Oral and Maxillofacial Surgery, Facial Laceration Repair
Oral and Maxillofacial Surgery, Facial Laceration Repair
Introduction
In the United States, approximately 2 million patients present annually to the emergency department for facial lacerations.[1][2] Given the prevalence of facial lacerations, understanding laceration management is crucial for primary and emergency care providers; poor wound care can lead to functional and aesthetic impairment, significantly reducing the patient's quality of life.[3]
Facial scarring can lead to negative social and functional impacts; timely facial soft tissue trauma management is crucial for maximizing aesthetic outcomes and overall patient satisfaction.[4] Not only does reconstruction of complex facial anatomy require careful attention to technical details, but the other aspects of wound care, such as thorough cleansing, tetanus prophylaxis, infection management, and scar prevention, are critical to optimizing outcomes after these injuries.
To provide optimal care for patients with facial lacerations, clinicians need to understand the relative anatomy of facial subunits and demonstrate competency in employing appropriate closure techniques and managing common complications associated with facial laceration repair.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The soft tissues of the head and face are divided into 8 distinct aesthetic and functional units with unique characteristics a clinician must understand to optimize laceration repair.[5][6]
Scalp
The scalp has 5 layers. From superficial to deep, the layers are skin, subcutaneous fat, galea aponeurotica, loose areolar tissue, and periosteum. The acronym SCALP can be used to help remember the layers.
The skin is the most external layer, containing hair follicles with a highly vascularized, fatty connective tissue layer underneath. Below this connective tissue is the galea aponeurotica; the galea overlies the vertex of the skull, carries the vessels that supply the subdermal plexus of the scalp, and serves as the insertion point for the occipitalis and frontalis bellies of the occipitofrontalis muscle. Laterally, the galea aponeurotica joins with the temporoparietal or superficial temporal fascia. This fascia is a loose areolar layer external to the fascia of the temporalis muscle or deep temporal fascia and the more superficial subcutaneous tissue. Beneath the galea aponeurotica is a layer of loose areolar tissue. Finally, the innermost layer of the scalp is the periosteum or pericranium, which is tightly adherent to the skull itself.
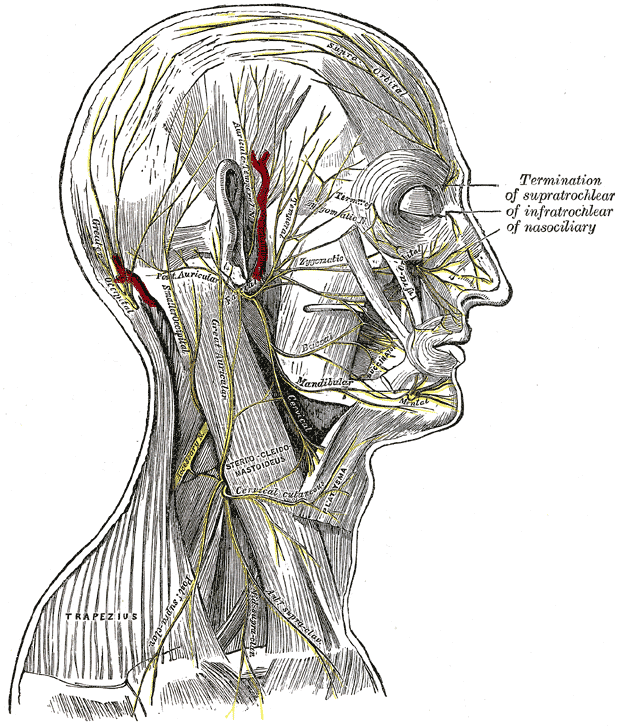
The scalp is a highly vascular region perfused by anastomoses of external and internal carotid artery branches; this robust vascularity is required to support the hair follicles, of which there are approximately 100,000. The superficial temporal, posterior auricular, and occipital arteries arise from the external carotid artery. These branches supply the frontotemporal scalp, the area superoposterior to the auricle, and the posterior scalp, respectively. The ophthalmic artery arises from the internal carotid artery and gives off the supraorbital and supratrochlear arteries, which perfuse the forehead and the anterior portion of the scalp. Outflow is supplied by superficial veins that run with their respectively named arteries. Because of this rich vascular network, the scalp can bleed profusely when injured due to the tight adherence of the vasculature to the fibrous galea aponeurotica, which impedes reactive vasoconstriction.[7]
Forehead
The forehead extends from the supraorbital rims inferiorly to the hairline superiorly or to the superior edge of the frontalis muscle if the natural hairline is not apparent. The forehead comprises layers analogous to the scalp; skin, subcutaneous connective tissue, the frontalis muscle contiguous with the galea aponeurotica, loose areolar tissue, and periosteum. Because the frontalis muscle contracts vertically, relaxed skin tension lines (RSTL) and skin creases or rhytids form transversely in the forehead. However, in the glabella, the action of the corrugator supercilii muscles causes vertical rhytids and RSTL. Therefore, orienting laceration repairs along RSTL, when possible, can be helpful for camouflaging scars.
The forehead is also supplied by branches of the internal and external carotid arteries. The paired supratrochlear and supraorbital arteries travel with their homonymous nerves to supply the central forehead. The supratrochlear artery exits the skull through a notch at the superomedial orbit roughly 2 cm from the midline and then courses through the corrugator supercilii muscle to reach the frontalis muscle and, ultimately, the skin.[8] The supraorbital artery exits the skull via a notch in 44% to 59% of patients or a foramen in 19% to 35% before coursing superiorly up the forehead.[9] The superficial temporal artery, a terminal branch of the external carotid artery, perfuses the lateral forehead.
The supraorbital and supratrochlear nerves, branches of the ophthalmic division of the trigeminal nerve (CN V Division V1), provide sensory innervation to the forehead and anterior scalp; the frontal or temporal branch of the facial nerve (CN VII) provides the motor innervation. This motor nerve lies within the subgaleal space and controls the frontalis, the superior portion of the orbicularis oculi, and corrugator supercilii muscles. The course of the frontal branch may be approximated by the Pitanguy line running from a point 5 mm inferior to the tragus to a point 15 mm above the lateral brow.[10][11][12]
Cheek
The cheek extends from the infraorbital rim inferiorly to the jawline, laterally to the preauricular crease, and medially to the nose. The layers of the cheek comprise the skin, subcutaneous tissue, superficial musculoaponeurotic system (SMAS), sub-SMAS areolar space, and the deep parotidomasseteric fascia.[13] The subcutaneous fat layer contains the facial fat pads that provide additional volume and contour to the face. Lacerations to the cheek that extend into the subcutaneous layer can lead to herniation of these fat pads. Deep to the subcutaneous layer is the SMAS, which is contiguous superiorly with the temporoparietal fascia and anteriorly with the mimetic muscles.[14] The sub-SMAS areolar space is a glide plane for the overlying facial muscles and facial ligaments that anchor the soft tissue to the bony facial framework.
The blood supply to the cheek comes from multiple sources. Branches of the ophthalmic artery, such as the zygomaticofacial, zygomaticotemporal, and infraorbital arteries, supply the medial cheek. The transverse facial artery, a branch of the superficial temporal artery, supplies the lateral cheek. Finally, the angular artery, the terminal branch of the facial artery, runs along the nasolabial fold to supply the inferomedial cheek and lateral nose.
