: 5 mL of normal saline (Add for patients with a documented UTI within 3 months)
- Heparin 10,000 IU in 10 mL
- Hydrocortisone 100 mg: 5 mL of normal saline
- Sodium Bicarbonate 8.4%: 40 mL–50 mL
- Schedule:
- Six weekly instillations. Should be administered during a premenstrual flare when possible.
- Appropriate for patients who can self-catheterize and administer at home.
Enhancing Healthcare Team Outcomes
IC/BPS is a long-term, chronic condition that can significantly negatively affect the patient's quality of life and physical, psychological, and social health. The condition is often not easily managed by 1 intervention alone, and many physical and psychological comorbidities often exist. Therefore, an interprofessional team approach involving different specialties is best suited to offer optimal care to the patient.
Urologists, pain management specialists, gynecologists, urogynecologists, primary care physicians, specialist nurses, mental health professionals, pharmacists, dieticians, social workers, physical therapists, and other healthcare professionals all play crucial roles in delivering comprehensive care.[161][208]
Healthcare professionals must possess specialized skills in recognizing, diagnosing, and managing IC/BPS. This includes proficiency in conducting cystoscopy and urodynamics to confirm the diagnosis and a deep understanding of the latest treatment modalities and therapeutic options.
Developing evidence-based treatment plans tailored to each patient's specific needs is vital. Strategies should encompass dietary modifications, physical therapy, and pharmacological interventions. Furthermore, clinicians must be well-versed in monitoring patient progress and adjusting treatment regimens accordingly.
Effective communication among team members is paramount. Clinicians should exchange information about patient progress, potential complications, and treatment adjustments to ensure cohesive and comprehensive care. Collaboration fosters a holistic approach to addressing the physical and emotional aspects of IC/BPS.
IC/BPS care is most effective when healthcare professionals collaborate to provide patient-centered, evidence-based care. The combined efforts of an interprofessional team are critical for enhancing the quality of life and overall well-being of individuals living with IC/BPS.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Clemens JQ, Erickson DR, Varela NP, Lai HH. Diagnosis and Treatment of Interstitial Cystitis/Bladder Pain Syndrome. The Journal of urology. 2022 Jul:208(1):34-42. doi: 10.1097/JU.0000000000002756. Epub 2022 May 10 [PubMed PMID: 35536143]
Hanno P, Dmochowski R. Status of international consensus on interstitial cystitis/bladder pain syndrome/painful bladder syndrome: 2008 snapshot. Neurourology and urodynamics. 2009:28(4):274-86. doi: 10.1002/nau.20687. Epub [PubMed PMID: 19260081]
Level 3 (low-level) evidenceBerger RE, Miller JE, Rothman I, Krieger JN, Muller CH. Bladder petechiae after cystoscopy and hydrodistension in men diagnosed with prostate pain. The Journal of urology. 1998 Jan:159(1):83-5 [PubMed PMID: 9400442]
Forrest JB, Schmidt S. Interstitial cystitis, chronic nonbacterial prostatitis and chronic pelvic pain syndrome in men: a common and frequently identical clinical entity. The Journal of urology. 2004 Dec:172(6 Pt 2):2561-2 [PubMed PMID: 15538208]
Forrest JB, Vo Q. Observations on the presentation, diagnosis, and treatment of interstitial cystitis in men. Urology. 2001 Jun:57(6 Suppl 1):26-9 [PubMed PMID: 11378046]
Level 2 (mid-level) evidenceClemens JQ, Clauw DJ, Kreder K, Krieger JN, Kusek JW, Lai HH, Rodriguez L, Williams DA, Hou X, Stephens A, Landis JR, MAPP Research Network. Comparison of baseline urological symptoms in men and women in the MAPP research cohort. The Journal of urology. 2015 May:193(5):1554-8. doi: 10.1016/j.juro.2014.11.016. Epub 2014 Nov 13 [PubMed PMID: 25463989]
Level 2 (mid-level) evidenceArora HC, Shoskes DA. The enigma of men with interstitial cystitis/bladder pain syndrome. Translational andrology and urology. 2015 Dec:4(6):668-76. doi: 10.3978/j.issn.2223-4683.2015.10.01. Epub [PubMed PMID: 26813678]
Bogart LM, Berry SH, Clemens JQ. Symptoms of interstitial cystitis, painful bladder syndrome and similar diseases in women: a systematic review. The Journal of urology. 2007 Feb:177(2):450-6 [PubMed PMID: 17222607]
Level 1 (high-level) evidenceBolla SR, Odeluga N, Amraei R, Jetti R. Histology, Bladder. StatPearls. 2024 Jan:(): [PubMed PMID: 31082007]
Sam P, Jiang J, Leslie SW, LaGrange CA. Anatomy, Abdomen and Pelvis, Sphincter Urethrae. StatPearls. 2024 Jan:(): [PubMed PMID: 29494045]
Sam P, Nassereddin A, LaGrange CA. Anatomy, Abdomen and Pelvis: Bladder Detrusor Muscle. StatPearls. 2024 Jan:(): [PubMed PMID: 29489195]
Shermadou ES, Rahman S, Leslie SW. Anatomy, Abdomen and Pelvis: Bladder. StatPearls. 2024 Jan:(): [PubMed PMID: 30285360]
Jhang JF, Jiang YH, Kuo HC. Current Understanding of the Pathophysiology and Novel Treatments of Interstitial Cystitis/Bladder Pain Syndrome. Biomedicines. 2022 Sep 23:10(10):. doi: 10.3390/biomedicines10102380. Epub 2022 Sep 23 [PubMed PMID: 36289642]
Level 3 (low-level) evidenceShie JH, Kuo HC. Higher levels of cell apoptosis and abnormal E-cadherin expression in the urothelium are associated with inflammation in patients with interstitial cystitis/painful bladder syndrome. BJU international. 2011 Jul:108(2 Pt 2):E136-41. doi: 10.1111/j.1464-410X.2010.09911.x. Epub 2010 Dec 16 [PubMed PMID: 21166752]
Level 2 (mid-level) evidenceJiang YH, Peng CH, Liu HT, Kuo HC. Increased pro-inflammatory cytokines, C-reactive protein and nerve growth factor expressions in serum of patients with interstitial cystitis/bladder pain syndrome. PloS one. 2013:8(10):e76779. doi: 10.1371/journal.pone.0076779. Epub 2013 Oct 17 [PubMed PMID: 24146927]
Tyagi P, Moon CH, Janicki J, Kaufman J, Chancellor M, Yoshimura N, Chermansky C. Recent advances in imaging and understanding interstitial cystitis. F1000Research. 2018:7():. pii: F1000 Faculty Rev-1771. doi: 10.12688/f1000research.16096.1. Epub 2018 Nov 9 [PubMed PMID: 30473772]
Level 3 (low-level) evidenceKairys AE, Schmidt-Wilcke T, Puiu T, Ichesco E, Labus JS, Martucci K, Farmer MA, Ness TJ, Deutsch G, Mayer EA, Mackey S, Apkarian AV, Maravilla K, Clauw DJ, Harris RE. Increased brain gray matter in the primary somatosensory cortex is associated with increased pain and mood disturbance in patients with interstitial cystitis/painful bladder syndrome. The Journal of urology. 2015 Jan:193(1):131-7. doi: 10.1016/j.juro.2014.08.042. Epub 2014 Aug 14 [PubMed PMID: 25132239]
Level 2 (mid-level) evidenceKilpatrick LA, Kutch JJ, Tillisch K, Naliboff BD, Labus JS, Jiang Z, Farmer MA, Apkarian AV, Mackey S, Martucci KT, Clauw DJ, Harris RE, Deutsch G, Ness TJ, Yang CC, Maravilla K, Mullins C, Mayer EA. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. The Journal of urology. 2014 Sep:192(3):947-55. doi: 10.1016/j.juro.2014.03.093. Epub 2014 Mar 26 [PubMed PMID: 24681331]
Misra S, Chetwood A, Coker C, Thomas P. Ketamine cystitis: practical considerations in management. Scandinavian journal of urology. 2014 Oct:48(5):482-8. doi: 10.3109/21681805.2014.909530. Epub 2014 Apr 30 [PubMed PMID: 24779452]
Level 2 (mid-level) evidenceArgade S, Chermansky C, Tyagi P. Biomarkers for interstitial cystitis/painful bladder syndrome. Women's health (London, England). 2016 Jan:12(1):87-90. doi: 10.2217/whe.15.93. Epub 2015 Dec 23 [PubMed PMID: 26696241]
