Introduction
Much like the skin and other mucosal surfaces, the human oral cavity is densely populated with microbial life that plays a variety of roles in our health and wellbeing. As with any colonized surface, it can also be susceptible to infection from native flora and invasive pathogens. Infections of dental origin—odontogenic infections—are among the most common infections worldwide.[1][2]
These infections can spread along fascial planes to involve the face and deep neck spaces; in adults, odontogenic infections are the primary cause of deep neck infections. These infections often require interprofessional treatment, with input from dentists, oral surgeons, otolaryngologists, primary care providers, and emergency medicine practitioners.[3]
Anatomy
The pediatric and adult oral cavity contains 20 and 32 teeth, respectively, divided evenly between the maxilla and mandible. Each tooth has between one and three roots that sit within the corresponding socket of the alveolus. The neck of each tooth sits above the alveolus and is surrounded by gingiva in the healthy adult. The crown, covered by enamel, is the visible portion of the tooth. Each tooth is fixed within the socket by periodontal ligaments and supplied by nerves and blood vessels through each root.[4]
There are many potential spaces between the muscles, bones, and fasciae of the face and neck to which odontogenic infections can spread.[5][6] Deep to the nasolabial fold, the canine space is formed by the insertion of the levator anguli oris muscle; it is bounded posteromedially by the maxilla and levator anguli oris and laterally by the facial skin. The root of the maxillary canine is often long and extends beyond this insertion.[7]
Infection of a maxillary canine can extend from the tooth root into the canine space. Similarly, the buccal space is formed by the insertion of the buccinator muscle on the maxilla and mandible; it is bordered medially by the buccinator muscle and maxilla and laterally by the skin. A maxillary molar root extending superiorly to the insertion can allow for the spread of infection into the subcutaneous tissues of the face.
Infections of the mandibular teeth can lead to infections in three different spaces.[6][5] The sublingual space is bounded superiorly by the lingual oral mucosa, medially by the extrinsic tongue muscles, inferiorly by the mylohyoid muscle, and anterolaterally by the lingual cortex of the mandible.
The submandibular space is bounded superiorly by the mylohyoid muscle, inferiorly by the neck skin, and laterally by the mandible. The posterior border of the mylohyoid is free, allowing infection to spread between the submandibular and sublingual spaces easily. Finally, the submental space is bounded superiorly by the mylohyoid muscle, inferiorly by the neck skin, and laterally by the anterior bellies of the digastric muscles.[8]
The spread of odontogenic infections of mandibular origin is largely based on the position of the tooth roots relative to the mylohyoid line.[6][5] The mylohyoid inserts on the superior surface of the hyoid bone and originates from the lingual cortex of the mandibular body at a point known as the mylohyoid line, which is an oblique line that slopes inferiorly from posterior to anterior. Infection spreading medially and inferiorly along dental roots superior to this line will result in infections of the sublingual space; spread along those roots inferior to the line will result in submandibular space infections.[6][5]
Spread to the submental space is unlikely to occur directly from the tooth root and often occurs due to spread from the submandibular space, sublingual space, or directly from the skin. Infection spreading to the buccal cortex will result in spread to the buccal space or superficial tissues of the neck along the insertion of the platysma on the mandible.[6][5][9]
Microbiology
The flora of the oral cavity is diverse, but it is dominated by bacteria, particularly anaerobes and facultative anaerobes.[10] It is estimated that over 700 types of bacteria live within the oral cavity and various types of fungi.[6] The most common bacterial genus by far is Streptococcus, a gram-positive coccus; common species include S. mitis, S. sanguinus, S. salivarius, and S. anginosus.[11]