Branches of the maxillary division of the trigeminal nerve (CN V Division V2) supply the sensory innervation to the cheek. The lateral cheek contains the parotid gland and the extratemporal facial nerve. Therefore, the parotid gland, duct, and extratemporal facial nerve must be evaluated in any soft tissue cheek injury. The facial nerve exits the stylomastoid foramen and enters the parotid gland, dividing the gland into superficial and deep lobes. The facial nerve divides within the parotid gland into the superior temporozygomatic and inferior cervicofacial divisions, which run beneath the SMAS on the superficial surface of the masseteric fascia until the branches reach their target muscles of facial expression. The terminal branches of the facial nerve include the frontal, zygomatic, buccal, marginal mandibular, and cervical nerves.[15]
All branches of the facial nerve should be assessed at the time of cheek injury; injuries proximal to the branching point of the nerve can lead to weakness in all divisions. Damage to facial nerve branches medial to the lateral canthus typically does not require repair, as cross-innervation among branches prevents significant deficits and promotes consistent recovery.[16]
The parotid gland lies within the parotidomasseteric fascia inferior to the zygomatic arch and superficial to the masseter muscle. The parotid or Stenson duct extends anteriorly from the parotid gland and courses medially with the buccal division of the facial nerve and the transverse facial artery. At the anterior border of the masseter muscle, the duct travels medially through the buccal fat pad, piercing the buccinator muscle. The duct then enters the oral cavity through a punctum adjacent to the second maxillary molar. The course of the parotid duct can be generally approximated by a line drawn from the tragus to the midline of the upper lip. Injury to the lateral cheek can violate the integrity of the parotid gland and duct. This must be addressed before laceration repair to prevent saliva from leaking into and collecting within the subcutaneous space, forming either a sialocele or cutaneous salivary fistula.[17][18][19][20][21][22]
Periorbital Region
The upper and lower eyelids are multilayered structures anchored to the medial and lateral orbital periosteum or periorbita by the canthal tendons. For convenience, the eyelids are often conceptualized as comprising anterior and posterior lamellae; some describe the orbital septum and tarsal plates as the middle lamella. The anterior lamella of the eyelid comprises the skin and underlying orbicularis oculi muscle. Eyelid skin is the thinnest in the body, contains no subcutaneous fat, and therefore requires gentle handling during laceration repair. Beneath the anterior lamella is the orbital septum, a fibrous connective tissue layer extending from the periosteum and inserting onto the tarsal plate. The orbital septum retains the orbital contents, particularly the fat, within the orbit. The tarsal plates are dense connective tissues that support the eyelids and contain the eyelash follicles.[23][24]
Beneath the tarsal plates lies the posterior lamella, comprising the levator palpebrae superioris muscle of the upper eyelid, capsulopalpebral fascia analogous to the levator palpebrae muscle in the lower eyelid, and conjunctiva. The conjunctiva lines the inner aspect of the eyelid and is closely associated with the globe to lubricate and protect the eye. Laterally, the lateral canthal tendons are bands of connective tissue that anchor the tarsus to the surrounding periosteum and suspend the eyelid in its appropriate position. The medial canthal tendon has a more complicated anatomic configuration than the lateral, surrounding the lacrimal drainage system and contributing to the lacrimal pump mechanism. The upper and lower eyelids each have one punctum along their margins, roughly where the lashes start medially, and these puncta communicate with the superior and inferior canaliculi that drain into the common lacrimal canaliculus. The common canaliculus collects into the lacrimal sac and drains tears through the nasolacrimal duct into the nose, underneath the inferior turbinate.
Medial canthal lacerations, therefore, should raise the index of suspicion for damage to the nasolacrimal drainage system, which must be evaluated before laceration repair. Physical examination findings indicative of medial canthal injuries are lateral displacement of the eyelid puncta, rounding of the medial canthal angle, telecanthus, and transverse shortening of the palpebral aperture.[25][26][27] Like other facial subunit boundaries, the eyelid margin is an essential landmark; disruptions must be carefully reapproximated to prevent unsightly eyelid notching.[28]
The eyelid receives a rich blood supply primarily from branches of the internal carotid artery, including the lateral palpebral artery from the lacrimal artery and the medial palpebral artery from the ophthalmic artery.[24]
Nose
The nasal soft tissue envelope comprises the skin, SMAS, fat, periosteum and perichondrium, bone and cartilage, and internal nasal mucosa. Functionally, the nose can be divided into upper, middle, and lower thirds. The nasal bones and frontal processes of the maxillae constitute the bony vault or upper third. The midvault or middle third comprises the paired upper lateral cartilages and dorsal septum. The lower third of the nose, or the tip, is supported by the 2 lower lateral cartilages.
For reconstructive purposes, the nose is often divided into 9 aesthetic subunits, following the work of Burget and Menick in 1985.[29] These subunits are the nasal tip, dorsum, columella, two nasal sidewalls, 2 soft tissue triangles or facets, and two nasal alae. The skin overlying the nose varies in thickness depending on the region, which is critical to consider for laceration repair; the skin of the midvault and rhinion is the thinnest, with thicker skin extending over the nasal root and tip.[30]
A robust anastomosis of arteries contributed by branches of the ophthalmic, maxillary, and facial arteries supply the external nose.[31]
The external nasal nerve from CN V Division 1 supplies sensory innervation to the nasal dorsum and nasal alae. The infraorbital nerve from CN V Division 2 supplies sensory innervation to lateral portions of the nose. The zygomatic and buccal branches of the facial nerve supply motor innervation.[32]
Ear
The auricle comprises 6 subunits: tragus, antitragus, helix including the helical crus, antihelix including the antihelical crura, conchal bowl divided into cymba concha and cavum concha by the helical crus, and lobule. From superficial to deep, the layers of the auricle are the skin, perichondrium, and underlying cartilage. The auricular lobule is unique as it comprises fibroadipose tissue without underlying cartilage.
Auricular blood supply is from the external carotid artery through the superficial temporal and posterior auricular arteries, which supply the perichondrium and that, in turn, nourishes the relatively avascular cartilage.
Several sensory nerves innervate the external ear: the auriculotemporal nerve from the trigeminal nerve, the lesser occipital nerve, and the greater auricular nerve.[33][34]
Lip
The perioral unit has 6 subunits, 4 in the upper lip and 2 in the lower. The perioral unit is dominated by the lips and spans the area between the nasolabial folds laterally, the labiomental sulcus inferiorly, and the nasal base superiorly. The subunits of the lower lip are the cutaneous lower lip or white lip and the vermillion or red lip. The subunits of the upper lip are the vermillion and the cutaneous portions, the latter of which is divided into bilateral lateral segments and a central philtrum. The transition from the white lip to the red lip is called the vermillion border, which must be precisely recreated when repairing a laceration, as this is a prominent aesthetic subunit boundary of the face. The white roll, just above the vermillion border, is less apparent in older patients but is a critical landmark in cleft lip repair. Another important transition zone is the wet-dry junction, where the red lip meets the labial mucosa.
The lips comprise 3 main layers, the skin or outer mucosal layer of the dry lip, the orbicularis oris muscle, and the oral mucosa.
The blood supply to the lips comes via the superior and inferior labial arteries, which are branches of the facial artery located deep to the muscle and are typically only injured in full-thickness lacerations.
