Antibiotic Prophylaxis in Dental and Oral Surgery Practice
Antibiotic Prophylaxis in Dental and Oral Surgery Practice
Introduction
The disruption of the dental mucosa can lead to transient bacteremia.[1] In turn, bacteremia is a risk factor for the development of infective endocarditis and other biofilm-associated infections, such as prosthetic joint infection.[2][3] The first suggestion that dental procedures could be associated with infective endocarditis came in 1923.[4]
In 1935, one study isolated S. viridans in blood cultures in 61% of patients with dental sepsis.[4] Recommendations for antimicrobial prophylaxis before dental procedures were first included in the American Heart Association (AHA) guidelines in 1955.[5] Antimicrobials have been offered to patients for various reasons before dental procedures and oral surgery. But, recent recommendations target prophylaxis toward the most vulnerable to infection.
Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Function
The microbiology of the oral cavity varies with the position in the mouth and with different systemic conditions. Nonmutans streptococcal species and organisms in the S. mitis group predominate near the root and in the teeth' cracks and fissures.[6] There is a minimal amount of mutans-group streptococcus in the healthy mouth.[6] Anaerobic flora predominates in periodontal disease, including Porphyromonas, Provotella, and Capnocytophaga.[6] Aggregatibacter is associated with aggressive periodontitis.[7]
Organisms involved in periodontal disease organize into complexes in conjunction with certain other species. Fusobacterium species belong to a complex that may herald early periodontal disease.[8] Mutans-group streptococci, Lactobacillus, and Actinomyces, predominate in biofilms associated with carious teeth.[9] Enterococcal species are also commonly found in the oral cavity.[10]
Organisms implicated in infective endocarditis that may be of oral origin include oral streptococci in 7 to 18%, enterococci in 5 to 10%, and organisms in the HACEK group that include oral anaerobes such as Eikinella corrodens and Aggrigatibacter in 1 to 2%.[11] Organisms potentially of an oral origin in prosthetic joint infection include streptococcal species in 8%, enterococcal species in 3%, and anaerobes in 4%.[12]
Most prosthetic joint infections involve Staphylococcus aureus and coagulase-negative Staphylococcus, which are generally considered rare in the human oral cavity.[12][13] However, staphylococcal species in the oral cavity may be involved in more oral infections than previously thought.[14]
Issues of Concern
Current guidelines regarding antibiotic prophylaxis before dental procedures have been influenced by two main issues: transient bacteremia's nature and the efficacy of antibiotics in reducing bacteremia and infective endocarditis.
Transient Bacteremia
Bacteremia refers to the presence of bacteria in the bloodstream.[15] Viable bacteria may reach the bloodstream after simple daily tasks like toothbrushing and during minor dental procedures and infectious processes.[15] The host's immunity normally clears these bacteria from the blood. However, transient bacteremia may lead to infection and sepsis when immune efforts are ineffective or under certain circumstances like anatomical defects, foreign material, or turbulent cardiac blood flow.[15]
As transient bacteremia occurs for many due to normal activities of daily living, this argues against the widespread use of antimicrobial prophylaxis in the general population. Furthermore, despite transient bacteremia, endocarditis remains a relatively rare diagnosis and has an incidence of 3 to 10 per 100,000 people.[16] Those with certain cardiac conditions, such as rheumatic heart disease, degenerative valve disease, and congenital heart disease, are more predisposed to the development of infective endocarditis.[16]
Advancements in cardiology have paved the way for prosthetic and bioprosthetic cardiac valves and other prosthetics, allowing for the correction of valvulopathy and congenital heart disease. Increased rates of endocarditis have also been noted in this population.[16] Specific populations develop endocarditis secondary to this transient bacteremia in a much higher proportion than others, suggesting that antibiotic prophylaxis may be more beneficial in these populations.