Other notable gram-positive bacteria include diphtheroids and Clostridium species, a common anaerobic bacterial genus native to the oral cavity. Gram-negative bacteria are also native but are more common in those with poor oral hygiene or diseased states; common gram-negative bacteria in the oral cavity include Prevotella, Fusobacterium, Haemophilus, and Neisseria. Given the diverse bacterial flora of the oral cavity, nearly all odontogenic infections are polymicrobial; treatment is therefore broadly directed even after culture results.[5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The oral cavity lies at the junction of many critical anatomic structures and the coalescence of many fascial planes.[7] As such, odontogenic infections can quickly spread and compromise neurovascular structures and airway patency. Prompt identification of dental caries, apical abscesses, and other minor dental infections can prevent these potentially catastrophic infections from developing. Failure to do so can lead to many preventable temporary and permanent sequelae that significantly burden both the patient and the healthcare system.[1]
Odontogenic infections are classified and treated based on the tissues and spaces involved and the severity or progression of infection. Dental caries is the leading cause of odontogenic infection, the most common of which is the periapical abscess.[12]
Treatment often involves a combination of enteral or intravenous antibiotics and surgical drainage or debridement. In any case of odontogenic or orofacial infection, treatment by definitive dental extraction is the gold standard, although select cases may be amenable to dental preservation through endodontic treatment.[13]
Epidemiology
Minor dental infection is common in the United States; by age 65, over 90% of people will have experienced dental caries.[14] Men are more commonly affected than women, and over 70% of infections occur in patients between 20 and 50 years old.[2]
Tobacco use and diabetes are the most commonly associated comorbid conditions with odontogenic neck infections. In the last few decades, dental, odontogenic, and deep neck infections have decreased with the widespread use and availability of antibiotics; however, the proportion of deep neck infections from odontogenic origin has increased and is currently the leading cause of deep neck infection in adults in the United States.[2]
History and Physical
Patients with odontogenic infections classically present with one to two weeks of worsening dental pain preceding facial or neck swelling.[13] Often these patients will present with fever and malaise with recently decreased oral intake.[7] A detailed history will often reveal poor dental hygiene and prior dental extraction or caries.[1]
A review of systems commonly reveals odynophagia, neck pain, facial pain, trismus, fever, and malaise. Depending on the tooth of origin and direction of spread, these patients can report paresthesias, ocular pain, diplopia, dyspnea, and sialorrhea.[7]
Patients who have had a rupture of the abscess intraorally may complain of foul tasting and smelling secretions as a result of pus in the oral cavity. As with any infection in the head and neck, a thorough neck and cranial nerve exam should be performed to assess for other possible sources and causes of infection.[13]
Evaluation
Untreated dental infection can readily spread to the surrounding gingiva and soft tissues of the face and neck. The spread of mandibular dental infections into the neck depends greatly on their location in relation to the mylohyoid muscle, as mentioned above.
Cellulitis in its early stages can be identified and treated in the outpatient setting and requires only a physical exam for evaluation.[1] Skin and mucosal findings consistent with cellulitis include erythema, edema, induration, tenderness, and acute, tender cervical lymphadenopathy.[15]
Point of care ultrasound (POCUS) can be used to evaluate for abscess formation if there is a concern.[16] A computed tomography (CT) scan is unnecessary but can be a useful adjunct in patients in whom bone shadow or body habitus makes POCUS an unreliable imaging modality. In this case, a CT scan with intravenous contrast can aid in differentiating cellulitis from an abscess.[7] Cellulitis will appear as fat stranding and local enhancement with contrast.[16][12]
A contrasted CT scan of the neck and face should be considered in cases where concern for complex abscess formation or spread to deep neck spaces is suspected.[7] In cases of prominent neck and oral cavity swelling, stridor, dyspnea, and frank respiratory distress, rapid evaluation of the upper airway must be performed in a controlled setting. This is best done by awake flexible laryngoscopy to evaluate for impending obstruction or edema, which may indicate the need for urgent airway intervention.[13]
Treatment / Management
Cellulitis
Odontogenic cellulitis is characteristically polymicrobial, consisting of gram-positive and -negative anaerobes and aerobes. As such, antibiosis should be broad-spectrum; commonly, these infections are effectively treated with amoxicillin-clavulanate or clindamycin in penicillin-allergic patients.[13] Timely dental extraction by a dentist can expedite treatment and halt progression as the necrotic tooth serves as the infectious nidus.