Separate branches of the trigeminal nerve supply the sensory innervation to the upper and lower lip. The infraorbital nerve from CN V Division 2 supplies the upper lip, and the mental nerve from CN V Division 3 supplies the lower lip. The buccal and marginal mandibular branches of the facial nerve provide motor innervation to the perioral musculature.[35][36]
Chin
The chin lies at the inferior central aspect of the mandible, just inferior to the labiomental sulcus. Much of the bulk of the chin comes from subcutaneous fibroadipose tissue and the mentalis muscle, which originates on the mandible and inserts into the dermis. Sensory innervation to the chin is supplied by the mental nerve of CN V Division 3, which exits the mental foramen of the mandible anterior to the first premolar. The marginal mandibular branch of the facial nerve provides motor innervation to the mentalis muscle.[6]
Indications
Any soft tissue injury to the head or face warrants a thorough history and physical examination. Superficial abrasions or injuries with minimal epidermal disruption may be suitable for management with local wound care and healing by secondary intention. However, all wounds require close inspection and evaluation of surrounding structural and concomitant injuries. Laceration repair can begin after associated facial injuries have been addressed. Wounds that violate multiple tissue planes require layered closure to restore tensile strength and resist dehiscence and unsightly scarring.[37]
Contraindications
Emergent injuries that require immediate attention, such as intracranial or intrathoracic trauma, take precedence over laceration repair. Securing the airway and maintaining hemodynamic stability remains the priority in a trauma evaluation. In a stable patient, head or facial lacerations should not be repaired until other bony or structural facial injuries have been assessed and the need for operative or specialty intervention has been determined. In some cases, facial lacerations provide optimal access to underlying bony injuries. Because the mechanisms of injury commonly resulting in facial lacerations include motor vehicle accidents, falls, and physical altercations, the cervical spine should always be evaluated and protected.
Wounds that appear grossly infected or highly contaminated should not be closed, as closure can create an abscess. A similar concern for infection exists with wounds evaluated 24 hours after injury. Lacerations that present more than 24 hours after injury without proper irrigation and repair carry a higher risk for wound infection and should be repaired in a delayed fashion or left to heal by secondary intention. Complex repair in the operating room should be considered when there is extensive soft tissue loss, need for debridement, or if laceration defects cannot be repaired tension-free at the bedside.[37][38]
Equipment
Laceration repair requires the following equipment:
- 1 L or more of sterile saline for irrigation
- Bulb syringe or 20 to 60 mL capacity catheter-tipped syringe for irrigation
- Povidone iodine, chlorhexidine, or antiseptic solution of choice
- Local anesthetic
- 18- and 27-gauge needles
- 10 mL syringe
- Sterile gloves
- Suction
- Gauze sponges
- Castroviejo or Halsey needle driver
- 0.5-mm Castroviejo or Adson-Brown forceps
- Mayo or Iris suture scissors
- Sutures of choice (a selection of 3-0 through 6-0 absorbable and nonabsorbable, monofilament and braided materials, depending on the location of the injury and what structures are involved)
- Antibiotic ointment or adhesive strip bandage tapes
Personnel
Emergency medicine or primary care providers who understand fundamental suturing techniques and tissue handling can repair simple facial lacerations. However, if there are underlying injuries to the globe, parotid duct, facial nerve, lacrimal drainage system, eyelid margin, a significant loss of tissue, or violation of multiple tissue planes requiring complex repair, the appropriate surgical specialist must be consulted for evaluation and management of the injury.[39]
Preparation
Informed consent must be obtained from the patient before laceration repair commences. The consent process must include a discussion of shared goals and expectations for the procedure, particularly if it is to be performed under local anesthesia, immediate postoperative period, and long-term recovery. Wound care instructions should be provided in written format and reviewed verbally, ideally before and after the repair, with the patient and a caregiver or escort. Modifiable and non-modifiable risk factors for poor wound healing should be discussed. Patients who are immunocompromised, chronically ill, or malnourished are predisposed to poor wound healing. Additionally, tobacco use or inadequate diabetes management can complicate wound healing. Preoperative counseling represents an opportunity to encourage patients to consider smoking cessation or improved glucose control to improve outcomes.[40][41]
A comprehensive medical history should be obtained, and a thorough physical examination should be conducted. In addition, other concomitant injuries should be identified, evaluated, or ruled out. Finally, photographs should be taken before and after laceration repair for medical documentation and potential legal purposes. These photographs also allow the patient, in the future, to look back at the injury and appreciate the fact that even if there is scarring and asymmetry in the long term, the repaired state typically appears and functions substantially better than the acutely injured state.
Additionally, limitations to patient participation in follow-up should be assessed. Patients with limited access to care or lacking reliable means to return to the clinic should have dissolvable sutures placed rather than non-dissolvable sutures that require removal.
Optimal laceration repair requires appropriate instrumentation and adequate lighting. Evaluation of the wound and associated injuries will determine if a bedside repair under local anesthesia or repair in the operating room under general anesthesia is preferable. Sensory and motor function must be evaluated before injecting local anesthetic to document baseline function and ensure that postoperative nerve deficits are only considered iatrogenic if they genuinely are.
Additionally, the need for any infection prophylaxis should be determined before beginning the repair. For example, bite wounds require prophylactic antibiotics and may require rabies vaccination. Amoxicillin-clavulanate is recommended for dog, cat, and human bites; other agents may be administered if patients are allergic to penicillins. In addition, tetanus toxoid injections, such as Td or TDaP, or human tetanus immunoglobulin may be required.
Regardless of the mechanism of injury, the wound should be thoroughly irrigated and any foreign debris removed. If the wounds are deeper than they are wide, as in puncture wounds, especially from animal bites, or the laceration occurred more than 24 hours before patient presentation, the wound should be irrigated, foreign bodies removed, and any infection prophylaxis provided. The wounds should undergo reassessment, and a decision should be made whether to close them in a delayed fashion or allow them to heal by secondary intention.
Technique or Treatment
Once the wound has been evaluated, anesthesia can decrease discomfort during wound preparation for repair by promoting better tolerance of wound manipulation and allowing the healthcare provider to be thorough with irrigation and debridement. There are various anesthetic options available. Topical 4% lidocaine, 0.1% epinephrine, 0.5% tetracaine (LET mixture), and tetracaine, adrenaline, and cocaine (TAC mixture) are aqueous or gel-based solutions that can be applied to the area before local anesthetic injection.[42][43][44]
Regional anesthesia or single-nerve blockade is a useful alternative to local infiltration because it can provide anesthesia to entire facial areas without distortion of wound edges, particularly when there is already posttraumatic edema. This is especially helpful in areas where approximating the wound edges based on anatomical landmarks is necessary, such as the border of the lip. The following list of nerve blocks will permit nearly complete anesthesia of the face.[36]
- Supraorbital and supratrochlear nerves: forehead and anterior scalp
- Infraorbital nerve: upper lip, lateral nose, lower eyelid
- Dorsal nasal nerve: nasal dorsum and tip
- Zygomaticofacial nerve: cheek
- Zygomaticotemporal nerve: temple
- Inferior alveolar nerve: lower lip, chin, teeth
- Mental nerve: lower lip and chin
- Auricular block: ear
When nerve blocks fail or are unnecessary, local infiltration through the wound edges should provide adequate anesthesia. Local infiltration with a mixture including epinephrine improves hemostasis, an advantage not conferred by regional anesthesia.
Local or regional application of anesthetic solutions may impact motor function; evaluating motor and sensory function before injection is critical. If a motor deficit requiring nerve repair is identified, laceration repair should be performed under general anesthesia. Any injection of local anesthetic or long-term paralytic medications will prohibit using a nerve stimulator to locate the distal nerve stump. However, plain 1:100,000 epinephrine may be injected to improve hemostasis, even without a local anesthetic agent.