Efficacy of Antimicrobial Prophylaxis in the Prevention of Bacteremia
Antimicrobial prophylaxis before dental procedures is not 100% effective at preventing transient bacteremia. A systemic review of twelve studies revealed that antimicrobial prophylaxis before tooth extraction reduced the risk of bacteremia by 50%.[17] Amoxicillin reduced the risk of bacteremia by 59%, azithromycin by 49%, and clindamycin by 11%.[17]
Efficacy of Antimicrobial Prophylaxis in the Prevention of Infective Endocarditis
Infective endocarditis is the infection of the innermost layer of the heart, known as the endocardium. It typically affects the cardiac or prosthetic valves and cardiac devices.[18]
To date, there has not been a randomized-controlled trial regarding antimicrobial prophylaxis before dental procedures to inform clinical decision-making. However, several studies have evaluated the incidence of infective endocarditis before and after changes in treatment guidelines from prominent medical organizations. In other words, much of the data informing on this topic are epidemiological studies before and after changes in recommendations.
Many epidemiological studies before and after the publication of antimicrobial prophylaxis recommendations have shown no change. A study in Olmstead County, Minnesota, in 2007 evaluated the incidence of infective endocarditis caused by viridans group streptococci before and after the change in the joint American Heart Association/American Dental Association guidelines (AHA/ADA), which narrowed recommendations for antimicrobial prophylaxis before dental procedures and did not reveal any change in the incidence of infective endocarditis.[19] Likewise, a study in France did not reveal an increased incidence of the condition after recommendations for antimicrobial prophylaxis were narrowed in 2002.[20]
However, some studies did reveal increased rates of endocarditis after the publication of guideline changes. Another US study revealed that the rate of streptococcal infective endocarditis has increased since the 2007 joint AHA/ADA guidelines but did not note an increase in valvular replacement.[21]
A study conducted on the incidence of infective endocarditis in Englandrevealedl an increase in the incidence of infective endocarditis after the guidelines in 2008 by the National Institute of Health and Clinical Excellence that did not recommend antimicrobial prophylaxis in any population.[22]