Dinis S, de Oliveira JT, Pinto R, Cruz F, Buffington CT, Dinis P. From bladder to systemic syndrome: concept and treatment evolution of interstitial cystitis. International journal of women's health. 2015:7():735-44. doi: 10.2147/IJWH.S60798. Epub 2015 Jul 23 [PubMed PMID: 26229509]
Gamper M, Regauer S, Welter J, Eberhard J, Viereck V. Are mast cells still good biomarkers for bladder pain syndrome/interstitial cystitis? The Journal of urology. 2015 Jun:193(6):1994-2000. doi: 10.1016/j.juro.2015.01.036. Epub 2015 Jan 14 [PubMed PMID: 25596361]
Ackerman AL, Lee UJ, Jellison FC, Tan N, Patel M, Raman SS, Rodriguez LV. MRI suggests increased tonicity of the levator ani in women with interstitial cystitis/bladder pain syndrome. International urogynecology journal. 2016 Jan:27(1):77-83. doi: 10.1007/s00192-015-2794-6. Epub 2015 Aug 1 [PubMed PMID: 26231233]
Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. The Journal of urology. 2011 Aug:186(2):540-4. doi: 10.1016/j.juro.2011.03.132. Epub 2011 Jun 16 [PubMed PMID: 21683389]
Suskind AM, Berry SH, Ewing BA, Elliott MN, Suttorp MJ, Clemens JQ. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND Interstitial Cystitis Epidemiology male study. The Journal of urology. 2013 Jan:189(1):141-5. doi: 10.1016/j.juro.2012.08.088. Epub 2012 Nov 16 [PubMed PMID: 23164386]
Curhan GC, Speizer FE, Hunter DJ, Curhan SG, Stampfer MJ. Epidemiology of interstitial cystitis: a population based study. The Journal of urology. 1999 Feb:161(2):549-52 [PubMed PMID: 9915446]
Konkle KS, Berry SH, Elliott MN, Hilton L, Suttorp MJ, Clauw DJ, Clemens JQ. Comparison of an interstitial cystitis/bladder pain syndrome clinical cohort with symptomatic community women from the RAND Interstitial Cystitis Epidemiology study. The Journal of urology. 2012 Feb:187(2):508-12. doi: 10.1016/j.juro.2011.10.040. Epub 2011 Dec 15 [PubMed PMID: 22177158]
Clemens JQ, Meenan RT, O'Keeffe Rosetti MC, Brown SO, Gao SY, Calhoun EA. Prevalence of interstitial cystitis symptoms in a managed care population. The Journal of urology. 2005 Aug:174(2):576-80 [PubMed PMID: 16006901]
Bade JJ, Rijcken B, Mensink HJ. Interstitial cystitis in The Netherlands: prevalence, diagnostic criteria and therapeutic preferences. The Journal of urology. 1995 Dec:154(6):2035-7; discussion 2037-8 [PubMed PMID: 7500452]
Chung KJ, Han AN, Kim KH. Recommendations to the primary care practitioners and the patients for managing pelvic pain, especially in painful bladder syndrome for early and better prognosis. Journal of exercise rehabilitation. 2015 Oct:11(5):251-4. doi: 10.12965/jer.150226. Epub 2015 Oct 30 [PubMed PMID: 26535214]
Jiang YH, Jhang JF, Kuo HC. The clinical application of intravesical botulinum toxin A injection in patients with overactive bladder and interstitial cystitis. Tzu chi medical journal. 2023 Jan-Mar:35(1):31-37. doi: 10.4103/tcmj.tcmj_313_21. Epub 2022 Mar 11 [PubMed PMID: 36866354]
Lopez SR, Mangır N. Current standard of care in treatment of bladder pain syndrome/interstitial cystitis. Therapeutic advances in urology. 2021 Jan-Dec:13():17562872211022478. doi: 10.1177/17562872211022478. Epub 2021 Jun 12 [PubMed PMID: 34178118]
Level 3 (low-level) evidenceJohansson SL, Fall M. Clinical features and spectrum of light microscopic changes in interstitial cystitis. The Journal of urology. 1990 Jun:143(6):1118-24 [PubMed PMID: 2342171]
Shie JH, Liu HT, Kuo HC. Increased cell apoptosis of urothelium mediated by inflammation in interstitial cystitis/painful bladder syndrome. Urology. 2012 Feb:79(2):484.e7-13. doi: 10.1016/j.urology.2011.09.049. Epub [PubMed PMID: 22310775]
Jiang YH, Jhang JF, Hsu YH, Kuo HC. Usefulness of Urinary Biomarkers for Assessing Bladder Condition and Histopathology in Patients with Interstitial Cystitis/Bladder Pain Syndrome. International journal of molecular sciences. 2022 Oct 10:23(19):. doi: 10.3390/ijms231912044. Epub 2022 Oct 10 [PubMed PMID: 36233356]
Birder LA. Pathophysiology of interstitial cystitis. International journal of urology : official journal of the Japanese Urological Association. 2019 Jun:26 Suppl 1():12-15. doi: 10.1111/iju.13985. Epub [PubMed PMID: 31144735]
Wen JY, Lo TS, Chuang YC, Ho CH, Long CY, Law KS, Tong YC, Wu MP. Risks of interstitial cystitis among patients with systemic lupus erythematosus: A population-based cohort study. International journal of urology : official journal of the Japanese Urological Association. 2019 Sep:26(9):897-902. doi: 10.1111/iju.14065. Epub 2019 Jul 16 [PubMed PMID: 31311067]
Yueh HZ, Yang MH, Huang JY, Wei JC. Risk of Autoimmune Diseases in Patients With Interstitial Cystitis/Bladder Pain Syndrome: A Nationwide Population-Based Study in Taiwan. Frontiers in medicine. 2021:8():747098. doi: 10.3389/fmed.2021.747098. Epub 2021 Sep 20 [PubMed PMID: 34616760]
Darrieutort-Laffite C, André V, Hayem G, Saraux A, Le Guern V, Le Jeunne C, Puéchal X. Sjögren's syndrome complicated by interstitial cystitis: A case series and literature review. Joint bone spine. 2015 Jul:82(4):245-50. doi: 10.1016/j.jbspin.2014.12.007. Epub 2015 Feb 10 [PubMed PMID: 25680227]
Level 3 (low-level) evidenceNickel JC, Tripp DA, Pontari M, Moldwin R, Mayer R, Carr LK, Doggweiler R, Yang CC, Mishra N, Nordling J. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. The Journal of urology. 2010 Oct:184(4):1358-63. doi: 10.1016/j.juro.2010.06.005. Epub 2010 Aug 17 [PubMed PMID: 20719340]
Level 2 (mid-level) evidencePereira E Silva R, Romão VC, Neves M, Garcia R, Oliveira S, Brites J, Ramos FO, Canhão H, Palma Dos Reis J, Pereira da Silva JA, Lopes T. Overactive bladder symptom bother and health-related quality of life in patients with systemic lupus erythematosus and primary Sjögren syndrome. Lupus. 2019 Jan:28(1):27-33. doi: 10.1177/0961203318811605. Epub 2018 Nov 12 [PubMed PMID: 30419773]
Level 2 (mid-level) evidenceKanter G, Komesu YM, Qaedan F, Jeppson PC, Dunivan GC, Cichowski SB, Rogers RG. Mindfulness-based stress reduction as a novel treatment for interstitial cystitis/bladder pain syndrome: a randomized controlled trial. International urogynecology journal. 2016 Nov:27(11):1705-1711 [PubMed PMID: 27116196]
Level 1 (high-level) evidenceHo NJ, Koziol JA, Parsons CL, Barlow W, Weiss NS. Natural history of interstitial cystitis in 274 patients receiving sulfated polysaccharide therapy. Urology. 1999 Jun:53(6):1133-9 [PubMed PMID: 10367841]
Lutgendorf SK, Kreder KJ, Rothrock NE, Ratliff TL, Zimmerman B. Stress and symptomatology in patients with interstitial cystitis: a laboratory stress model. The Journal of urology. 2000 Oct:164(4):1265-9 [PubMed PMID: 10992377]
Pierce AN, Christianson JA. Stress and chronic pelvic pain. Progress in molecular biology and translational science. 2015:131():509-35. doi: 10.1016/bs.pmbts.2014.11.009. Epub 2015 Feb 2 [PubMed PMID: 25744684]
Level 3 (low-level) evidenceLovick TA. Central control of visceral pain and urinary tract function. Autonomic neuroscience : basic & clinical. 2016 Oct:200():35-42. doi: 10.1016/j.autneu.2016.02.001. Epub 2016 Feb 6 [PubMed PMID: 26905459]
Dupont MC, Spitsbergen JM, Kim KB, Tuttle JB, Steers WD. Histological and neurotrophic changes triggered by varying models of bladder inflammation. The Journal of urology. 2001 Sep:166(3):1111-8 [PubMed PMID: 11490308]
Level 3 (low-level) evidenceLynes WL, Flynn SD, Shortliffe LD, Stamey TA. The histology of interstitial cystitis. The American journal of surgical pathology. 1990 Oct:14(10):969-76 [PubMed PMID: 2403198]