After obtaining adequate anesthesia and before any suturing, the wound must be thoroughly irrigated to remove debris and blood. Cleaning the wound decreases the risk of infection and improves visualization of tissue layers and surface landmarks. Warm sterile saline irrigation alone is adequate to cleanse facial lacerations. Antiseptic solutions such as dilute povidone-iodine and sodium hypochlorite have bactericidal properties but can inhibit fibroblast cell growth and delay overall wound healing.[45][46] Thorough irrigation is also important to prevent traumatic tattooing. Leaving pigmented debris such as gravel or shrapnel in a wound bed covered by epithelium can cause traumatic tattoos with continued wound manipulation.[47]
After irrigation and debris removal, the wound bed must be inspected for hemostasis. Active bleeding may require direct pressure or vessel ligation.
Next, the wound should be examined to determine if a tension-free closure will be possible. Surrounding hair can be shaved to improve visualization; care must be taken with eyebrow hair, which can take six months to regrow.[48] Conservative tissue undermining can bring wound edges together, but devitalizing and forceful handling of the soft tissue must be minimized.
Below are technical considerations specific to different aesthetic and functional subunits.
Scalp
Evaluation of intracranial injuries must occur before scalp laceration repair. Significant tissue loss requiring complex reconstruction necessitates plastic surgery or otolaryngology consultation. Violation of the cranial vault warrants neurosurgical consultation. The scalp often bleeds profusely; transfusion may be necessary. After hemostasis is achieved, superficial scalp lacerations can be closed with staples or sutures, with outpatient removal scheduled in 7-10 days. Absorbable sutures such as 5-0 plain gut may be used if follow-up may be difficult.[49][50] Wounds that violate multiple tissue layers require a layered closure using 5-0 on the skin surface, 4-0 in the subdermis, and 3-0 in the galea aponeurotica. Calvarial bone without periosteum should not be exposed after the repair; if laceration repair is insufficient to prevent this situation, a reconstruction with flap transfer may be required.
Forehead
Like scalp wounds, forehead lacerations require layered closure if multiple tissue layers are violated. A 6-0 non-dissolvable or dissolvable suture may be used for the skin, with a 5-0 dissolvable suture in the subdermis and, if necessary, a 4-0 in the frontalis muscle. If possible, orienting repair within the RSTL will help camouflage the scar. Wounds that cannot be closed without tension due to substantial tissue loss should be repaired in a delayed fashion with grafts or flaps as necessary. If the supraorbital or supratrochlear nerves are transected, repair should be attempted; most patients will recover at least some sensation even without neurorrhaphy. Transection of the frontal branch of the facial nerve should also be repaired, if possible; good functional outcomes, even after neurorrhaphy, are far from guaranteed. Neurorrhaphies are best performed under an operating microscope with 10-0 interrupted sutures placed into the epineurium.[51]
Cheek
Cheek lacerations require evaluation of the parotid gland, duct, and extratemporal facial nerve before repair can begin. The parotid gland may be milked while observing the Stensen ductal papilla to verify that saliva is expressed. If milking produces blood, a gland or a duct injury should be assumed. A lacrimal probe or small angiocatheter may be used to cannulate the parotid duct intraorally, taking care not to injure the duct itself. A ductal or parotid gland injury requires further evaluation and repair before repair of the cutaneous laceration. Saliva may collect subcutaneously and cause a sialocele or cutaneous salivary fistula if a skin laceration is repaired without addressing a ductal injury.[52] Sialoceles can be managed with serial aspiration and placement of a bolster to prevent fluid accumulation.[17][21] A drain can also be placed to allow the saliva to egress, and botulinum toxin injections may also help reduce saliva production, thereby slowing fluid accumulation.[53] If the etiology of posttraumatic subcutaneous fluid accumulation is unclear, the aspirate or drainage can be analyzed for amylase, differentiating a sialocele from a seroma.
The extratemporal branches of the facial nerve should be evaluated before the injection of a local anesthetic to determine baseline facial nerve function. If there is a transection of the facial nerve or a branch thereof, the nerve stumps should be coapted under an operating microscope using 10-0 interrupted epineural sutures, provided the nerve stumps can be located. For injuries to major branches, a formal facial nerve exploration may be required. If there is significant tissue loss and the nerve ends cannot be coapted in a tension-free fashion, using a collagen nerve sheath for gaps less than 6 mm or an interposition cable graft for gaps greater than 6 mm of the greater auricular or sural nerve should be considered. Immediate repair is preferable to delayed repair; the ability to locate the distal nerve stump by electrical stimulation will be lost after roughly 72 hours due to Wallerian degeneration.[51][54] When nerve branches are transected medial to a vertical line drawn through the lateral canthus, however, there is sufficient redundancy of innervation that repair is unnecessary; more distal nerve branches are challenging to repair due to their very small caliber.
The skin of the cheek may be sutured with a 6-0 running or interrupted, non-dissolvable or dissolvable suture, taking care to produce adequate wound edge eversion; adequate eversion may require a vertical or horizontal mattress suture technique. Buried 5-0 dissolvable sutures should be placed in the subdermis, and a 4-0 dissolvable suture should be used in the SMAS, if necessary.
Periorbital Region
Periorbital trauma requires an ocular exam to evaluate visual acuity and rule out globe injury. Visual deficits or gross injury to the globe warrant emergent ophthalmology consultation. Next, the canthal ligaments and the nasolacrimal duct should be evaluated. Any deformity of the medial canthus or lateralization, bleeding from, or direct involvement of the punctum or the medial canthus in the laceration warrants emergent ophthalmology consultation to evaluate the lacrimal drainage system. An oculoplastic surgery consultation is warranted if there is canthal ligament disruption, orbital fat herniation, or involvement of the eyelid margin. Orbital fat herniation signifies postseptal injury and potential damage to deeper orbital structures that must be evaluated and addressed, and eyelid margin injuries require careful closure to prevent notching.
The goal for eyelid repair is a tension-free closure that prevents lagophthalmos and exposure keratitis. Careful eversion and approximation of wound edges are especially important for the eyelid because scar contraction close to the margin can cause cicatricial ectropion, particularly in full-thickness lacerations. When suturing eyelid lacerations, suture placement approximately 1 mm from the skin edge with 6-0 or 7-0 suture; deeper layers can be repaired with 5-0 or 6-0 absorbable sutures, avoiding excess tension on the thin eyelid skin. Passing suture through the full thickness of the eyelid incorporating the conjunctivae should be avoided; exposed conjunctival suture can cause corneal abrasions. Minor, superficial wounds that comprise less than 25% of the lid surface area may heal by secondary intention in 2 to 6 weeks. The oculoplastic surgery team may employ complex grafts or flaps for larger tissue defects.[55]
Nose
Control of epistaxis, ruling out cerebral spinal fluid leaks, and identifying nasal septal hematomas are of the utmost importance with nasal trauma. If no other injuries are apparent, nasal lacerations must be repaired in layers, especially if the cartilaginous framework is violated. First, the nasal mucosa should be reapproximated with 5-0 dissolvable sutures, followed by the cartilage with a long-lasting 5-0 dissolvable suture, then the muscle and subcutaneous tissue with a 5-0 dissolvable suture, and finally, the skin with a 6-0 suture. If the laceration comes within 5 mm of the alar or columellar margin, a cartilage graft from the septum or auricle may be required to provide additional support and prevent alar notching or columellar retraction.