These guidelines were updated in 2016 to indicate that the organization does not generally recommend antimicrobial prophylaxis before dental procedures.[23]
Clinical Significance
Indications for Antimicrobial Prophylaxis in Healthy Patients
Antimicrobial prophylaxis is not routinely indicated in healthy patients undergoing dental or oral surgery procedures. However, there are some exceptions when prophylaxis is recommended, as it reduces the risk of postoperative complications, including implant failure, local infection, or sinusitis.
Indications for Antimicrobial Prophylaxis in Healthy patients: Dental Implants
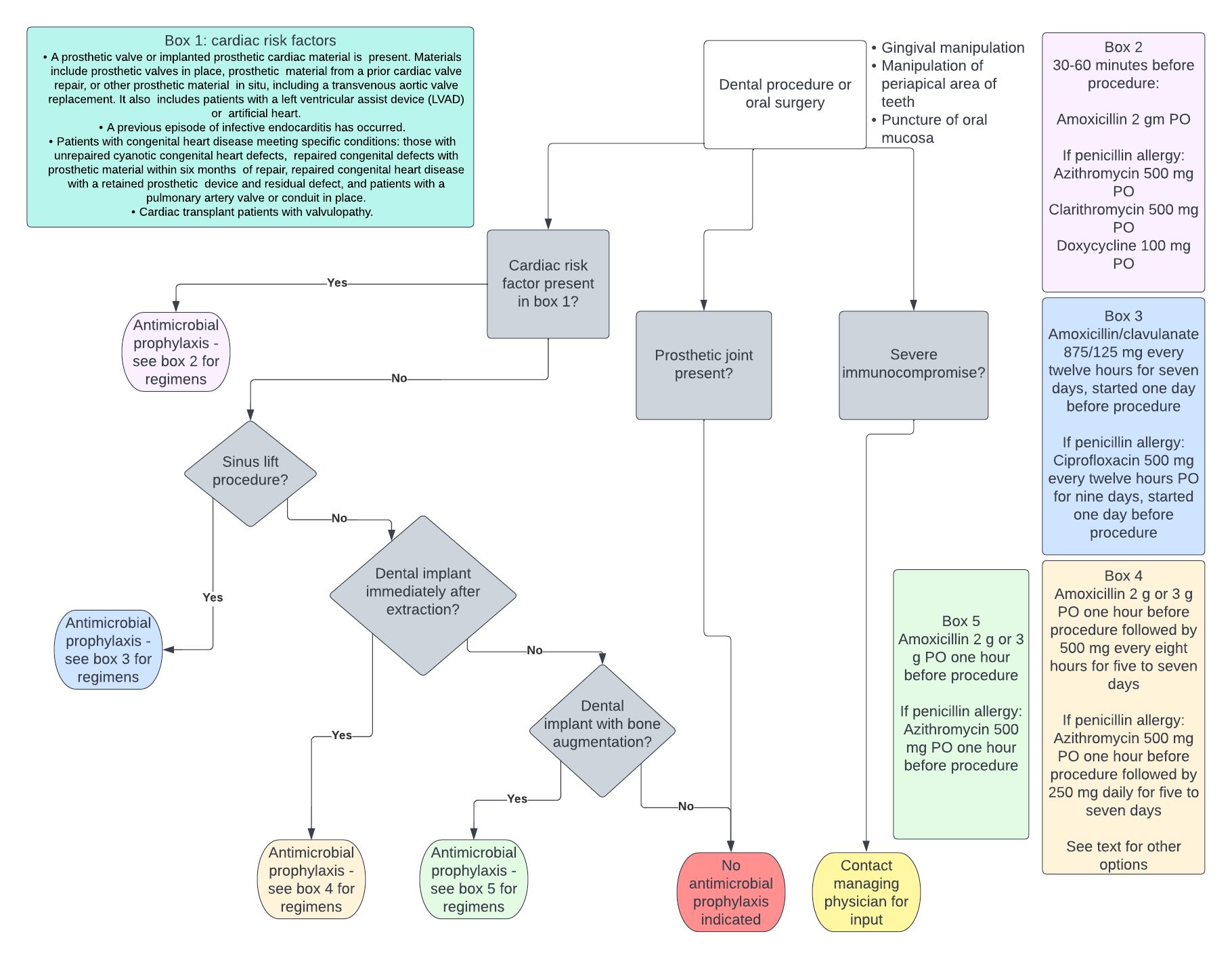
Antimicrobial prophylaxis is recommended before dental implants are placed immediately after dental extraction, with or without clinical signs of infection. These recommendations stem from concerns regarding the placement of implants into a potentially infected wound bed, which may affect osseointegration.[24] Amoxicillin 2 g to 3 g one hour before the procedure, followed by amoxicillin 500 mg every eight hours, continued for five to seven days, reduces the risk of early failure.[25]
In penicillin-allergic patients, alternatives include azithromycin or clarithromycin 500 mg one hour before the procedure followed by 250 mg daily for 5 to 7 days, or metronidazole 1 g one hour before the procedure followed by 500 mg every six hours for five to seven days.[25]
Antimicrobial prophylaxis during the second phase, or prosthetic phase, of dental implants is not justified.[25] This recommendation was extrapolated from studies involving periodontal access surgeries, which noted that the number needed to treat was 117 to prevent postoperative infection.[25]
For dental implant placements done concurrently with sinus lift procedures, amoxicillin/clavulanate 875/125 mg every twelve hours started one day before the procedure and continued for seven days is recommended. Antimicrobial prophylaxis in this situation is to prevent sinusitis in case of perforation of Schneider's membrane, resulting in communication between the oral cavity and the maxillary sinus.[25] However, studies evaluating this subject are quite limited. Similarly, patients with oro-antral communication or fistula should receive antimicrobial therapy.[26] Ciprofloxacin 500 mg every twelve hours, started one day before the procedure, is recommended for penicillin-allergic patients for nine days.