Jhang JF, Hsu YH, Jiang YH, Ho HC, Kuo HC. Clinical Relevance of Bladder Histopathological Findings and Their Impact on Treatment Outcomes among Patients with Interstitial Cystitis/Bladder Pain Syndrome: An Investigation of the European Society for the Study of Interstitial Cystitis Histopathological Classification. The Journal of urology. 2021 Jan:205(1):226-235. doi: 10.1097/JU.0000000000001334. Epub 2020 Aug 28 [PubMed PMID: 32856961]
Kim HJ. Update on the Pathology and Diagnosis of Interstitial Cystitis/Bladder Pain Syndrome: A Review. International neurourology journal. 2016 Mar:20(1):13-7. doi: 10.5213/inj.1632522.261. Epub 2016 Mar 23 [PubMed PMID: 27032552]
Natale F, Campagna G, Marturano M, Caramazza D, Panico G, Vacca L, Torcia E, Cervigni M, Scambia G, Ercoli A. Is There a Role for Bladder Biopsy in the Diagnosis of Non-Hunner Lesions Interstitial Cystitis? Urologia internationalis. 2023:107(3):257-262. doi: 10.1159/000525849. Epub 2022 Jul 27 [PubMed PMID: 35896088]
Diggs C, Meyer WA, Langenberg P, Greenberg P, Horne L, Warren JW. Assessing urgency in interstitial cystitis/painful bladder syndrome. Urology. 2007 Feb:69(2):210-4 [PubMed PMID: 17275075]
Level 2 (mid-level) evidenceClemens JQ, Calhoun EA, Litwin MS, McNaughton-Collins M, Kusek JW, Crowley EM, Landis JR, Urologic Pelvic Pain Collaborative Research Network. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology. 2009 Nov:74(5):983-7, quiz 987.e1-3. doi: 10.1016/j.urology.2009.06.078. Epub 2009 Oct 2 [PubMed PMID: 19800663]
Level 1 (high-level) evidenceBallstaedt L, Leslie SW, Woodbury B. Bladder Post Void Residual Volume. StatPearls. 2024 Jan:(): [PubMed PMID: 30969661]
Siltberg H, Larsson G, Victor A. Frequency/volume chart: the basic tool for investigating urinary symptoms. Acta obstetricia et gynecologica Scandinavica. Supplement. 1997:166():24-7 [PubMed PMID: 9253374]
Abrams P, Klevmark B. Frequency volume charts: an indispensable part of lower urinary tract assessment. Scandinavian journal of urology and nephrology. Supplementum. 1996:179():47-53 [PubMed PMID: 8908664]
Clemens JQ, Brown SO, Calhoun EA. Mental health diagnoses in patients with interstitial cystitis/painful bladder syndrome and chronic prostatitis/chronic pelvic pain syndrome: a case/control study. The Journal of urology. 2008 Oct:180(4):1378-82. doi: 10.1016/j.juro.2008.06.032. Epub 2008 Aug 15 [PubMed PMID: 18707716]
Level 2 (mid-level) evidenceBarocas DA, Boorjian SA, Alvarez RD, Downs TM, Gross CP, Hamilton BD, Kobashi KC, Lipman RR, Lotan Y, Ng CK, Nielsen ME, Peterson AC, Raman JD, Smith-Bindman R, Souter LH. Microhematuria: AUA/SUFU Guideline. The Journal of urology. 2020 Oct:204(4):778-786. doi: 10.1097/JU.0000000000001297. Epub 2020 Jul 23 [PubMed PMID: 32698717]
Erickson DR, Kunselman AR, Bentley CM, Peters KM, Rovner ES, Demers LM, Wheeler MA, Keay SK. Changes in urine markers and symptoms after bladder distention for interstitial cystitis. The Journal of urology. 2007 Feb:177(2):556-60 [PubMed PMID: 17222633]
Novicki DE, Larson TR, Swanson SK. Interstitial cystitis in men. Urology. 1998 Oct:52(4):621-4 [PubMed PMID: 9763081]
Forrest JB, Nickel JC, Moldwin RM. Chronic prostatitis/chronic pelvic pain syndrome and male interstitial cystitis: enigmas and opportunities. Urology. 2007 Apr:69(4 Suppl):60-3 [PubMed PMID: 17462482]
. . :(): [PubMed PMID: 37057400]
Parsons CL, Zupkas P, Parsons JK. Intravesical potassium sensitivity in patients with interstitial cystitis and urethral syndrome. Urology. 2001 Mar:57(3):428-32; discussion 432-3 [PubMed PMID: 11248610]
Level 2 (mid-level) evidenceLeslie SW, Antolak S, Feloney MP, Soon-Sutton TL. Pudendal Neuralgia. StatPearls. 2024 Jan:(): [PubMed PMID: 32965917]
Watanabe D, Akiyama Y, Niimi A, Nomiya A, Yamada Y, Sato Y, Nakamura M, Kawai T, Yamada D, Suzuki M, Igawa Y, Kume H, Homma Y. Clinical characterization of interstitial cystitis/bladder pain syndrome in women based on the presence or absence of Hunner lesions and glomerulations. Lower urinary tract symptoms. 2021 Jan:13(1):139-143. doi: 10.1111/luts.12344. Epub 2020 Aug 23 [PubMed PMID: 32830459]
Chen YC, Jiang YH, Jhang JF, Kuo HC. Using Urine Biomarkers to Differentiate Bladder Dysfunctions in Women with Sensory Bladder Disorders. International journal of molecular sciences. 2024 Aug 29:25(17):. doi: 10.3390/ijms25179359. Epub 2024 Aug 29 [PubMed PMID: 39273307]
Gross J, Vetter J, Lai HH. Clinical Presentation of Urologic Chronic Pelvic Pain Syndrome (UCPPS) Varies With Presenting Age - Implication on Patient Evaluation. Urology. 2021 Dec:158():66-73. doi: 10.1016/j.urology.2021.07.007. Epub 2021 Jul 22 [PubMed PMID: 34302833]
Wennevik GE, Meijlink JM, Hanno P, Nordling J. The Role of Glomerulations in Bladder Pain Syndrome: A Review. The Journal of urology. 2016 Jan:195(1):19-25. doi: 10.1016/j.juro.2015.06.112. Epub 2015 Aug 28 [PubMed PMID: 26318984]
Ronstrom C, Lai HH. Presenting an atlas of Hunner lesions in interstitial cystitis which can be identified with office cystoscopy. Neurourology and urodynamics. 2020 Nov:39(8):2394-2400. doi: 10.1002/nau.24500. Epub 2020 Sep 9 [PubMed PMID: 32902893]
Ko KJ, Cho WJ, Lee YS, Choi J, Byun HJ, Lee KS. Comparison of the Efficacy Between Transurethral Coagulation and Transurethral Resection of Hunner Lesion in Interstitial Cystitis/Bladder Pain Syndrome Patients: A Prospective Randomized Controlled Trial. European urology. 2020 May:77(5):644-651. doi: 10.1016/j.eururo.2020.01.002. Epub 2020 Jan 17 [PubMed PMID: 31959549]
Level 1 (high-level) evidencePeeker R, Aldenborg F, Fall M. Complete transurethral resection of ulcers in classic interstitial cystitis. International urogynecology journal and pelvic floor dysfunction. 2000:11(5):290-5 [PubMed PMID: 11052564]
Level 2 (mid-level) evidenceSairanen J, Tammela TL, Leppilahti M, Multanen M, Paananen I, Lehtoranta K, Ruutu M. Cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. The Journal of urology. 2005 Dec:174(6):2235-8 [PubMed PMID: 16280777]
Level 1 (high-level) evidenceTissot WD, Diokno AC, Peters KM. A referral center's experience with transitional cell carcinoma misdiagnosed as interstitial cystitis. The Journal of urology. 2004 Aug:172(2):478-80 [PubMed PMID: 15247708]
Level 2 (mid-level) evidenceChen Y, Ying Z, Xiao Y, Liu Y, Wu S. The diagnostic and therapeutic efficacy of cystoscopy with hydrodistension and random biopsies in clinically suspected interstitial cystitis/bladder pain syndrome. European journal of obstetrics, gynecology, and reproductive biology. 2021 Oct:265():156-161. doi: 10.1016/j.ejogrb.2021.08.025. Epub 2021 Aug 27 [PubMed PMID: 34492610]
Sholan R. Clinical manifestations and results of cystoscopy in women with interstitial cystitis/bladder pain syndrome. Northern clinics of Istanbul. 2020:7(5):417-424. doi: 10.14744/nci.2020.23245. Epub 2020 Aug 18 [PubMed PMID: 33163875]
Grover S, Srivastava A, Lee R, Tewari AK, Te AE. Role of inflammation in bladder function and interstitial cystitis. Therapeutic advances in urology. 2011 Feb:3(1):19-33. doi: 10.1177/1756287211398255. Epub [PubMed PMID: 21789096]
Level 3 (low-level) evidenceFitzGerald MP, Payne CK, Lukacz ES, Yang CC, Peters KM, Chai TC, Nickel JC, Hanno PM, Kreder KJ, Burks DA, Mayer R, Kotarinos R, Fortman C, Allen TM, Fraser L, Mason-Cover M, Furey C, Odabachian L, Sanfield A, Chu J, Huestis K, Tata GE, Dugan N, Sheth H, Bewyer K, Anaeme A, Newton K, Featherstone W, Halle-Podell R, Cen L, Landis JR, Propert KJ, Foster HE Jr, Kusek JW, Nyberg LM, Interstitial Cystitis Collaborative Research Network. Randomized multicenter clinical trial of myofascial physical therapy in women with interstitial cystitis/painful bladder syndrome and pelvic floor tenderness. The Journal of urology. 2012 Jun:187(6):2113-8. doi: 10.1016/j.juro.2012.01.123. Epub 2012 Apr 12 [PubMed PMID: 22503015]