Abscess
If treatment is delayed, cellulitis can progress to suppuration and abscess formation. Pus will often collect alongside the lingual or buccal cortex of the mandible or maxilla, depending on the tooth affected. An abscess clinically appears similar to cellulitis with tenderness, induration, and erythema. Physical exam findings more suggestive of an abscess include fluctuance, significant mass effect, and suppurative dental sockets. Most minor abscesses do not require a CT scan and can be identified with POCUS; an abscess will appear as an anechoic, occasionally loculated mass commonly along the cortex of its corresponding bone.[17][6][5] (B2)
Drainage of an abscess is recommended both for source control and palliation.[13] Odontogenic abscesses can be drained through transoral, transfacial, transcervical, or transnasal routes. However, transoral drainage confers the least morbidity and effectively conceals incisions; it should be attempted whenever feasible. Needle aspiration, incision and drainage, and temporary drain placement can be used in combination depending on the size, location, and patient. Often, ultrasound-guided needle aspiration can be used to confirm the location of the abscess cavity before formal incision and drainage are attempted, minimizing the possibility of a negative attempt. As with cellulitis, extraction of the offending tooth coupled with antibiosis is indicated.[6][5] (B2)
A culture swab of the abscess cavity should be taken, although due to the aforementioned polymicrobial nature of these infections, it rarely affects management. Many patients with odontogenic abscesses can be treated as an outpatient with oral antibiotics, but those with more extensive infections, immunodeficiency, or comorbid medical conditions should be given intravenous antibiotics and resuscitated and monitored as an inpatient.[15][1](B2)
Ludwig Angina
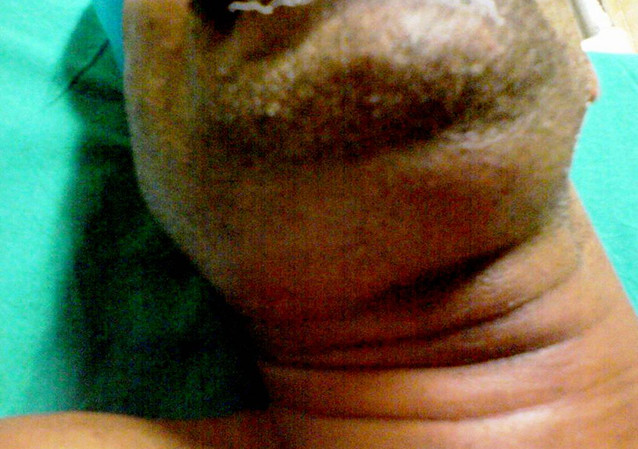
Many of the anatomic spaces contain a boundary that does not physically separate two spaces, allowing for the uninhibited spread to that space.[12] The upper cervical spaces (submandibular, sublingual, and submental) have few true fascial boundaries, and rapid spread of infection between all six of the three paired spaces can occur. Ludwig angina is a rare clinical entity that was first described in 1836 by Fredrick von Ludwig as a rapid and phlegmonous infection of bilateral submandibular, submental, and sublingual spaces.[18] The infection manifests most commonly as cellulitis as it presents too early to suppurate.[6][5] (B2)
Ludwig angina is an emergency given the mass effect of the infection on the upper airway. The infection promotes edema of the six spaces as well as the tongue, which can drastically decrease the distance between the base of the tongue to the soft palate and posterior pharyngeal wall leading to a potential airway catastrophe. A classic clinical appearance of the patient with Ludwig’s angina is respiratory distress, drooling, and a “bullfrog” appearance of the anterior neck.[12]
Management should prioritize airway assessment and stability with the simultaneous implementation of both short- and long-term airway stabilizing strategies. After securing the airway, these patients warrant prompt, broad-spectrum intravenous antibiotics and inpatient admission. Surgical intervention may be necessary for either airway concerns or infectious drainage, or debridement.[19]
Odontogenic Sinusitis
Dental infections (or normal oral flora) can spread superiorly along the maxilla, causing sinusitis. Sinusitis of odontogenic origin is common, accounting for nearly 40% of all acute bacterial maxillary sinusitis.[7][13](B3)
The maxillary teeth, particularly the molars and pre-molars, are closely associated with the maxillary sinus. Rarely on CT imaging or endoscopy, tooth roots and dental implants can be seen within the maxillary sinus. While this is often an incidental finding, occasionally, poor dental hygiene or a malpositioned implant can allow for the spread of infection into the maxillary sinus. In some patients with a history of oral or maxillofacial surgery, persistent oroantral fistulas can occur, serving as a conduit for opportunistic infection.[20][12]