Antimicrobial prophylaxis before routine dental implants remains controversial. They have been recommended to reduce the risk of osseointegration failure caused by a microorganism affecting the wound healing process.[27] A Cochrane review performed in 2013 concluded that antimicrobial prophylaxis before dental implant placement reduced the risk of failure.[28] Other studies suggest that antibiotics decrease the risk of early failure but do not reduce infection risk.[29]
On the other hand, a meta-analysis including three studies found that the number needed to treat to prevent one implant failure was thirty-three.[30] Clinical practice guidelines developed by the Spanish Society of Implants acknowledge the disagreement about the subject and state that amoxicillin, either 2 g or 3 g one hour before the procedure or no antibiotic prophylaxis, are both acceptable in routine dental implant placements in healthy patients.[25] In penicillin-allergic patients, azithromycin 500 mg is an alternative to amoxicillin.[25]
Infected bone grafts are a considerable problem as they often lead to total or partial graft loss.[31] Antibiotic prophylaxis must be prescribed before bone regeneration procedures, and the recommended regime is amoxicillin (2 or 3 g) or azithromycin 500 mg one hour before surgery.[25]
Clindamycin should not be used to prevent dental implant-related infections as worse outcomes, including infection and osseointegration failure, are noted compared to when amoxicillin is used.[25]
Indications for Antimicrobial Prophylaxis in Healthy patients: Facial Fractures
Infection rates of mandibular fractures range from 3.3% to 43.9%, while infections involving fractures of the upper facial, third, and mid-face occur in 0% to 12.5%.[32] Antimicrobials are generally offered to prevent hardware-associated infection in these cases[33]. A review of all available studies involving infection after these fractures conducted in 2022 did not reveal any benefit to extending prophylactic antibiotics beyond twenty-four hours in these cases.[32]
Antimicrobial Prophylaxis in Special Populations
Severely immunocompromised patients, such as those on chemotherapy, should avoid invasive dental procedures whenever possible.[34] If considered, dentists and oral surgeons should seek the opinion of the patient's managing physician.[34]
There is a lack of evidence regarding patients with severe neutropenia, high doses of immunosuppressants, or patients with immunodeficiencies.[35] Consulting with the patient's managing physician is helpful in such cases.
Previous guidelines have recommended antimicrobial prophylaxis be provided to patients with diabetes mellitus.[36] However, a recent systematic review found that guidelines have been inconsistent, and there is no evidence of the effectiveness of antimicrobial prophylaxis in this population.[37]
There is no consensus regarding the need for antimicrobial prophylaxis before dental procedures in patients that have received a splenectomy.[38] Dentists and oral surgeons should seek an opinion from the patient's managing physician.
The American Dental Association does not routinely recommend antimicrobial prophylaxis in patients with implanted prosthetic joints before dental procedures.[39] The American Academy of Orthopedic Surgeons also does not recommend antimicrobial prophylaxis in this population, citing that there has not been any study that demonstrates an association between bacteremia induced by dental procedures and prosthetic joint infection. To date, no study has shown that antimicrobial prophylaxis decreased the rate of prosthetic joint infection.
Indications for Antimicrobial Prophylaxis in Patients With Cardiac Risk Factors
Due to the increased risk for infective endocarditis in patients with certain cardiac risk factors, The American Heart Association recommends antimicrobial prophylaxis before specific dental procedures if patients meet one of the below criteria.[40]
- A prosthetic valve or implanted prosthetic cardiac material is present. Materials include prosthetic valves in place, prosthetic material from a prior cardiac valve repair, or other prosthetic material in situ, including a transvenous aortic valve replacement. It also includes patients with a left ventricular assist device (LVAD) or artificial heart.[40]
- A previous episode of infective endocarditis has occurred.[40]
- Certain patients with congenital heart disease meeting specific conditions: those with unrepaired cyanotic congenital heart defects, repaired congenital defects with prosthetic material within six months of repair, repaired congenital heart disease with a retained prosthetic device and residual defect, and patients with a pulmonary artery valve or conduit in place.[40]
- Cardiac transplant patients with valvulopathy.[40]
In patients not meeting the above conditions, the American Heart Association does not recommend antimicrobial prophylaxis for infective endocarditis before dental procedures.[40] Examples of such patients include the following:[40]
- Patients using pacemakers or automatic cardioverter-defibrillators
- Patients having undergone prior septal defect closure with success
- Patients with peripheral vascular grafts (such as those used for hemodialysis access) or vascular stents
- Patients with ventriculoatrial shunts
- Patients with vena cava filters
- Patients with pledgets
Procedures that carry sufficient risk to justify antimicrobial prophylaxis in the groups outlined above involve gingival or periapical manipulation and cases that require perforation of the oral mucosa.[40] Examples of procedures that carry sufficient risk and those considered not risky are outlined in the table below.[41][42] It is worth noting that this is not an exhaustive list, and each case must be judged individually by the treating clinician. See Figure. Antibiotic Prophylaxis.