Level 1 (high-level) evidenceWeiss JM. Pelvic floor myofascial trigger points: manual therapy for interstitial cystitis and the urgency-frequency syndrome. The Journal of urology. 2001 Dec:166(6):2226-31 [PubMed PMID: 11696740]
Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA. 2016 Apr 19:315(15):1624-45. doi: 10.1001/jama.2016.1464. Epub [PubMed PMID: 26977696]
Santos TGD, Miranda IAS, Nygaard CC, Schreiner L, Castro RA, Haddad JM. Systematic Review of Oral Therapy for the Treatment of Symptoms of Bladder Pain Syndrome: The Brazilian Guidelines. Revista brasileira de ginecologia e obstetricia : revista da Federacao Brasileira das Sociedades de Ginecologia e Obstetricia. 2018 Feb:40(2):96-102. doi: 10.1055/s-0037-1609049. Epub 2017 Dec 14 [PubMed PMID: 29241263]
Level 1 (high-level) evidenceVollstedt A, Tennyson L, Turner K, Hasenau D, Saon M, McCartney T, Beck D, Gilleran J, Peters K. Evidence for Early Cyclosporine Treatment for Hunner Lesion Interstitial Cystitis. Female pelvic medicine & reconstructive surgery. 2022 Jan 1:28(1):e1-e5. doi: 10.1097/SPV.0000000000001108. Epub [PubMed PMID: 34608034]
Chermansky CJ, Guirguis MO. Pharmacologic Management of Interstitial Cystitis/Bladder Pain Syndrome. The Urologic clinics of North America. 2022 May:49(2):273-282. doi: 10.1016/j.ucl.2022.01.003. Epub [PubMed PMID: 35428433]
Thour A, Marwaha R. Amitriptyline. StatPearls. 2024 Jan:(): [PubMed PMID: 30725910]
van Ophoven A, Pokupic S, Heinecke A, Hertle L. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. The Journal of urology. 2004 Aug:172(2):533-6 [PubMed PMID: 15247722]
Level 1 (high-level) evidenceFoster HE Jr, Hanno PM, Nickel JC, Payne CK, Mayer RD, Burks DA, Yang CC, Chai TC, Kreder KJ, Peters KM, Lukacz ES, FitzGerald MP, Cen L, Landis JR, Propert KJ, Yang W, Kusek JW, Nyberg LM, Interstitial Cystitis Collaborative Research Network. Effect of amitriptyline on symptoms in treatment naïve patients with interstitial cystitis/painful bladder syndrome. The Journal of urology. 2010 May:183(5):1853-8. doi: 10.1016/j.juro.2009.12.106. Epub 2010 Mar 29 [PubMed PMID: 20303115]
Level 1 (high-level) evidenceAbat D, Altunkol A, Gökalp F. The effect of intravesical cocktail therapy combined with low-dose amitriptyline on primary bladder pain syndrome. International urogynecology journal. 2022 May:33(5):1225-1230. doi: 10.1007/s00192-021-05043-y. Epub 2022 Jan 3 [PubMed PMID: 34977954]
Dasgupta P, Sharma SD, Womack C, Blackford HN, Dennis P. Cimetidine in painful bladder syndrome: a histopathological study. BJU international. 2001 Aug:88(3):183-6 [PubMed PMID: 11488726]
Pino MA, Azer SA. Cimetidine. StatPearls. 2024 Jan:(): [PubMed PMID: 31334975]
Thilagarajah R, Witherow RO, Walker MM. Oral cimetidine gives effective symptom relief in painful bladder disease: a prospective, randomized, double-blind placebo-controlled trial. BJU international. 2001 Feb:87(3):207-12 [PubMed PMID: 11167643]
Level 1 (high-level) evidenceLewi H. Cimetidine in treatment of interstitial cystitis. Urology. 1995 Jun:45(6):1088 [PubMed PMID: 7771018]
Level 3 (low-level) evidenceForrest JB, Payne CK, Erickson DR. Cyclosporine A for refractory interstitial cystitis/bladder pain syndrome: experience of 3 tertiary centers. The Journal of urology. 2012 Oct:188(4):1186-91. doi: 10.1016/j.juro.2012.06.023. Epub 2012 Aug 15 [PubMed PMID: 22901569]
Level 2 (mid-level) evidenceDi XP, Luo DY, Jin X, Zhao WY, Li H, Wang KJ. Efficacy and safety comparison of pharmacotherapies for interstitial cystitis and bladder pain syndrome: a systematic review and Bayesian network meta-analysis. International urogynecology journal. 2021 May:32(5):1129-1141. doi: 10.1007/s00192-020-04659-w. Epub 2021 Feb 27 [PubMed PMID: 33638677]
Level 1 (high-level) evidenceTapia C, Nessel TA, Zito PM. Cyclosporine. StatPearls. 2024 Jan:(): [PubMed PMID: 29494057]
Brière R, Bouchard F, Ismail S, Gareau Labelle AK, Tu LM. A pilot study on oral cyclosporine A in association with fulguration for the treatment of interstitial cystitis with Hunner's lesions. Neurourology and urodynamics. 2022 Aug:41(6):1498-1504. doi: 10.1002/nau.24997. Epub 2022 Jun 22 [PubMed PMID: 35731015]
Level 3 (low-level) evidenceSpeer LM, Mushkbar S, Erbele T. Chronic Pelvic Pain in Women. American family physician. 2016 Mar 1:93(5):380-7 [PubMed PMID: 26926975]
Hansen HC. Interstitial cystitis and the potential role of gabapentin. Southern medical journal. 2000 Feb:93(2):238-42 [PubMed PMID: 10701800]
Level 3 (low-level) evidenceSasaki K, Smith CP, Chuang YC, Lee JY, Kim JC, Chancellor MB. Oral gabapentin (neurontin) treatment of refractory genitourinary tract pain. Techniques in urology. 2001 Mar:7(1):47-9 [PubMed PMID: 11272678]
Kim YT, Kwon DD, Kim J, Kim DK, Lee JY, Chancellor MB. Gabapentin for overactive bladder and nocturia after anticholinergic failure. International braz j urol : official journal of the Brazilian Society of Urology. 2004 Jul-Aug:30(4):275-8 [PubMed PMID: 15679954]
Yasaei R, Katta S, Patel P, Saadabadi A. Gabapentin. StatPearls. 2024 Jan:(): [PubMed PMID: 29630280]
Sant GR, Propert KJ, Hanno PM, Burks D, Culkin D, Diokno AC, Hardy C, Landis JR, Mayer R, Madigan R, Messing EM, Peters K, Theoharides TC, Warren J, Wein AJ, Steers W, Kusek JW, Nyberg LM, Interstitial Cystitis Clinical Trials Group. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. The Journal of urology. 2003 Sep:170(3):810-5 [PubMed PMID: 12913705]
Level 3 (low-level) evidenceMinogiannis P, El-Mansoury M, Betances JA, Sant GR, Theoharides TC. Hydroxyzine inhibits neurogenic bladder mast cell activation. International journal of immunopharmacology. 1998 Oct:20(10):553-63 [PubMed PMID: 9839659]
Level 3 (low-level) evidenceDobberfuhl AD. Pathophysiology, assessment, and treatment of overactive bladder symptoms in patients with interstitial cystitis/bladder pain syndrome. Neurourology and urodynamics. 2022 Nov:41(8):1958-1966. doi: 10.1002/nau.24958. Epub 2022 May 24 [PubMed PMID: 35607890]
Loloi J, Clearwater W, Schulz A, Suadicani SO, Abraham N. Medical Treatment of Overactive Bladder. The Urologic clinics of North America. 2022 May:49(2):249-261. doi: 10.1016/j.ucl.2021.12.005. Epub [PubMed PMID: 35428431]
Holm-Bentzen M, Jacobsen F, Nerstrøm B, Lose G, Kristensen JK, Pedersen RH, Krarup T, Feggetter J, Bates P, Barnard R. A prospective double-blind clinically controlled multicenter trial of sodium pentosanpolysulfate in the treatment of interstitial cystitis and related painful bladder disease. The Journal of urology. 1987 Sep:138(3):503-7 [PubMed PMID: 2442415]
Level 1 (high-level) evidenceNickel JC, Herschorn S, Whitmore KE, Forrest JB, Hu P, Friedman AJ, Baseman AS. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: insights from a randomized, double-blind, placebo controlled study. The Journal of urology. 2015 Mar:193(3):857-62. doi: 10.1016/j.juro.2014.09.036. Epub 2014 Sep 20 [PubMed PMID: 25245489]
Level 1 (high-level) evidenceMulholland SG, Hanno P, Parsons CL, Sant GR, Staskin DR. Pentosan polysulfate sodium for therapy of interstitial cystitis. A double-blind placebo-controlled clinical study. Urology. 1990 Jun:35(6):552-8 [PubMed PMID: 1693797]