Ear
Complex ear lacerations also require layered closure. If the underlying cartilage is violated, it should be repaired with a 5-0 dissolvable suture. Auricular cartilage is fragile, and care must be taken not to tear or avulse the cartilage and overlying perichondrium during reapproximation. Next, the skin must be aligned and repaired with 5-0 or 6-0 sutures. Lacerations along the helical rim or lobule should be closed with significant eversion, as with vertical mattress sutures, to avoid notching. Exposed cartilage without sufficient soft tissue for coverage may be left in continuity with the auricle and buried via an incision in the scalp; this may improve the chance that the cartilage survives and permit reconstruction in a delayed, staged manner with skin grafting or local flap transfer. In the event of complete avulsion of all or part of the auricle, the chance that reimplanting the avulsed segment will lead to its survival is meager; however, if the avulsed segment remains attached by even a small skin bridge, reimplantation may be successful. If the laceration extends into the external auditory canal, an ear wick or Ambrose pack should be placed to prevent postrepair stenosis of the canal.
A quilting suture with or without a bolster may be placed to prevent auricular hematoma and can be removed roughly 1 week later. Although there is no consensus on antibiotic coverage for ear lacerations, antibiotic prophylaxis may be considered, particularly when the cartilage has been exposed; fluoroquinolones are effective against Pseudomonas aeruginosa, a common pathogen in cartilage infections.[33][34]
Lip
Lip injuries require an examination of the oral cavity to evaluate for intraoral lacerations and broken teeth. Sensorimotor deficits should be assessed and noted before injection of local anesthetic. These nerves should be repaired as described above, as scar contracture from perioral lacerations can exacerbate oral incompetence and smile asymmetry. Minor lacerations of the lip may be left to heal by secondary intention, but deep lip lacerations require reapproximation of the muscle underneath. Undermining subcutaneous tissue allows for the development of muscle flaps to facilitate repair. If there is a significant amount of tissue loss, as in partial avulsion, and the edges of the lip cannot come together without tension, delayed closure should be considered. Forcing the edges of the lip together for closure can cause unnecessary scarring and subsequent microstomia, limiting the functionality of the mouth.
Lip lacerations that cross the vermillion border should be approximated with care and ideally less than 1 mm of error; this prevents noticeable step-offs or peaking of this obvious aesthetic subunit boundary. Aligning the vermillion border with an interrupted suture should be the first step in laceration repair and facilitates the appropriate placement of the remaining soft tissues; this suture may even be placed before reapproximating the deeper tissues. When injecting a local anesthetic, it is best to avoid infiltration near the vermillion border; any epinephrine will blanch the vermillion and make distinguishing it from the cutaneous lip difficult. Additionally, the volume of local anesthetic injected may distort the lip, making precise suturing unnecessarily challenging.
The wet lip and dry lip require different suture materials. The wet lip should be closed with a 4-0 or 5-0 chromic gut or polyglactin suture; a non-dissolvable 5-0 or 6-0 polypropylene suture is appropriate for the dry lip. If the patient cannot return for suture removal, a dissolvable suture can be used on the dry lip.[35]
Chin
Chin lacerations can be closed similarly to cheek lacerations, with 6-0 dissolvable or non-dissolvable sutures in the skin, 5-0 deep dermal sutures, and 4-0 sutures in the mentalis muscle. If the mentalis muscle is involved, repairing it is very important because failure to do so may result in chin ptosis.
Special Considerations for Suture Choice
The choice of suture material depends on the patient's ability to return for outpatient suture removal. If the patient cannot return for suture removal, an absorbable suture is a good alternative to polypropylene and nylon. Dissolvable sutures remain intact for varying lengths of time, depending upon composition and whether they are braided or monofilamentous.[56]
- Nonabsorbable, non-braided suture includes polypropylene, hexafluoropropylene, and nylon. Non-braided sutures have lower tissue reactivity than braided sutures, theoretically decreasing the risk of scarring. Non-braided sutures are typically removed from the face six days after placement. Adhesive strip tapes or antibiotic ointment are typically placed on top of these sutures. Braided nonabsorbable sutures include silk, polyester, and braided nylon, but these are not typically used for skin closure, except for silk, which is often used on eyelids.
- Absorbable sutures persist for different lengths of time and require living mammalian tissue and a functioning immune system to produce the inflammatory reaction necessary to dissolve the suture material. Braided synthetic sutures typically dissolve faster than monofilaments, but gut sutures dissolve faster than braided synthetic sutures. Polydioxanone monofilament suture dissolves over 6 to 7 months, poliglecaprone monofilament suture over about 3 months, and polyglactin braided suture over about 2 months. Chromic gut dissolves in 2-3 weeks, plain gut in 10-14 days, and fast gut in about 7 days.
Other non-suture closure methods, such as skin staples and glue, may be considered. Staples are frequently employed on the scalp and occasionally on the neck and should be removed roughly one week after placement to avoid characteristic "train track" punctate marks along the incision. Cyanoacrylate is a glue commonly used instead of skin sutures; it tends to fall off after roughly 7-10 days but provides a water-tight seal and tensile strength to the wound for its duration of action. Both staples and cyanoacrylate are easier and faster to use than suture, but they are far less precise for wound edge approximation; staples work well for wound edge eversion. However, skin staples and glue are only useful for superficial lacerations. A layered suture closure is necessary for deeper lacerations, although staples or skin glue may be used for the most superficial tissue layer. Cyanoacrylate may be placed after removing nonabsorbable sutures to provide prolonged wound support and prevent dehiscence or scar widening.[57]
The goals of laceration repair are adequate wound cleansing, hemostasis, gentle tissue handling, tension-free, everted approximation, and, ultimately, an aesthetically aligned scar. To optimize the final appearance of the scar, the technical considerations and suture material selection decisions are critical, but attentive postrepair care is nearly as crucial.
Scars will continue to mature for approximately 12 months; avoiding sunlight for that period will help prevent hyperpigmentation. If scars appear excessively erythematous in the initial 2 to 3 weeks postrepair, treatment with a pulsed-dye laser will help to decrease the redness. Raised or widened scars may be treated with steroid injections or potentially with ablative lasers like carbon dioxide or erbium: YAG lasers. Some patients, particularly those predisposed to keloid development, may also benefit from silicone sheeting or topical gel, which should be used consistently for the first year after injury.
Complications
Scarring and keloid formation are common complications after laceration repair. Excessive tension, insufficient eversion, and suboptimal wound edge level matching can cause poor cosmetic outcomes. If patients are susceptible to keloids or hypertrophic scarring, as evidenced by keloid or hypertrophic scar formation after prior surgery or trauma, it is important to manage expectations and discuss the likelihood of suboptimal scarring despite optimal tissue handling and repair. Fortunately, keloid formation on the face itself is uncommon, although keloids of the scalp, posterior neck, and ears occur commonly in predisposed patients.
Scar revision options include intralesional corticosteroid injections, dermabrasion, laser resurfacing, and surgical revision. Intralesional steroid injections can help reduce inflammation and soften the scars or reduce fibrosis by inhibiting fibroblast activity; however, they may also cause dermal thinning, fat atrophy, and telangiectasia development. Dermabrasion is a skin resurfacing technique that smooths contour irregularities by ablating the epidermis and superficial dermis to promote re-epithelization and collagenesis.[58] Laser resurfacing with carbon dioxide or erbium: YAG lasers work similarly to dermabrasion to resurface scars and promote re-epithelization; the tissue ablation is accomplished by vaporization of intracellular water rather than with mechanical friction.[59][60] Surgical scar revision involves the excision of the scars, often combined with techniques that better orient the scar for improved aesthetics. Examples of reorienting techniques include z-plasty, w-plasty, and geometric broken line closure.[61] These techniques are often combined; for example, a scar may be treated with serial steroid injections, then excised surgically and revised, and the surgery may be followed by laser resurfacing of the final scar.