| Dental procedures that warrant antibiotic prophylaxis in patients with specific cardiac risk factors | Dental procedures that do not justify antibiotic prophylaxis in patients with specific cardiac risk factors |
| Periodontal procedures, e.g., scaling and root planning, placement of subgingival antibiotic strips, bone grafting, gingivectomy, gingivoplasty, gingival grafts, periodontal flaps | Taking oral impressions, taking oral radiographs, and placing rubber dams |
| Endodontic instrumentation beyond the apex of the tooth and apicoectomies | Intra-canal endodontic treatment |
| Dental implant placement | Restorative dentistry with or without a retraction cord |
| Reimplantation of avulsed teeth | Shedding of primary teeth |
| Intraligamentary local anesthetic injection | Non-ligamentary local anesthetic injections |
| Dental cleaning where bleeding is anticipated | Placement of removable prosthodontic or orthodontics |
| Postoperative suture removal |
Recommended Regimens for Patients with Cardiac Risk Factors
Antimicrobials should be administered as a single dose thirty to sixty minutes before the procedure.[40] The preferred regimen in adults is amoxicillin 2 g PO.[40] Practitioners should adjust amoxicillin dosing to 50 mg/kg in the pediatric population.[40] Practitioners can give ampicillin 2 g IV or PO for those unable to take oral medications.[40]
Alternative regimens include cephalexin 2 g, azithromycin 500 mg, clarithromycin 500 mg, or doxycycline 100 mg for those with penicillin allergy.[40] In children with penicillin allergy, cephalexin 50 mg/kg, azithromycin or clarithromycin 15 mg/kg, or doxycycline 2.2 mg/kg in patients weighing less than 45 kg, and 100 mg for patients weighing more than 45 kg can be prescribed.[40]
In patients with type I hypersensitivity to penicillins, including reactions of anaphylaxis, angioedema, or urticaria, cephalexin should not be administered.[40] Instead, prescribers should utilize an alternative agent, such as azithromycin, clarithromycin, or doxycycline.[40] The recommended regimen in patients with a history of type I IgE-mediated penicillin allergy that cannot take oral medications includes cefazolin or ceftriaxone 1 gm IV or IM in adults and 50 mg/kg IV or IM in children.[40]
Clindamycin is not recommended for antibiotic prophylaxis before dental procedures and oral surgery due to the high incidence of adverse events, such as C. difficile colitis.[40] C. difficile colitis can manifest with symptoms ranging from asymptomatic carriage to life-threatening colitis, resulting in toxic megacolon, which may require surgical intervention or result in death.[43] Additionally, C. difficile colitis is often characterized by a high recurrence rate, making this infection particularly difficult to treat.[43]
Other Issues
Antimicrobial Stewardship in Dentistry and Oral Surgery
Dentists prescribe 10% of all outpatient antibiotic prescriptions and prescribe the most antibiotics as a specialty in the United States.[44] Due to this, increased attention to antimicrobial stewardship in dentistry and oral surgery can create a significant impact. A study evaluating antibiotic prophylaxis at 168,420 dental visits in the United States between 2011 and 2015 found that only 19.1% of these prescriptions were concordant with guidelines.[45] The study reported that 1.4% of these prescriptions were associated with an adverse event within fourteen days.[46] These adverse events included emergency room visits in 83% and antibiotic allergy in 16%.[46]
Clostridioides difficile colitis occurred in 0.007 per 1000 patient days, and anaphylaxis occurred in 0.003 per 1000 person days.[46] Although these events affected a small portion of those prescribed unnecessary antibiotic prophylaxis, these events were entirely preventable by compliance with current guidelines.