Level 1 (high-level) evidenceParsons CL, Benson G, Childs SJ, Hanno P, Sant GR, Webster G. A quantitatively controlled method to study prospectively interstitial cystitis and demonstrate the efficacy of pentosanpolysulfate. The Journal of urology. 1993 Sep:150(3):845-8 [PubMed PMID: 7688432]
Level 1 (high-level) evidenceParsons CL, Mulholland SG. Successful therapy of interstitial cystitis with pentosanpolysulfate. The Journal of urology. 1987 Sep:138(3):513-6 [PubMed PMID: 2442417]
Level 1 (high-level) evidenceHanno PM. Analysis of long-term Elmiron therapy for interstitial cystitis. Urology. 1997 May:49(5A Suppl):93-9 [PubMed PMID: 9146008]
Margines JB, Tripathy K, Hobbs SD. Pentosan Polysulfate Maculopathy. StatPearls. 2024 Jan:(): [PubMed PMID: 36944010]
Pearce WA, Chen R, Jain N. Pigmentary Maculopathy Associated with Chronic Exposure to Pentosan Polysulfate Sodium. Ophthalmology. 2018 Nov:125(11):1793-1802. doi: 10.1016/j.ophtha.2018.04.026. Epub 2018 May 22 [PubMed PMID: 29801663]
Ohning C, Skopis G, Levinson J, Kasi S. Characteristics of Pentosan Polysulfate Sodium-Associated Maculopathy and Similarities With Other Maculopathies Commonly Managed in a Retina Practice. Journal of vitreoretinal diseases. 2022 Mar-Apr:6(2):104-110. doi: 10.1177/24741264211020259. Epub 2021 Jul 30 [PubMed PMID: 37008666]
Hanif AM, Armenti ST, Taylor SC, Shah RA, Igelman AD, Jayasundera KT, Pennesi ME, Khurana RN, Foote JE, O'Keefe GA, Yang P, Hubbard GB 3rd, Hwang TS, Flaxel CJ, Stein JD, Yan J, Jain N. Phenotypic Spectrum of Pentosan Polysulfate Sodium-Associated Maculopathy: A Multicenter Study. JAMA ophthalmology. 2019 Nov 1:137(11):1275-1282. doi: 10.1001/jamaophthalmol.2019.3392. Epub [PubMed PMID: 31486843]
Level 2 (mid-level) evidenceJain N, Li AL, Yu Y, VanderBeek BL. Association of macular disease with long-term use of pentosan polysulfate sodium: findings from a US cohort. The British journal of ophthalmology. 2020 Aug:104(8):1093-1097. doi: 10.1136/bjophthalmol-2019-314765. Epub 2019 Nov 6 [PubMed PMID: 31694837]
Mukhopadhyay C, Boyce TM, Gehrs KM, Folk JC, Mullins RF, Luo Y, Kreder K, Sohn EH. Age-Related Macular Degeneration Masquerade: A Review of Pentosan Polysulfate Maculopathy and Implications for Clinical Practice. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2022 Mar-Apr 01:11(2):100-110. doi: 10.1097/APO.0000000000000504. Epub [PubMed PMID: 35533330]
Priluck A, Fung AT, Singh MS. Pentosan polysulfate maculopathy: a brief primer for general practitioners, ophthalmologists, optometrists and urologists. The Medical journal of Australia. 2023 May 1:218(8):348-350. doi: 10.5694/mja2.51913. Epub 2023 Mar 29 [PubMed PMID: 36990107]
Jung EH, Lindeke-Myers A, Jain N. Two-Year Outcomes After Variable Duration of Drug Cessation in Patients With Maculopathy Associated With Pentosan Polysulfate Use. JAMA ophthalmology. 2023 Mar 1:141(3):260-266. doi: 10.1001/jamaophthalmol.2022.6093. Epub [PubMed PMID: 36729449]
Greig J, Mak Q, Furrer MA, Sahai A, Raison N. Sacral neuromodulation in the management of chronic pelvic pain: A systematic review and meta-analysis. Neurourology and urodynamics. 2023 Apr:42(4):822-836. doi: 10.1002/nau.25167. Epub 2023 Mar 6 [PubMed PMID: 36877182]
Level 1 (high-level) evidenceFeloney MP, Stauss K, Leslie SW. Sacral Neuromodulation. StatPearls. 2024 Jan:(): [PubMed PMID: 33620828]
Peters KM, Konstandt D. Sacral neuromodulation decreases narcotic requirements in refractory interstitial cystitis. BJU international. 2004 Apr:93(6):777-9 [PubMed PMID: 15049989]
Level 2 (mid-level) evidenceAlkis O, Aras B, Sevim M, Kartal İG, Sönmez OY, İvelik Hİ. Efficacy of transcutaneous tibial nerve stimulation in the treatment of bladder pain syndrome. Current urology. 2022 Jun:16(2):83-87. doi: 10.1097/CU9.0000000000000082. Epub 2022 Jan 27 [PubMed PMID: 36570363]
Padilla-Fernández B, Hernández-Hernández D, Castro-Díaz DM. Current role of neuromodulation in bladder pain syndrome/interstitial cystitis. Therapeutic advances in urology. 2022 Jan-Dec:14():17562872221135941. doi: 10.1177/17562872221135941. Epub 2022 Nov 21 [PubMed PMID: 36438605]
Level 3 (low-level) evidenceElhilali MM, Khaled SM, Kashiwabara T, Elzayat E, Corcos J. Sacral neuromodulation: long-term experience of one center. Urology. 2005 Jun:65(6):1114-7 [PubMed PMID: 15913732]
Sogutdelen E, Citamak B. Efficacy of intravesical cocktail therapy with or without dimethyl sulphoxide in interstitial cystitis. Central European journal of urology. 2022:75(3):299-304. doi: 10.5173/ceju.2022.129. Epub 2022 Sep 24 [PubMed PMID: 36381159]
Perez-Marrero R, Emerson LE, Feltis JT. A controlled study of dimethyl sulfoxide in interstitial cystitis. The Journal of urology. 1988 Jul:140(1):36-9 [PubMed PMID: 3288775]
Level 1 (high-level) evidenceMoss NP, Chill HH, Sand PK, Chang C, Goldberg RP, Gafni-Kane A. A prospective, randomized trial comparing intravesical dimethyl sulfoxide (DMSO) to bupivacaine, triamcinolone, and heparin (BTH), for newly diagnosed interstitial cystitis/painful bladder syndrome (IC/PBS). Neurourology and urodynamics. 2023 Mar:42(3):615-622. doi: 10.1002/nau.25142. Epub 2023 Feb 6 [PubMed PMID: 36747494]
Level 1 (high-level) evidenceYoshimura N, Homma Y, Tomoe H, Otsuka A, Kitta T, Masumori N, Akiyama Y, Niimi A, Mitsui T, Nanri M, Namima T, Takei M, Yamaguchi A, Sekiguchi Y, Kajiwara M, Kobayashi S, Ameda K, Ohashi Y, Sakamoto S, Muraki O, Shishido T, Kageyama S, Kokura K, Okazoe H, Yamanishi T, Watanabe T, Uno T, Ohinata A, Ueda T. Efficacy and safety of intravesical instillation of KRP-116D (50% dimethyl sulfoxide solution) for interstitial cystitis/bladder pain syndrome in Japanese patients: A multicenter, randomized, double-blind, placebo-controlled, clinical study. International journal of urology : official journal of the Japanese Urological Association. 2021 May:28(5):545-553. doi: 10.1111/iju.14505. Epub 2021 Feb 12 [PubMed PMID: 33580603]
Level 1 (high-level) evidenceHung MJ, Chen YT, Shen PS, Hsu ST, Chen GD, Ho ES. Risk factors that affect the treatment of interstitial cystitis using intravesical therapy with a dimethyl sulfoxide cocktail. International urogynecology journal. 2012 Nov:23(11):1533-9. doi: 10.1007/s00192-012-1699-x. Epub 2012 Mar 17 [PubMed PMID: 22426874]
Level 2 (mid-level) evidenceIyer S, Lotsof E, Zhou Y, Tran A, Botros C, Sand P, Goldberg R, Tomezsko J, Gafni-Kane A, Botros S. Which bladder instillations are more effective? DMSO vs. bupivacaine/heparin/triamcinolone: a retrospective study. International urogynecology journal. 2017 Sep:28(9):1335-1340. doi: 10.1007/s00192-017-3266-y. Epub 2017 Feb 1 [PubMed PMID: 28150028]
Level 2 (mid-level) evidenceTomoe H. In what type of interstitial cystitis/bladder pain syndrome is DMSO intravesical instillation therapy effective? Translational andrology and urology. 2015 Dec:4(6):600-4. doi: 10.3978/j.issn.2223-4683.2015.09.01. Epub [PubMed PMID: 26816859]
Parsons CL, Housley T, Schmidt JD, Lebow D. Treatment of interstitial cystitis with intravesical heparin. British journal of urology. 1994 May:73(5):504-7 [PubMed PMID: 8012771]
Parsons CL, Zupkas P, Proctor J, Koziol J, Franklin A, Giesing D, Davis E, Lakin CM, Kahn BS, Garner WJ. Alkalinized lidocaine and heparin provide immediate relief of pain and urgency in patients with interstitial cystitis. The journal of sexual medicine. 2012 Jan:9(1):207-12. doi: 10.1111/j.1743-6109.2011.02542.x. Epub 2011 Nov 14 [PubMed PMID: 22082303]