While unsightly or uncomfortable scarring may occur in the long term, the most common short-term complications are hematoma and wound infection with or without dehiscence. Other complications unique to each anatomical site are outlined above.
Failure to achieve adequate hemostasis before laceration repair predisposes to hematoma formation. The wound must be systematically examined for persistent bleeding before closure and include the removal of any retained foreign bodies, as their persistence significantly raises the risk of wound infection and dehiscence. If a wound infection occurs, appropriate antibiotics should be administered; the laceration will need to be opened and irrigated aggressively, debrided if necessary, then potentially reclosed over a drain, with or without a pressure dressing to prevent accumulation of purulent exudate. In some cases, however, the wound will need to be closed in a delayed fashion to permit the infection to clear completely. Expanding hematomas will also require reopening and wound exploration with meticulous hemostasis. After hematoma evacuation, the wound may need to be reclosed over a suction drain with a pressure dressing. Non-expanding hematomas may be managed conservatively with pressure dressings and warm compresses, with or without needle aspiration.[62][41]
Clinical Significance
Optimal facial laceration repair requires understanding the affected anatomy and soft tissue handling techniques. Poor aesthetic outcomes from improper management of facial lacerations can have negative psychological impacts on patients, who may be left with stress or anxiety disorders, scars, facial subunit distortion, or functional deficits. Discussing repair options and counseling patients regarding expected outcomes, when possible, builds the therapeutic relationship and improves long-term satisfaction for both patients and clinicians.
Enhancing Healthcare Team Outcomes
Because of the complexity of facial anatomy, there is the potential for myriad different complications to arise when major lacerations occur. Numerous specialists may be required to manage more complex injuries: oculoplastic surgeons and ophthalmologists can address periorbital and lacrimal duct injuries, neurosurgeons will repair violations of the cranial vault, and plastic surgeons, facial plastic surgeons, or otolaryngologists manage facial nerve and parotid duct injuries a well as performing complex soft tissue repair. Patient satisfaction with facial laceration management is mainly dependent on cosmesis, and poor cosmesis can have a negative psychological impact; for this reason, early involvement of an interprofessional team is crucial.[63] [Level 3]
Surgeons do not work alone but rather partner with skilled staff who monitor the patient before and after the procedure and reiterate postprocedural care instructions upon discharge. Case managers address psychosocial sequelae and patient needs after facial laceration repair, particularly when the lacerations occur in the context of polytrauma or in patients who require assistance accessing healthcare. Collaboration among all interprofessional healthcare team members is essential to provide optimal patient care, promote aesthetically pleasing wound healing, and minimize complications. [Level 5]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
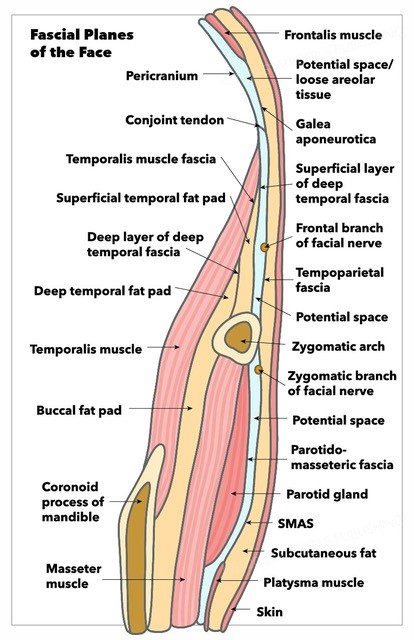
Fascial Planes of the Face and Musculature. This illustration depicts the fascial planes of the face, highlighting the continuity of the frontalis muscle, galea aponeurotica, temporoparietal fascia, superficial musculoaponeurotic system, platysma, and the location of the facial nerve.
Contributed by K Humphreys and MH Hohman, MD, FACS
(Click Image to Enlarge)
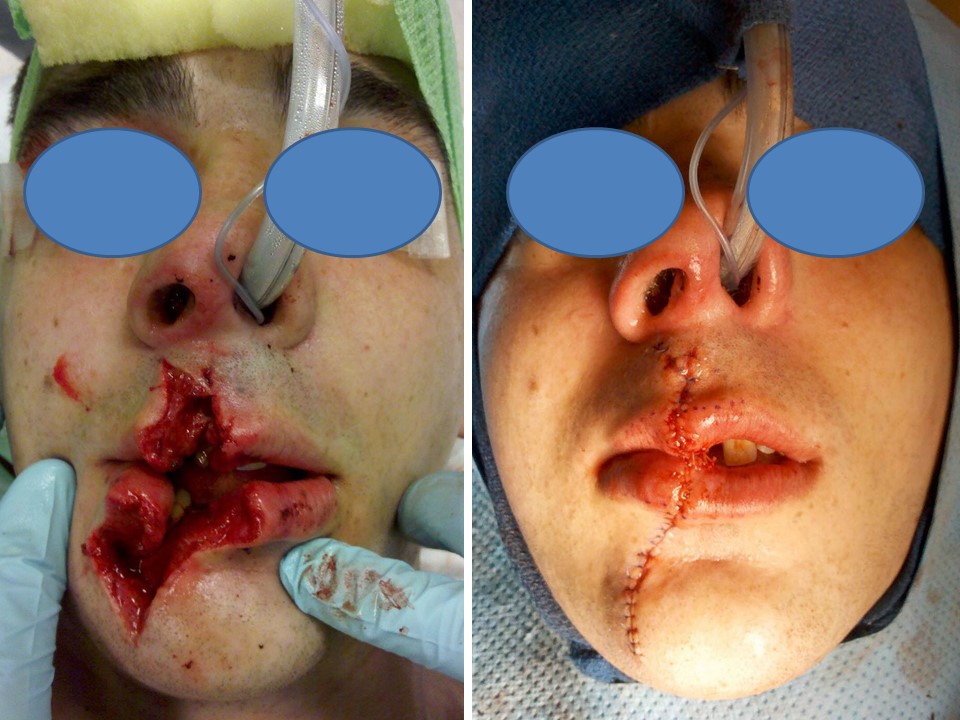
19-year-old male struck in the face by the back end of a heavy machine gun that was dislodged from its mount when the barrel struck an overpass as the vehicle was driving. Note the use of nasotracheal intubation in order to avoid distortion of the lips and also the markings along the vermilion border that were used to ensure precise alignment of the aesthetic subunit boundary. Contributed by Marc H Hohman, MD, FACS
(Click Image to Enlarge)

16-year-old male with a full-thickness stellate facial laceration due to a motor vehicle collision. The skin flaps were tacked in place first, in order to establish soft tissue orientation, and then the closure was performed in layers, from deep to superficial. Contributed by Marc H Hohman, MD, FACS
(Click Image to Enlarge)
References
Singer AJ, Thode HC Jr, Hollander JE. National trends in ED lacerations between 1992 and 2002. The American journal of emergency medicine. 2006 Mar:24(2):183-8 [PubMed PMID: 16490648]
McCaig LF, Burt CW. National Hospital Ambulatory Medical Care Survey: 2001 emergency department summary. Advance data. 2003 Jun 4:(335):1-29 [PubMed PMID: 12822264]
Level 3 (low-level) evidenceLevine E, Degutis L, Pruzinsky T, Shin J, Persing JA. Quality of life and facial trauma: psychological and body image effects. Annals of plastic surgery. 2005 May:54(5):502-10 [PubMed PMID: 15838211]
Level 2 (mid-level) evidenceTebble NJ, Adams R, Thomas DW, Price P. Anxiety and self-consciousness in patients with facial lacerations one week and six months later. The British journal of oral & maxillofacial surgery. 2006 Dec:44(6):520-5 [PubMed PMID: 16343707]
GONZALEZ-ULLOA M, CASTILLO A, STEVENS E, ALVAREZ FUERTES G, LEONELLI F, UBALDO F. Preliminary study of the total restoration of the facial skin. Plastic and reconstructive surgery (1946). 1954 Mar:13(3):151-61 [PubMed PMID: 13145324]