Unneeded antimicrobials contribute to antimicrobial resistance, a growing threat in the community. In the United States alone, antibiotic-resistant bacteria result in the deaths of 23,000 people per year.[47]
Dentists working in academic hospitals or university settings were found more likely to prescribe antibiotic prophylaxis correctly when compared to dentists practicing elsewhere.[48] Additionally, dentists practicing for fifteen years or more had a higher likelihood of deviating from guideline-directed antibiotic prophylaxis prescribing.[48]
Increased education regarding the dangers of antibiotic-related events, including Clostridiodes difficile colitis and antimicrobial resistance, could make a difference. A small prospective cohort study involving infectious diseases antimicrobial stewardship personnel outreach to private practice dentists showed an increase in appropriate antibiotic prescribing from 19% pre-education to 87.9% post-education.[49]
Enhancing Healthcare Team Outcomes
Appropriate antimicrobial administration involves the entire healthcare team: dentists, oral surgeons, orthopedic surgeons, infectious diseases specialists, cardiologists, primary care physicians, and pharmacists. Dentists play a crucial role in the prescribing of antimicrobial prophylaxis. A panel of experts formed the American Dental Association's guidelines on antibiotic prophylaxis before dental procedures to prevent prosthetic joint infection in 2014.[39] [Level 4]
However, impediments to guideline-adherent prescribing include a lack of information regarding the complete cardiac histories of patients presenting for dental procedures, pressure from other physicians and the patients themselves to prescribe antimicrobial prophylaxis, and lack of education regarding the most up-to-date guidelines. The current infective endocarditis prophylaxis guidelines from the American Heart Society were developed after an exhaustive review of all available data.[40] [Level 4]
Cardiologists are crucial in advising patients and dentists about when patients do and do not require antimicrobial prophylaxis before dental procedures and oral surgery. Orthopedic surgeons also play a role when advising patients with implanted prosthetic joints. Primary care providers also contribute, as patients commonly ask whether antimicrobial prophylaxis is necessary before patients present to their dentists for dental work.
Infectious disease physicians have a critical role in advising their patients and other physicians and have also played a significant role in education and outreach. Pharmacists also have a considerable role to play in helping with antimicrobial selection in more challenging situations, such as antibiotic allergy, and especially antimicrobial stewardship pharmacists significantly contribute via outreach and education. Many physicians, surgeons, and pharmacists contribute to the breadth of knowledge regarding this subject with significant research contributions.
As multiple physicians are often involved in these situations, in addition to the dentist or oral surgeon, all members of the patient's healthcare team must provide consistent messaging and education to the patient to avoid antibiotic overprescribing that leads to increased antimicrobial resistance and unnecessary adverse events in situations where antimicrobial prophylaxis does not have benefit.