Level 1 (high-level) evidenceParsons CL, Koziol JA, Proctor JG, Zupkas P, Argade S. Heparin and alkalinized lidocaine versus alkalinized lidocaine for treatment of interstitial cystitis symptoms. The Canadian journal of urology. 2015 Apr:22(2):7739-44 [PubMed PMID: 25891339]
Digesu GA, Tailor V, Bhide AA, Khullar V. The role of bladder instillation in the treatment of bladder pain syndrome: Is intravesical treatment an effective option for patients with bladder pain as well as LUTS? International urogynecology journal. 2020 Jul:31(7):1387-1392. doi: 10.1007/s00192-020-04303-7. Epub 2020 May 1 [PubMed PMID: 32358624]
Madurga Patuel B, González-López R, Resel Folkersma L, Machado Fernández G, Adot Zurbano JM, Bonillo MÁ, Vozmediano Chicharro R, Zubiaur Líbano C. Recommendations on the use of intravesical hyaluronic acid instillations in bladder pain syndrome. Actas urologicas espanolas. 2022 Apr:46(3):131-137. doi: 10.1016/j.acuroe.2022.02.007. Epub 2022 Mar 4 [PubMed PMID: 35256323]
Han XM, Wu XH, Li B, Pan F, Li WC, Liu SL, Zeng FQ, Chen M. The effects of intravesical therapy with hyaluronic acid for painful bladder syndrome: Preliminary Chinese experience and systematic review. Taiwanese journal of obstetrics & gynecology. 2015 Jun:54(3):240-7. doi: 10.1016/j.tjog.2014.09.007. Epub [PubMed PMID: 26166334]
Level 1 (high-level) evidencePyo JS, Cho WJ. Systematic Review and Meta-Analysis of Intravesical Hyaluronic Acid and Hyaluronic Acid/Chondroitin Sulfate Instillation for Interstitial Cystitis/Painful Bladder Syndrome. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2016:39(4):1618-25. doi: 10.1159/000447863. Epub 2016 Sep 15 [PubMed PMID: 27627755]
Level 1 (high-level) evidenceRooney P, Srivastava A, Watson L, Quinlan LR, Pandit A. Hyaluronic acid decreases IL-6 and IL-8 secretion and permeability in an inflammatory model of interstitial cystitis. Acta biomaterialia. 2015 Jun:19():66-75. doi: 10.1016/j.actbio.2015.02.030. Epub 2015 Mar 26 [PubMed PMID: 25818949]
Liang CC, Lin YH, Hsieh WC, Huang L. Urinary and psychological outcomes in women with interstitial cystitis/bladder pain syndrome following hyaluronic acid treatment. Taiwanese journal of obstetrics & gynecology. 2018 Jun:57(3):360-363. doi: 10.1016/j.tjog.2018.04.006. Epub [PubMed PMID: 29880165]
Hung MJ, Tsai CP, Lin YH, Huang WC, Chen GD, Shen PS. Hyaluronic acid improves pain symptoms more than bladder storage symptoms in women with interstitial cystitis. Taiwanese journal of obstetrics & gynecology. 2019 May:58(3):417-422. doi: 10.1016/j.tjog.2018.11.033. Epub [PubMed PMID: 31122535]
Henry RA, Morales A, Cahill CM. Beyond a Simple Anesthetic Effect: Lidocaine in the Diagnosis and Treatment of Interstitial Cystitis/bladder Pain Syndrome. Urology. 2015 May:85(5):1025-1033. doi: 10.1016/j.urology.2015.01.021. Epub [PubMed PMID: 25917728]
Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal transduction and targeted therapy. 2017:2():17023-. doi: 10.1038/sigtrans.2017.23. Epub 2017 Jul 14 [PubMed PMID: 29158945]
Abdel-Mageed AB, Bajwa A, Shenassa BB, Human L, Ghoniem GM. NF-kappaB-dependent gene expression of proinflammatory cytokines in T24 cells: possible role in interstitial cystitis. Urological research. 2003 Oct:31(5):300-5 [PubMed PMID: 14574533]
Davies NM, Longstreth J, Jamali F. Misoprostol therapeutics revisited. Pharmacotherapy. 2001 Jan:21(1):60-73 [PubMed PMID: 11191738]
Level 3 (low-level) evidenceTyagi P, Li Z, Chancellor M, De Groat WC, Yoshimura N, Huang L. Sustained intravesical drug delivery using thermosensitive hydrogel. Pharmaceutical research. 2004 May:21(5):832-7 [PubMed PMID: 15180342]
Level 3 (low-level) evidenceGray KJ, Engelmann UH, Johnson EH, Fishman IJ. Evaluation of misoprostol cytoprotection of the bladder with cyclophosphamide (Cytoxan) therapy. The Journal of urology. 1986 Aug:136(2):497-500 [PubMed PMID: 3090278]
Level 3 (low-level) evidenceKelly JD, Young MR, Johnston SR, Keane PF. Clinical response to an oral prostaglandin analogue in patients with interstitial cystitis. European urology. 1998:34(1):53-6 [PubMed PMID: 9676414]
Cardenas-Trowers OO, Abraham AG, Dotson TK, Houlette BA, Gaskins JT, Francis SL. Bladder Instillations With Triamcinolone Acetonide for Interstitial Cystitis-Bladder Pain Syndrome: A Randomized Controlled Trial. Obstetrics and gynecology. 2021 May 1:137(5):810-819. doi: 10.1097/AOG.0000000000004348. Epub [PubMed PMID: 33831942]
Level 1 (high-level) evidenceJiang T, Zhou X, Chen Z, Xiong T, Fu J, Liu Z, Yan D, Zhou Z, Shen W. Clinical efficacy of submucosal injection of triamcinolone acetonide in the treatment of type II/III interstitial cystitis/bladder pain syndrome. BMC urology. 2020 Mar 30:20(1):36. doi: 10.1186/s12894-020-00597-3. Epub 2020 Mar 30 [PubMed PMID: 32228552]
Gülpınar O, Haliloğlu AH, Gökce Mİ, Arıkan N. Instillation of Hyaluronic Acid via Electromotive Drug Administration Can Improve the Efficacy of Treatment in Patients With Interstitial Cystitis/Painful Bladder Syndrome: A Randomized Prospective Study. Korean journal of urology. 2014 May:55(5):354-9. doi: 10.4111/kju.2014.55.5.354. Epub 2014 May 12 [PubMed PMID: 24868341]
Level 1 (high-level) evidenceHashemi S, Sahai A, Malde S. Applications of electromotive drug administration in urology. Urology annals. 2020 Oct-Dec:12(4):301-308. doi: 10.4103/UA.UA_152_19. Epub 2020 Oct 15 [PubMed PMID: 33776323]
Kuo HC. Repeated intravesical onabotulinumtoxinA injections are effective in treatment of refractory interstitial cystitis/bladder pain syndrome. International journal of clinical practice. 2013 May:67(5):427-34. doi: 10.1111/ijcp.12113. Epub [PubMed PMID: 23574103]
Pinto RA, Costa D, Morgado A, Pereira P, Charrua A, Silva J, Cruz F. Intratrigonal OnabotulinumtoxinA Improves Bladder Symptoms and Quality of Life in Patients with Bladder Pain Syndrome/Interstitial Cystitis: A Pilot, Single Center, Randomized, Double-Blind, Placebo Controlled Trial. The Journal of urology. 2018 Apr:199(4):998-1003. doi: 10.1016/j.juro.2017.10.018. Epub 2017 Oct 13 [PubMed PMID: 29031769]
Level 1 (high-level) evidenceKuo HC, Chancellor MB. Comparison of intravesical botulinum toxin type A injections plus hydrodistention with hydrodistention alone for the treatment of refractory interstitial cystitis/painful bladder syndrome. BJU international. 2009 Sep:104(5):657-61. doi: 10.1111/j.1464-410X.2009.08495.x. Epub 2009 Mar 30 [PubMed PMID: 19338543]
Level 2 (mid-level) evidenceGiannantoni A, Gubbiotti M, Bini V. Botulinum Neurotoxin A Intravesical Injections in Interstitial Cystitis/Bladder Painful Syndrome: A Systematic Review with Meta-Analysis. Toxins. 2019 Aug 30:11(9):. doi: 10.3390/toxins11090510. Epub 2019 Aug 30 [PubMed PMID: 31480323]
Level 1 (high-level) evidencePanunzio A, Tafuri A, Mazzucato G, Cerrato C, Orlando R, Pagliarulo V, Antonelli A, Cerruto MA. Botulinum Toxin-A Injection in Chronic Pelvic Pain Syndrome Treatment: A Systematic Review and Pooled Meta-Analysis. Toxins. 2022 Jan 1:14(1):. doi: 10.3390/toxins14010025. Epub 2022 Jan 1 [PubMed PMID: 35051002]
Level 1 (high-level) evidenceLee CL, Kuo HC. Long-Term Efficacy and Safety of Repeated Intravescial OnabotulinumtoxinA Injections Plus Hydrodistention in the Treatment of Interstitial Cystitis/Bladder Pain Syndrome. Toxins. 2015 Oct 22:7(10):4283-93. doi: 10.3390/toxins7104283. Epub 2015 Oct 22 [PubMed PMID: 26506388]
Wang Y, Zheng J, Li Z, Jiang Y, Yu J, Li S, Chen X. Modified Botulinum Toxin Type A Injections Improve Symptoms Associated With Interstitial Cystitis/Bladder Pain Syndrome in Women: A Retrospective Cohort Study. Urology. 2024 Jul:189():27-33. doi: 10.1016/j.urology.2024.04.039. Epub 2024 May 4 [PubMed PMID: 38710455]