Patients with odontogenic sinusitis often present with facial pain and pressure, fever, and malaise. Imaging often shows unilateral isolated maxillary sinusitis, whereas non-odontogenic rhinosinusitis is often bilateral and involves other sinuses.[20]
Treatment consists similarly of dental or implant extraction, antibiosis, and possible surgical intervention of the sinuses (endoscopic sinus surgery).[7] Patients with odontogenic sinusitis resulting from persistent oroantral fistula benefit most from repair of the fistula following resolution of the infection.[20](B3)
Differential Diagnosis
While odontogenic infections are often evident in their etiology, repeated infections or infection in an otherwise healthy individual can be an indicator of oral cavity malignancy.[1]
Dental or surgical follow-up should assess for resolution of any mucosal or bony irregularities and maintain a low threshold for biopsy. Occasionally, infection presumably of dental origin can originate from other adjacent sites such as the paranasal sinuses, temporal bone, congenital or acquired neck mass, or aerodigestive tract.[15]
When there is no obvious infected tooth or the history of dental pain is questionable, a thorough investigation of the nearby anatomy should be performed through a physical exam or imaging studies when applicable. Patients presenting with odontogenic infections as one of many soft tissue infections should be evaluated for possible immunodeficiencies, either congenital or acquired.[1]
Prognosis
Depending on the severity of the infection, the prognosis for odontogenic infections is generally positive.[7]
In a 2022 study of 200 patients who presented to the emergency department with odontogenic infections, over half were discharged same-day with medical therapy. Less than half necessitated inpatient admission, and approximately 50% of those admitted necessitated surgical intervention.[2] Of the entire cohort, there was only one morbidity and two patients with necrotizing soft tissue infections requiring multiple debridements.[2]
Complications
If left untreated, odontogenic infections that are easily treated as an outpatient with minimal intervention can progress to more serious infections requiring admission, invasive procedures, and moderate to severe morbidity.
Deep Space Neck Infections
Infections of the upper and lower jaws can quickly become cellulitis and abscesses of the surrounding soft tissues in the masticator and submandibular spaces, respectively.[12] These spaces have open boundaries with other deep neck spaces allowing for direct spread to the parapharyngeal, retropharyngeal, and carotid spaces of the neck. The prevertebral space, if infected, provides a rapid conduit into the mediastinum and chest, which cause mediastinitis or empyema. Infections of this nature often cannot effectively be treated without thoracic surgical intervention, which conveys significant additional morbidity and mortality.[15]
Osteomyelitis
The proximity of teeth to bone permits frequent, low-grade infection of the alveolus and other portions of the upper and lower jaw bones, known as acute osteomyelitis. These infections often resolve and permit bony regrowth when recognized and treated promptly. However, delayed treatment, poor nutrition, poor oral hygiene, and tobacco use can promote the development of chronic osteomyelitis. The presentation of chronic osteomyelitis is typically insidious, longstanding jaw pain and can include fistula formation, paresthesia from nerve involvement, or pathologic fracture.[6][5]
CT scan can identify sequestration of infected bone, osteolysis, and areas of frank necrosis. Treatment consists of long-term culture-directed antibiotic therapy with debridement of necrotic bone. In severe cases, reconstruction with bone grafts or free tissue transfer may be necessary.