Media
(Click Image to Enlarge)
References
Carroll GC, Sebor RJ. Dental flossing and its relationship to transient bacteremia. Journal of periodontology. 1980 Dec:51(12):691-2 [PubMed PMID: 6937641]
Andersen MH, Holle SLK, Klein CF, Bruun NE, Arpi M, Bundgaard H, Tønder N, Iversen KK. Risk for infective endocarditis in bacteremia with Gram positive cocci. Infection. 2020 Dec:48(6):905-912. doi: 10.1007/s15010-020-01504-6. Epub 2020 Aug 25 [PubMed PMID: 32844380]
Honkanen M, Jämsen E, Karppelin M, Huttunen R, Eskelinen A, Syrjänen J. Periprosthetic Joint Infections as a Consequence of Bacteremia. Open forum infectious diseases. 2019 Jun:6(6):ofz218. doi: 10.1093/ofid/ofz218. Epub 2019 May 7 [PubMed PMID: 31214625]
Dayer M, Thornhill M. Is antibiotic prophylaxis to prevent infective endocarditis worthwhile? Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy. 2018 Jan:24(1):18-24. doi: 10.1016/j.jiac.2017.10.006. Epub 2017 Oct 26 [PubMed PMID: 29107651]
. PREVENTION of rheumatic fever and bacterial endocarditis through control of streptococcal infections. Modern concepts of cardiovascular disease. 1956 Dec:25(12 Suppl):365-9 [PubMed PMID: 13387501]
Patil S, Rao RS, Sanketh DS, Amrutha N. Microbial flora in oral diseases. The journal of contemporary dental practice. 2013 Nov 1:14(6):1202-8 [PubMed PMID: 24858777]
Fine DH, Markowitz K, Furgang D, Fairlie K, Ferrandiz J, Nasri C, McKiernan M, Gunsolley J. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: longitudinal cohort study of initially healthy adolescents. Journal of clinical microbiology. 2007 Dec:45(12):3859-69 [PubMed PMID: 17942658]
Level 2 (mid-level) evidenceSocransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. Microbial complexes in subgingival plaque. Journal of clinical periodontology. 1998 Feb:25(2):134-44 [PubMed PMID: 9495612]
Struzycka I. The oral microbiome in dental caries. Polish journal of microbiology. 2014:63(2):127-35 [PubMed PMID: 25115106]
Level 3 (low-level) evidenceKomiyama EY, Lepesqueur LS, Yassuda CG, Samaranayake LP, Parahitiyawa NB, Balducci I, Koga-Ito CY. Enterococcus Species in the Oral Cavity: Prevalence, Virulence Factors and Antimicrobial Susceptibility. PloS one. 2016:11(9):e0163001. doi: 10.1371/journal.pone.0163001. Epub 2016 Sep 15 [PubMed PMID: 27631785]
Selton-Suty C, Célard M, Le Moing V, Doco-Lecompte T, Chirouze C, Iung B, Strady C, Revest M, Vandenesch F, Bouvet A, Delahaye F, Alla F, Duval X, Hoen B, AEPEI Study Group. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012 May:54(9):1230-9. doi: 10.1093/cid/cis199. Epub [PubMed PMID: 22492317]
Level 3 (low-level) evidenceTande AJ, Patel R. Prosthetic joint infection. Clinical microbiology reviews. 2014 Apr:27(2):302-45. doi: 10.1128/CMR.00111-13. Epub [PubMed PMID: 24696437]
Morris AM, Howie S. Recommendations for antibiotics in patients with joint prosthesis are irresponsible and indefensible. Journal (Canadian Dental Association). 2009 Sep:75(7):513-5 [PubMed PMID: 19744360]
Smith AJ, Jackson MS, Bagg J. The ecology of Staphylococcus species in the oral cavity. Journal of medical microbiology. 2001 Nov:50(11):940-946. doi: 10.1099/0022-1317-50-11-940. Epub [PubMed PMID: 11699589]
Christaki E, Giamarellos-Bourboulis EJ. The complex pathogenesis of bacteremia: from antimicrobial clearance mechanisms to the genetic background of the host. Virulence. 2014 Jan 1:5(1):57-65. doi: 10.4161/viru.26514. Epub 2013 Sep 25 [PubMed PMID: 24067507]