Fattahi TT. An overview of facial aesthetic units. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2003 Oct:61(10):1207-11 [PubMed PMID: 14586859]
Level 3 (low-level) evidenceSeery GE. Surgical anatomy of the scalp. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2002 Jul:28(7):581-7 [PubMed PMID: 12135510]
Shumrick KA, Smith TL. The anatomic basis for the design of forehead flaps in nasal reconstruction. Archives of otolaryngology--head & neck surgery. 1992 Apr:118(4):373-9 [PubMed PMID: 1554465]
Tomaszewska A, Kwiatkowska B, Jankauskas R. The localization of the supraorbital notch or foramen is crucial for headache and supraorbital neuralgia avoiding and treatment. Anatomical record (Hoboken, N.J. : 2007). 2012 Sep:295(9):1494-503. doi: 10.1002/ar.22534. Epub 2012 Jul 16 [PubMed PMID: 22807312]
Seline PC, Siegle RJ. Forehead reconstruction. Dermatologic clinics. 2005 Jan:23(1):1-11, v [PubMed PMID: 15620615]
Garritano FG, Quatela VC. Surgical Anatomy of the Upper Face and Forehead. Facial plastic surgery : FPS. 2018 Apr:34(2):109-113. doi: 10.1055/s-0038-1637727. Epub 2018 Apr 9 [PubMed PMID: 29631278]
Pitanguy I, Ramos AS. The frontal branch of the facial nerve: the importance of its variations in face lifting. Plastic and reconstructive surgery. 1966 Oct:38(4):352-6 [PubMed PMID: 5926990]
Mendelson BC, Jacobson SR. Surgical anatomy of the midcheek: facial layers, spaces, and the midcheek segments. Clinics in plastic surgery. 2008 Jul:35(3):395-404; discussion 393. doi: 10.1016/j.cps.2008.02.003. Epub [PubMed PMID: 18558234]
Mitz V, Peyronie M. The superficial musculo-aponeurotic system (SMAS) in the parotid and cheek area. Plastic and reconstructive surgery. 1976 Jul:58(1):80-8 [PubMed PMID: 935283]
Gosain AK. Surgical anatomy of the facial nerve. Clinics in plastic surgery. 1995 Apr:22(2):241-51 [PubMed PMID: 7634735]
Phillips CD, Bubash LA. The facial nerve: anatomy and common pathology. Seminars in ultrasound, CT, and MR. 2002 Jun:23(3):202-17 [PubMed PMID: 12168997]
Steinberg MJ, Herréra AF. Management of parotid duct injuries. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2005 Feb:99(2):136-41 [PubMed PMID: 15660081]
De Cordier BC, de la Torre JI, Al-Hakeem MS, Rosenberg LZ, Costa-Ferreira A, Gardner PM, Fix RJ, Vasconez LO. Rejuvenation of the midface by elevating the malar fat pad: review of technique, cases, and complications. Plastic and reconstructive surgery. 2002 Nov:110(6):1526-36; discussion 1537-40 [PubMed PMID: 12409773]
Level 2 (mid-level) evidenceFalk J, Borgwardt R, MacLeod S. Posttraumatic Parotid Sialocele Complicating a Mandibular Fracture: A Case Report. Craniomaxillofacial trauma & reconstruction. 2017 Dec:10(4):314-317. doi: 10.1055/s-0036-1593891. Epub 2016 Dec 13 [PubMed PMID: 29109843]
Level 3 (low-level) evidenceCanosa A, Cohen MA. Post-traumatic parotid sialocele: report of two cases. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 1999 Jun:57(6):742-5 [PubMed PMID: 10368103]
Level 3 (low-level) evidenceHwang J, You YC, Burm JS. Treatment of intractable parotid sialocele occurred after open reduction-fixation of mandibular subcondylar fracture. Archives of craniofacial surgery. 2018 Jun:19(2):157-161. doi: 10.7181/acfs.2018.01802. Epub 2018 Jun 22 [PubMed PMID: 29925226]
Lazaridis N, Iordanides S, Mangoudi E, Letsis I, Karakasis D. [End-to-end anastomosis of the lacerated parotid duct]. Deutsche Zeitschrift fur Mund-, Kiefer- und Gesichts-Chirurgie. 1990 Mar-Apr:14(2):90-1 [PubMed PMID: 2102419]
Level 3 (low-level) evidenceChandler DB, Gausas RE. Lower eyelid reconstruction. Otolaryngologic clinics of North America. 2005 Oct:38(5):1033-42 [PubMed PMID: 16214572]
Burkat CN, Lemke BN. Anatomy of the orbit and its related structures. Otolaryngologic clinics of North America. 2005 Oct:38(5):825-56 [PubMed PMID: 16214562]
Ko AC, Satterfield KR, Korn BS, Kikkawa DO. Eyelid and Periorbital Soft Tissue Trauma. Facial plastic surgery clinics of North America. 2017 Nov:25(4):605-616. doi: 10.1016/j.fsc.2017.06.011. Epub [PubMed PMID: 28941512]
Kang H, Takahashi Y, Ichinose A, Nakano T, Asamoto K, Ikeda H, Iwaki M, Kakizaki H. Lateral canthal anatomy: a review. Orbit (Amsterdam, Netherlands). 2012 Aug:31(4):279-85. doi: 10.3109/01676830.2012.694957. Epub 2012 Jun 12 [PubMed PMID: 22690873]
Robinson TJ, Stranc MF. The anatomy of the medial canthal ligament. British journal of plastic surgery. 1970 Jan:23(1):1-7 [PubMed PMID: 5413491]
Chang EL, Rubin PA. Management of complex eyelid lacerations. International ophthalmology clinics. 2002 Summer:42(3):187-201 [PubMed PMID: 12131595]
Burget GC, Menick FJ. The subunit principle in nasal reconstruction. Plastic and reconstructive surgery. 1985 Aug:76(2):239-47 [PubMed PMID: 4023097]
Cerci FB. Usefulness of the subunit principle in nasal reconstruction. Anais brasileiros de dermatologia. 2017:92(5 Suppl 1):159-162. doi: 10.1590/abd1806-4841.20175278. Epub [PubMed PMID: 29267479]
Oneal RM, Beil Jr RJ, Schlesinger J. Surgical anatomy of the nose. Otolaryngologic clinics of North America. 1999 Feb:32(1):145-81 [PubMed PMID: 10196443]
Stevens MR, Emam HA. Applied surgical anatomy of the nose. Oral and maxillofacial surgery clinics of North America. 2012 Feb:24(1):25-38. doi: 10.1016/j.coms.2011.10.007. Epub [PubMed PMID: 22284395]
Lavasani L, Leventhal D, Constantinides M, Krein H. Management of acute soft tissue injury to the auricle. Facial plastic surgery : FPS. 2010 Dec:26(6):445-50. doi: 10.1055/s-0030-1267718. Epub 2010 Nov 17 [PubMed PMID: 21086230]
Alvord LS, Farmer BL. Anatomy and orientation of the human external ear. Journal of the American Academy of Audiology. 1997 Dec:8(6):383-90 [PubMed PMID: 9433684]