Emergencies Secondary to Odontogenic Infections
Severe sequelae of dental infections are uncommon but require swift action by an interprofessional team. The most common emergent sequela of odontogenic infections is airway compromise, either from Ludwig’s angina or a variant thereof. Prompt recognition of respiratory distress and the level of airway obstruction by the emergency department can appropriately triage the patient and initiate aggressive and/or conservative strategies as appropriate.[15]
There are many long-acting strategies to improve obstruction. Surveillance with continuous pulse oximetry, telemetry, and high-acuity nursing is critical. Securing intravenous access is imperative as these patients may require much intravenous fluid resuscitation and medication. Some patients may require immediate supplemental oxygen, and this should be readily available.[15][19]
Intravenous administration of antibiotics and corticosteroids can improve airway edema and obstruction, but these medications require significant time to take effect.[21] Nebulized racemic epinephrine, while primarily used for idiopathic or hereditary angioedema and epiglottitis, can be somewhat effective in reducing edema of the tongue and oropharynx in the setting of airway obstruction secondary to an odontogenic infection; however, its utility is limited given the inability to reduce the mass effect of abscesses or deep tissue edema. Prompt assessment of the level and nature of obstruction guides therapy. An oral exam and neck exam can aid in evaluation, but often the exam is incomplete without an awake flexible laryngoscopy.[19]
Different levels of obstruction can be temporized readily with conservative measures; placement of a nasopharyngeal airway or (in the obtunded patient) an oropharyngeal airway can alleviate tongue base obstruction. Obstruction secondary to parapharyngeal or retropharyngeal spread may not be effectively treated by these methods.[19][21]
Based on the overall clinical picture, it may be necessary to definitively secure the airway by intubation or by surgical intervention. Most patients with severe enough obstruction to cause respiratory distress will not be able to be intubated with direct laryngoscopy or video-assisted indirect laryngoscopy.[21] In these cases, awake fiberoptic intubation is safe and effective if the glottis can be visualized. Some patients with acute respiratory distress and severe disease or unfavorable anatomy will require surgical intervention such as a cricothyroidotomy or tracheostomy.[19]
Rarely, odontogenic infections can progress to necrotizing soft tissue infections such as necrotizing fasciitis. As with any anatomic site, this is a true surgical emergency, and rapid airway assessment and surgical debridement should be performed. Necrotizing fasciitis is often caused by Group A Streptococcal species, but other anaerobic bacteria and oral flora have been implicated.[6][5][15][12]
Deterrence and Patient Education
The mainstay of the prevention of odontogenic and orofacial infections is proper dental hygiene.[3] Primary prevention strategies include education from primary care providers, public service information, and dental counseling. Patients should be instructed to perform regular dental brushing and flossing as well as biannual dental visits.
Since dental hygiene is of critical importance, discussion of poor dental habits is appropriate. These include consumption of highly acidic or sweet foods, consumption of tobacco products, consumption of alcohol, and tooth grinding (particularly bruxism). Secondary prevention is performed exclusively by dental hygienists and dentists with plaque removal and prompt recognition and treatment of dental caries.[3]
Enhancing Healthcare Team Outcomes
In 2000, the Surgeon General proclaimed that the United States was in a silent epidemic of dental disease. [3] While dental care is primarily managed by dentists and dental hygienists, dental evaluation and treatment in the United States depend on more than dentists alone.
Primary care providers, dentists, oral surgeons, and otolaryngologists regularly evaluate and treat oral and dental pathologies, providing ample opportunities for primary prevention and early recognition of poor hygiene and infection.[2] Maintaining a routine of regular oral examination is an easy and effective strategy to monitor patient dental disease, which is an overall marker of patient health.[3][12]