Level 2 (mid-level) evidenceKuo HC, Jiang YH, Tsai YC, Kuo YC. Intravesical botulinum toxin-A injections reduce bladder pain of interstitial cystitis/bladder pain syndrome refractory to conventional treatment - A prospective, multicenter, randomized, double-blind, placebo-controlled clinical trial. Neurourology and urodynamics. 2016 Jun:35(5):609-14. doi: 10.1002/nau.22760. Epub 2015 Apr 24 [PubMed PMID: 25914337]
Level 1 (high-level) evidencePinto R, Lopes T, Silva J, Silva C, Dinis P, Cruz F. Persistent therapeutic effect of repeated injections of onabotulinum toxin a in refractory bladder pain syndrome/interstitial cystitis. The Journal of urology. 2013 Feb:189(2):548-53. doi: 10.1016/j.juro.2012.09.027. Epub 2012 Dec 14 [PubMed PMID: 23253961]
Imamura M, Scott NW, Wallace SA, Ogah JA, Ford AA, Dubos YA, Brazzelli M. Interventions for treating people with symptoms of bladder pain syndrome: a network meta-analysis. The Cochrane database of systematic reviews. 2020 Jul 30:7(7):CD013325. doi: 10.1002/14651858.CD013325.pub2. Epub 2020 Jul 30 [PubMed PMID: 32734597]
Level 1 (high-level) evidenceWalker SJ, Plair A, Hemal K, Langefeld CD, Matthews C, Badlani G, Zambon J, Heath H, Evans RJ. Bladder Hydrodistention Does Not Result in a Significant Change in Bladder Capacity for Interstitial Cystitis/Bladder Pain Syndrome Patients. Urology. 2019 Oct:132():81-86. doi: 10.1016/j.urology.2019.06.031. Epub 2019 Jul 9 [PubMed PMID: 31299328]
Glemain P, Rivière C, Lenormand L, Karam G, Bouchot O, Buzelin JM. Prolonged hydrodistention of the bladder for symptomatic treatment of interstitial cystitis: efficacy at 6 months and 1 year. European urology. 2002 Jan:41(1):79-84 [PubMed PMID: 11999471]
Level 2 (mid-level) evidencePeters KM. Neuromodulation for the treatment of refractory interstitial cystitis. Reviews in urology. 2002:4 Suppl 1(Suppl 1):S36-43 [PubMed PMID: 16986033]
Wang J, Chen Y, Chen J, Zhang G, Wu P. Sacral Neuromodulation for Refractory Bladder Pain Syndrome/Interstitial Cystitis: a Global Systematic Review and Meta-analysis. Scientific reports. 2017 Sep 8:7(1):11031. doi: 10.1038/s41598-017-11062-x. Epub 2017 Sep 8 [PubMed PMID: 28887515]
Level 1 (high-level) evidenceMahran A, Baaklini G, Hassani D, Abolella HA, Safwat AS, Neudecker M, Hijaz AK, Mahajan ST, Siegel SW, El-Nashar SA. Sacral neuromodulation treating chronic pelvic pain: a meta-analysis and systematic review of the literature. International urogynecology journal. 2019 Jul:30(7):1023-1035. doi: 10.1007/s00192-019-03898-w. Epub 2019 Mar 14 [PubMed PMID: 30874835]
Level 1 (high-level) evidenceYang TX, Luo DY, Li H, Wang KJ, Shen H. Is Urethrectomy Necessary During Cystectomy in Patients With Interstitial Cystitis or Bladder Pain Syndrome? Urology. 2016 Nov:97():73-79. doi: 10.1016/j.urology.2016.07.003. Epub 2016 Jul 14 [PubMed PMID: 27424120]
Costello AJ, Crowe H, Agarwal D. Supratrigonal cystectomy and ileocystoplasty in management of interstitial cystitis. The Australian and New Zealand journal of surgery. 2000 Jan:70(1):34-8 [PubMed PMID: 10696940]
Kim HJ, Lee JS, Cho WJ, Lee HS, Lee HN, You HW, Jung W, Lee KS. Efficacy and safety of augmentation ileocystoplasty combined with supratrigonal cystectomy for the treatment of refractory bladder pain syndrome/interstitial cystitis with Hunner's lesion. International journal of urology : official journal of the Japanese Urological Association. 2014 Apr:21 Suppl 1():69-73. doi: 10.1111/iju.12320. Epub [PubMed PMID: 24807503]
Level 2 (mid-level) evidenceMadec FX, Hedhli O, Perrouin-Verbe MA, Levesque A, Le Normand L, Rigaud J. Feasibility, Morbidity, and Functional Results of Supratrigonal Cystectomy with Augmentation Ileocystoplasty by Combined Robot-Assisted Laparoscopy and Mini-Laparotomy Approach. Journal of endourology. 2017 Jul:31(7):655-660. doi: 10.1089/end.2017.0107. Epub 2017 Jun 6 [PubMed PMID: 28467725]
Level 2 (mid-level) evidenceQueissert F, Bruecher B, van Ophoven A, Schrader AJ. Supratrigonal cystectomy and augmentation cystoplasty with ileum or ileocecum in the treatment of ulcerative interstitial cystitis/bladder pain syndrome: a 14-year follow-up. International urogynecology journal. 2022 May:33(5):1267-1272. doi: 10.1007/s00192-022-05110-y. Epub 2022 Mar 1 [PubMed PMID: 35230481]
Osman NI, Bratt DG, Downey AP, Esperto F, Inman RD, Chapple CR. A Systematic Review of Surgical interventions for the Treatment of Bladder Pain Syndrome/Interstitial Cystitis. European urology focus. 2021 Jul:7(4):877-885. doi: 10.1016/j.euf.2020.02.014. Epub 2020 Mar 1 [PubMed PMID: 32127327]
Mykoniatis I, Tsiakaras S, Samarinas M, Anastasiadis A, Symeonidis EN, Sountoulides P. Monoclonal Antibody Therapy for the Treatment of Interstitial Cystitis. Biologics : targets & therapy. 2022:16():47-55. doi: 10.2147/BTT.S290286. Epub 2022 May 20 [PubMed PMID: 35619987]
Kocatürk H, Atasoy N, Bedir F, Altay MS, Demirdöğen ŞO, Koç E, Yilmaz S. Questionnaire-guided evaluation of the effectiveness of long-term intravesical 0.2% chondroitin sulfate therapy in interstitial cystitis. International urogynecology journal. 2021 May:32(5):1293-1298. doi: 10.1007/s00192-020-04245-0. Epub 2020 Feb 11 [PubMed PMID: 32047969]
Danacioglu YO, Erol B, Ozkanli S, Yildirim A, Atis RG, Silay MS, Caskurlu T. Comparison of Intravesical Hyaluronic Acid, Chondroitin Sulfate, and Combination of Hyaluronic Acid-Chondroitin Sulfate Therapies in Animal Model of Interstitial Cystitis. International neurourology journal. 2021 Mar:25(1):42-50. doi: 10.5213/inj.1938176.088. Epub 2021 Jan 19 [PubMed PMID: 33504136]
Level 3 (low-level) evidenceÖzkıdık M. Assessment of long-term intravesical hyaluronic acid, chondroitin sulfate and combination therapy for patients with bladder pain syndrome. Central European journal of urology. 2019:72(3):270-275. doi: 10.5173/ceju.2019.0007. Epub 2019 Sep 16 [PubMed PMID: 31720029]
Stellavato A, Pirozzi AVA, Diana P, Reale S, Vassallo V, Fusco A, Donnarumma G, De Rosa M, Schiraldi C. Hyaluronic acid and chondroitin sulfate, alone or in combination, efficiently counteract induced bladder cell damage and inflammation. PloS one. 2019:14(6):e0218475. doi: 10.1371/journal.pone.0218475. Epub 2019 Jun 25 [PubMed PMID: 31237905]
Lee YK, Jiang YH, Jhang JF, Ho HC, Kuo HC. Changes in the Ultrastructure of the Bladder Urothelium in Patients with Interstitial Cystitis after Intravesical Injections of Platelet-Rich Plasma. Biomedicines. 2022 May 20:10(5):. doi: 10.3390/biomedicines10051182. Epub 2022 May 20 [PubMed PMID: 35625918]
Gallego-Vilar D, García-Fadrique G, Povo-Martin I, Salvador-Marin M, Gallego-Gomez J. Maintenance of the response to dimethyl sulfoxide treatment using hyperbaric oxygen in interstitial cystitis/painful bladder syndrome: a prospective, randomized, comparative study. Urologia internationalis. 2013:90(4):411-6. doi: 10.1159/000343697. Epub 2013 Mar 13 [PubMed PMID: 23485788]
Level 3 (low-level) evidenceChuang YC, Lee WC, Lee WC, Chiang PH. Intravesical liposome versus oral pentosan polysulfate for interstitial cystitis/painful bladder syndrome. The Journal of urology. 2009 Oct:182(4):1393-400. doi: 10.1016/j.juro.2009.06.024. Epub 2009 Aug 15 [PubMed PMID: 19683290]
Tyagi P, Kashyap M, Majima T, Kawamorita N, Yoshizawa T, Yoshimura N. Intravesical liposome therapy for interstitial cystitis. International journal of urology : official journal of the Japanese Urological Association. 2017 Apr:24(4):262-271. doi: 10.1111/iju.13317. Epub 2017 Mar 4 [PubMed PMID: 28258657]