Grunebaum LD, Smith JE, Hoosien GE. Lip and perioral trauma. Facial plastic surgery : FPS. 2010 Dec:26(6):433-44. doi: 10.1055/s-0030-1267717. Epub 2010 Nov 17 [PubMed PMID: 21086229]
Zide BM, Swift R. How to block and tackle the face. Plastic and reconstructive surgery. 1998 Mar:101(3):840-51 [PubMed PMID: 9500408]
Leach J. Proper handling of soft tissue in the acute phase. Facial plastic surgery : FPS. 2001 Nov:17(4):227-38 [PubMed PMID: 11735055]
Waseem M, Lakdawala V, Patel R, Kapoor R, Leber M, Sun X. Is there a relationship between wound infections and laceration closure times? International journal of emergency medicine. 2012 Jul 26:5(1):32. doi: 10.1186/1865-1380-5-32. Epub 2012 Jul 26 [PubMed PMID: 22835090]
Lee SJ, Cho YD, Park SJ, Kim JY, Yoon YH, Choi SH. Satisfaction with facial laceration repair by provider specialty in the emergency department. Clinical and experimental emergency medicine. 2015 Sep:2(3):179-183 [PubMed PMID: 27752594]
Bayat A, McGrouther DA, Ferguson MW. Skin scarring. BMJ (Clinical research ed.). 2003 Jan 11:326(7380):88-92 [PubMed PMID: 12521975]
Singer AJ, Quinn JV, Thode HC Jr, Hollander JE, TraumaSeal Study Group. Determinants of poor outcome after laceration and surgical incision repair. Plastic and reconstructive surgery. 2002 Aug:110(2):429-35; discussion 436-7 [PubMed PMID: 12142655]
Level 1 (high-level) evidenceSinger AJ, Hollander JE, Quinn JV. Evaluation and management of traumatic lacerations. The New England journal of medicine. 1997 Oct 16:337(16):1142-8 [PubMed PMID: 9329936]
Smith GA, Strausbaugh SD, Harbeck-Weber C, Shields BJ, Powers JD. Comparison of topical anesthetics with lidocaine infiltration during laceration repair in children. Clinical pediatrics. 1997 Jan:36(1):17-23 [PubMed PMID: 9007343]
Level 1 (high-level) evidenceSchilling CG, Bank DE, Borchert BA, Klatzko MD, Uden DL. Tetracaine, epinephrine (adrenalin), and cocaine (TAC) versus lidocaine, epinephrine, and tetracaine (LET) for anesthesia of lacerations in children. Annals of emergency medicine. 1995 Feb:25(2):203-8 [PubMed PMID: 7832348]
Level 1 (high-level) evidenceBalin AK, Pratt L. Dilute povidone-iodine solutions inhibit human skin fibroblast growth. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2002 Mar:28(3):210-4 [PubMed PMID: 11896770]
Rodeheaver G, Bellamy W, Kody M, Spatafora G, Fitton L, Leyden K, Edlich R. Bactericidal activity and toxicity of iodine-containing solutions in wounds. Archives of surgery (Chicago, Ill. : 1960). 1982 Feb:117(2):181-6 [PubMed PMID: 7034678]
Level 3 (low-level) evidenceWilkins RG, Unverdorben M. Wound cleaning and wound healing: a concise review. Advances in skin & wound care. 2013 Apr:26(4):160-3. doi: 10.1097/01.ASW.0000428861.26671.41. Epub [PubMed PMID: 23507692]
Level 3 (low-level) evidenceFezza JP, Klippenstein KA, Wesley RE. Cilia regrowth of shaven eyebrows. Archives of facial plastic surgery. 1999 Jul-Sep:1(3):223-4 [PubMed PMID: 10937110]
Level 1 (high-level) evidenceKanegaye JT, Vance CW, Chan L, Schonfeld N. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. The Journal of pediatrics. 1997 May:130(5):808-13 [PubMed PMID: 9152292]
Level 1 (high-level) evidenceKhan AN, Dayan PS, Miller S, Rosen M, Rubin DH. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatric emergency care. 2002 Jun:18(3):171-3 [PubMed PMID: 12066001]
Level 1 (high-level) evidenceMistry RK, Hohman MH, Al-Sayed AA. Facial Nerve Trauma. StatPearls. 2025 Jan:(): [PubMed PMID: 31971735]
Van Sickels JE. Management of parotid gland and duct injuries. Oral and maxillofacial surgery clinics of North America. 2009 May:21(2):243-6. doi: 10.1016/j.coms.2008.12.010. Epub [PubMed PMID: 19348990]
Maharaj S, Mungul S, Laher A. Botulinum toxin A is an effective therapeutic tool for the management of parotid sialocele and fistula: A systematic review. Laryngoscope investigative otolaryngology. 2020 Feb:5(1):37-45. doi: 10.1002/lio2.350. Epub 2020 Jan 23 [PubMed PMID: 32128429]
Level 1 (high-level) evidenceHohman MH, De Jesus O. Facial Nerve Repair. StatPearls. 2025 Jan:(): [PubMed PMID: 32809458]
Subramanian N. Reconstructions of eyelid defects. Indian journal of plastic surgery : official publication of the Association of Plastic Surgeons of India. 2011 Jan:44(1):5-13. doi: 10.4103/0970-0358.81437. Epub [PubMed PMID: 21713158]
Lo S, Aslam N. A review of tissue glue use in facial lacerations: potential problems with wound selection in accident and emergency. European journal of emergency medicine : official journal of the European Society for Emergency Medicine. 2004 Oct:11(5):277-9 [PubMed PMID: 15359201]
Level 2 (mid-level) evidenceFarion K, Osmond MH, Hartling L, Russell K, Klassen T, Crumley E, Wiebe N. Tissue adhesives for traumatic lacerations in children and adults. The Cochrane database of systematic reviews. 2002:2002(3):CD003326 [PubMed PMID: 12137689]
Level 1 (high-level) evidenceBedford L, Daveluy S. Skin Resurfacing Dermabrasion. StatPearls. 2023 Jan:(): [PubMed PMID: 32644381]
Yumeen S, Khan T. Laser Carbon Dioxide Resurfacing. StatPearls. 2023 Jan:(): [PubMed PMID: 32809379]
Yumeen S, Hohman MH, Khan T. Laser Erbium-Yag Resurfacing. StatPearls. 2023 Jan:(): [PubMed PMID: 32809766]
Tullington JE, Gemma R. Scar Revision. StatPearls. 2023 Jan:(): [PubMed PMID: 31194458]
Newberry CI, Thomas JR, Cerrati EW. Facial Scar Improvement Procedures. Facial plastic surgery : FPS. 2018 Oct:34(5):448-457. doi: 10.1055/s-0038-1669400. Epub 2018 Oct 8 [PubMed PMID: 30296796]
Glynn SM, Shetty V, Elliot-Brown K, Leathers R, Belin TR, Wang J. Chronic posttraumatic stress disorder after facial injury: a 1-year prospective cohort study. The Journal of trauma. 2007 Feb:62(2):410-8; discussion 418 [PubMed PMID: 17297333]