Odontogenic infections can often be evaluated and managed at the bedside with a clinical exam alone. Many equivocal exams can be further clarified using POCUS to assess for the presence of an abscess and guide surgical intervention.[13]
CT scan with contrast is often not indicated and can expose patients to unnecessary neck radiation, risk contrast-associated complications, and unveil incidental findings that prompt unnecessary, costly workup.[16]
Close collaboration with the emergency department, urgent care, and primary care providers, operating as an interprofessional team, can improve diagnostic accuracy and minimize physical and financial harm to the patient.[17]
Media
(Click Image to Enlarge)
References
Zawiślak E, Nowak R. Odontogenic Head and Neck Region Infections Requiring Hospitalization: An 18-Month Retrospective Analysis. BioMed research international. 2021:2021():7086763. doi: 10.1155/2021/7086763. Epub 2021 Jan 18 [PubMed PMID: 33532496]
Level 2 (mid-level) evidenceEisler L,Wearda K,Romatoski K,Odland RM, Morbidity and cost of odontogenic infections. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2013 Jul; [PubMed PMID: 23585157]
Level 2 (mid-level) evidenceStephens MB,Wiedemer JP,Kushner GM, Dental Problems in Primary Care. American family physician. 2018 Dec 1; [PubMed PMID: 30485039]
Sobkowska Ł,Sobkowska J,Dudek D,Grabarek BO,Czajka-Jakubowska A,Przystańska A, Symptoms of the Eruption of Permanent Teeth. International journal of environmental research and public health. 2022 Mar 11; [PubMed PMID: 35328989]
Rega AJ,Aziz SR,Ziccardi VB, Microbiology and antibiotic sensitivities of head and neck space infections of odontogenic origin. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2006 Sep; [PubMed PMID: 16916672]
Level 2 (mid-level) evidenceDøving M,Handal T,Galteland P, Bacterial odontogenic infections. Tidsskrift for den Norske laegeforening : tidsskrift for praktisk medicin, ny raekke. 2020 May 5; [PubMed PMID: 32378841]
Workman AD,Granquist EJ,Adappa ND, Odontogenic sinusitis: developments in diagnosis, microbiology, and treatment. Current opinion in otolaryngology [PubMed PMID: 29084007]
Level 3 (low-level) evidenceToth J,Lappin SL, Anatomy, Head and Neck, Mylohyoid Muscle StatPearls. 2022 Jan; [PubMed PMID: 31424877]
Hoerter JE,Patel BC, Anatomy, Head and Neck, Platysma StatPearls. 2022 Jan; [PubMed PMID: 31424878]
Riggio MP,Aga H,Murray CA,Jackson MS,Lennon A,Hammersley N,Bagg J, Identification of bacteria associated with spreading odontogenic infections by 16S rRNA gene sequencing. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2007 May; [PubMed PMID: 17141534]
Dewhirst FE,Chen T,Izard J,Paster BJ,Tanner AC,Yu WH,Lakshmanan A,Wade WG, The human oral microbiome. Journal of bacteriology. 2010 Oct; [PubMed PMID: 20656903]
Ogle OE, Odontogenic Infections. Dental clinics of North America. 2017 Apr; [PubMed PMID: 28317564]
Bhatia S,Kohli S, Tips for odontogenic infections. British dental journal. 2020 Dec; [PubMed PMID: 33339909]
Su S,Lipsky MS,Licari FW,Hung M, Comparing oral health behaviours of men and women in the United States. Journal of dentistry. 2022 Jul [PubMed PMID: 35545161]
Jevon P,Abdelrahman A,Pigadas N, Management of odontogenic infections and sepsis: an update. British dental journal. 2020 Sep; [PubMed PMID: 32978579]
Mardini S,Gohel A, Imaging of Odontogenic Infections. Radiologic clinics of North America. 2018 Jan; [PubMed PMID: 29157547]
Weyh A,Busby E,Smotherman C,Gautam S,Salman SO, Overutilization of Computed Tomography for Odontogenic Infections. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2019 Mar; [PubMed PMID: 30503981]
Derber CJ,Troy SB, Head and neck emergencies: bacterial meningitis, encephalitis, brain abscess, upper airway obstruction, and jugular septic thrombophlebitis. The Medical clinics of North America. 2012 Nov; [PubMed PMID: 23102480]
Vytla S, Gebauer D. Clinical guideline for the management of odontogenic infections in the tertiary setting. Australian dental journal. 2017 Dec:62(4):464-470. doi: 10.1111/adj.12538. Epub 2017 Jul 24 [PubMed PMID: 28621799]
Aukštakalnis R,Simonavičiūtė R,Simuntis R, Treatment options for odontogenic maxillary sinusitis: a review. Stomatologija. 2018; [PubMed PMID: 29806655]
Ovassapian A, Tuncbilek M, Weitzel EK, Joshi CW. Airway management in adult patients with deep neck infections: a case series and review of the literature. Anesthesia and analgesia. 2005 Feb:100(2):585-589. doi: 10.1213/01.ANE.0000141526.32741.CF. Epub [PubMed PMID: 15673898]
Level 2 (mid-level) evidence