Wen C, Xie L, Hu C. Roles of mesenchymal stem cells and exosomes in interstitial cystitis/bladder pain syndrome. Journal of cellular and molecular medicine. 2022 Feb:26(3):624-635. doi: 10.1111/jcmm.17132. Epub 2021 Dec 24 [PubMed PMID: 34953040]
Etulain J. Platelets in wound healing and regenerative medicine. Platelets. 2018 Sep:29(6):556-568. doi: 10.1080/09537104.2018.1430357. Epub 2018 Feb 14 [PubMed PMID: 29442539]
Mussano F, Genova T, Munaron L, Petrillo S, Erovigni F, Carossa S. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets. 2016 Jul:27(5):467-71. doi: 10.3109/09537104.2016.1143922. Epub 2016 Mar 7 [PubMed PMID: 26950533]
Kuffler DP. Platelet-rich plasma and the elimination of neuropathic pain. Molecular neurobiology. 2013 Oct:48(2):315-32. doi: 10.1007/s12035-013-8494-7. Epub 2013 Jul 7 [PubMed PMID: 23832571]
Level 3 (low-level) evidenceJhang JF, Wu SY, Lin TY, Kuo HC. Repeated intravesical injections of platelet-rich plasma are effective in the treatment of interstitial cystitis: a case control pilot study. Lower urinary tract symptoms. 2019 Apr:11(2):O42-O47. doi: 10.1111/luts.12212. Epub 2017 Dec 19 [PubMed PMID: 29265766]
Level 2 (mid-level) evidenceJhang JF, Lin TY, Kuo HC. Intravesical injections of platelet-rich plasma is effective and safe in treatment of interstitial cystitis refractory to conventional treatment-A prospective clinical trial. Neurourology and urodynamics. 2019 Feb:38(2):703-709. doi: 10.1002/nau.23898. Epub 2018 Dec 21 [PubMed PMID: 30576011]
Jiang YH, Kuo YC, Jhang JF, Lee CL, Hsu YH, Ho HC, Kuo HC. Repeated intravesical injections of platelet-rich plasma improve symptoms and alter urinary functional proteins in patients with refractory interstitial cystitis. Scientific reports. 2020 Sep 16:10(1):15218. doi: 10.1038/s41598-020-72292-0. Epub 2020 Sep 16 [PubMed PMID: 32939046]
Jhang JF, Jiang YH, Hsu YH, Ho HC, Birder LA, Lin TY, Kuo HC. Improved Urothelial Cell Proliferation, Cytoskeleton and Barrier Function Protein Expression in the Patients With Interstitial Cystitis/Bladder Pain Syndrome After Intravesical Platelet-Rich Plasma Injection. International neurourology journal. 2022 Feb:26(Suppl 1):S57-67. doi: 10.5213/inj.2142100.050. Epub 2022 Jan 20 [PubMed PMID: 35073671]
Ke QS, Jhang JF, Lin TY, Ho HC, Jiang YH, Hsu YH, Kuo HC. Therapeutic potential of intravesical injections of platelet-rich plasma in the treatment of lower urinary tract disorders due to regenerative deficiency. Ci ji yi xue za zhi = Tzu-chi medical journal. 2019 Jul-Sep:31(3):135-143. doi: 10.4103/tcmj.tcmj_92_19. Epub [PubMed PMID: 31258287]
Li H, Zhang Z, Peng J, Xin Z, Li M, Yang B, Fang D, Tang Y, Guo Y. Treatment with low-energy shock wave alleviates pain in an animal model of uroplakin 3A-induced autoimmune interstitial cystitis/painful bladder syndrome. Investigative and clinical urology. 2019 Sep:60(5):359-366. doi: 10.4111/icu.2019.60.5.359. Epub 2019 Aug 30 [PubMed PMID: 31501798]
Level 3 (low-level) evidenceJiang YH, Jhang JF, Lee YK, Kuo HC. Low-Energy Shock Wave Plus Intravesical Instillation of Botulinum Toxin A for Interstitial Cystitis/Bladder Pain Syndrome: Pathophysiology and Preliminary Result of a Novel Minimally Invasive Treatment. Biomedicines. 2022 Feb 7:10(2):. doi: 10.3390/biomedicines10020396. Epub 2022 Feb 7 [PubMed PMID: 35203604]
Jhang JF, Hsu YH, Peng CW, Jiang YH, Ho HC, Kuo HC. Epstein-Barr Virus as a Potential Etiology of Persistent Bladder Inflammation in Human Interstitial Cystitis/Bladder Pain Syndrome. The Journal of urology. 2018 Sep:200(3):590-596. doi: 10.1016/j.juro.2018.03.133. Epub 2018 Apr 11 [PubMed PMID: 29653163]
Marcu I, Campian EC, Tu FF. Interstitial Cystitis/Bladder Pain Syndrome. Seminars in reproductive medicine. 2018 Mar:36(2):123-135. doi: 10.1055/s-0038-1676089. Epub 2018 Dec 19 [PubMed PMID: 30566978]
Cvach K, Rosamilia A. Review of intravesical therapies for bladder pain syndrome/interstitial cystitis. Translational andrology and urology. 2015 Dec:4(6):629-37. doi: 10.3978/j.issn.2223-4683.2015.10.07. Epub [PubMed PMID: 26816864]
Bradley CS, Gallop R, Sutcliffe S, Kreder KJ, Lai HH, Clemens JQ, Naliboff BD, Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. Long-Term Symptom Trajectories in Urologic Chronic Pelvic Pain Syndrome: A MAPP Research Network Study. Urology. 2022 Nov:169():58-64. doi: 10.1016/j.urology.2022.07.045. Epub 2022 Aug 9 [PubMed PMID: 35961564]
Engelsgjerd JS, Deibert CM. Cystoscopy. StatPearls. 2024 Jan:(): [PubMed PMID: 29630232]
Axiotakis LG Jr, Youngerman BE, Casals RK, Cooke TS, Winston GM, Chang CL, Boyett DM, Lalwani AK, McKhann GM. Risk of Acquiring Perioperative COVID-19 During the Initial Pandemic Peak: A Retrospective Cohort Study. Annals of surgery. 2021 Jan 1:273(1):41-48. doi: 10.1097/SLA.0000000000004586. Epub [PubMed PMID: 33156061]
Level 2 (mid-level) evidenceGottschalk A, Van Aken H, Zenz M, Standl T. Is anesthesia dangerous? Deutsches Arzteblatt international. 2011 Jul:108(27):469-74. doi: 10.3238/arztebl.2011.0469. Epub 2011 Jul 8 [PubMed PMID: 21814522]
Fasting S. [Risk in anaesthesia]. Tidsskrift for den Norske laegeforening : tidsskrift for praktisk medicin, ny raekke. 2010 Mar 11:130(5):498-502. doi: 10.4045/tidsskr.08.0666. Epub [PubMed PMID: 20224619]
Breau RH, McGrath PJ, Norman RW. Assessing self-help issues for patients with prostate cancer, interstitial cystitis, erectile dysfunction and urinary diversion. BJU international. 2003 Nov:92(7):736-40 [PubMed PMID: 14616457]
Gohritz A, Dellon AL. Bladder Pain Syndome/Interstitial Cystitis due to Pudendal Nerve Compression: Described in 1915-A Reminder for Treating Pelvic Pain a Century Later. Journal of brachial plexus and peripheral nerve injury. 2020 Jan:15(1):e5-e8. doi: 10.1055/s-0039-1700538. Epub 2020 Mar 6 [PubMed PMID: 32153650]
Korting GE, Smith SD, Wheeler MA, Weiss RM, Foster HE Jr. A randomized double-blind trial of oral L-arginine for treatment of interstitial cystitis. The Journal of urology. 1999 Feb:161(2):558-65 [PubMed PMID: 9915448]
Level 1 (high-level) evidenceMurina F, Graziottin A, Felice R, Gambini D. Alpha Lipoic Acid Plus Omega-3 Fatty Acids for Vestibulodynia Associated With Painful Bladder Syndrome. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC. 2017 Mar:39(3):131-137. doi: 10.1016/j.jogc.2016.12.035. Epub [PubMed PMID: 28343553]
Di Tucci C, Di Feliciantonio M, Vena F, Capone C, Schiavi MC, Pietrangeli D, Muzii L, Benedetti Panici P. Alpha lipoic acid in obstetrics and gynecology. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2018 Sep:34(9):729-733. doi: 10.1080/09513590.2018.1462320. Epub 2018 May 4 [PubMed PMID: 29726290]
Level 2 (mid-level) evidenceKatske F, Shoskes DA, Sender M, Poliakin R, Gagliano K, Rajfer J. Treatment of interstitial cystitis with a quercetin supplement. Techniques in urology. 2001 Mar:7(1):44-6 [PubMed PMID: 11272677]
Bologna RA, Gomelsky A, Lukban JC, Tu LM, Holzberg AS, Whitmore KE. The efficacy of calcium glycerophosphate in the prevention of food-related flares in interstitial cystitis. Urology. 2001 Jun:57(6 Suppl 1):119-20 [PubMed PMID: 11378102]
Gupta P, Gaines N, Sirls LT, Peters KM. A multidisciplinary approach to the evaluation and management of interstitial cystitis/bladder pain syndrome: an ideal model of care. Translational andrology and urology. 2015 Dec:4(6):611-9. doi: 10.3978/j.issn.2223-4683.2015.10.10. Epub [PubMed PMID: 26816861]