Cahill TJ, Prendergast BD. Infective endocarditis. Lancet (London, England). 2016 Feb 27:387(10021):882-93. doi: 10.1016/S0140-6736(15)00067-7. Epub 2015 Sep 1 [PubMed PMID: 26341945]
Lafaurie GI, Noriega LA, Torres CC, Castillo Y, Moscoso SB, Mosquera S, Díaz-Báez D, Chambrone L. Impact of antibiotic prophylaxis on the incidence, nature, magnitude, and duration of bacteremia associated with dental procedures: A systematic review. Journal of the American Dental Association (1939). 2019 Nov:150(11):948-959.e4. doi: 10.1016/j.adaj.2019.06.017. Epub 2019 Sep 25 [PubMed PMID: 31561837]
Level 1 (high-level) evidenceHubers SA, DeSimone DC, Gersh BJ, Anavekar NS. Infective Endocarditis: A Contemporary Review. Mayo Clinic proceedings. 2020 May:95(5):982-997. doi: 10.1016/j.mayocp.2019.12.008. Epub 2020 Apr 13 [PubMed PMID: 32299668]
Desimone DC, Tleyjeh IM, Correa de Sa DD, Anavekar NS, Lahr BD, Sohail MR, Steckelberg JM, Wilson WR, Baddour LM, Mayo Cardiovascular Infections Study Group. Incidence of infective endocarditis caused by viridans group streptococci before and after publication of the 2007 American Heart Association's endocarditis prevention guidelines. Circulation. 2012 Jul 3:126(1):60-4. doi: 10.1161/CIRCULATIONAHA.112.095281. Epub 2012 Jun 11 [PubMed PMID: 22689929]
Duval X, Delahaye F, Alla F, Tattevin P, Obadia JF, Le Moing V, Doco-Lecompte T, Celard M, Poyart C, Strady C, Chirouze C, Bes M, Cambau E, Iung B, Selton-Suty C, Hoen B, AEPEI Study Group. Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: three successive population-based surveys. Journal of the American College of Cardiology. 2012 May 29:59(22):1968-76. doi: 10.1016/j.jacc.2012.02.029. Epub [PubMed PMID: 22624837]
Level 2 (mid-level) evidencePant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, Hirsch GA, Mehta JL. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. Journal of the American College of Cardiology. 2015 May 19:65(19):2070-6. doi: 10.1016/j.jacc.2015.03.518. Epub [PubMed PMID: 25975469]
Dayer MJ, Jones S, Prendergast B, Baddour LM, Lockhart PB, Thornhill MH. Incidence of infective endocarditis in England, 2000-13: a secular trend, interrupted time-series analysis. Lancet (London, England). 2015 Mar 28:385(9974):1219-28. doi: 10.1016/S0140-6736(14)62007-9. Epub 2014 Nov 18 [PubMed PMID: 25467569]
Level 2 (mid-level) evidenceThornhill MH, Dayer M, Lockhart PB, McGurk M, Shanson D, Prendergast B, Chambers JB. A change in the NICE guidelines on antibiotic prophylaxis. British dental journal. 2016 Aug 12:221(3):112-4. doi: 10.1038/sj.bdj.2016.554. Epub [PubMed PMID: 27514339]
Waasdorp JA, Evian CI, Mandracchia M. Immediate placement of implants into infected sites: a systematic review of the literature. Journal of periodontology. 2010 Jun:81(6):801-8. doi: 10.1902/jop.2010.090706. Epub [PubMed PMID: 20192616]
Level 3 (low-level) evidenceSalgado-Peralvo AO, Garcia-Sanchez A, Kewalramani N, Barone A, Martínez-González JM, Velasco-Ortega E, López-López J, Kaiser-Cifuentes R, Guerra F, Matos-Garrido N, Moreno-Muñoz J, Núñez-Márquez E, Ortiz-García I, Jiménez-Guerra Á, Monsalve-Guil L. Consensus Report on Preventive Antibiotic Therapy in Dental Implant Procedures: Summary of Recommendations from the Spanish Society of Implants. Antibiotics (Basel, Switzerland). 2022 May 13:11(5):. doi: 10.3390/antibiotics11050655. Epub 2022 May 13 [PubMed PMID: 35625298]
Level 3 (low-level) evidenceKhandelwal P, Hajira N. Management of Oro-antral Communication and Fistula: Various Surgical Options. World journal of plastic surgery. 2017 Jan:6(1):3-8 [PubMed PMID: 28289607]
Tonetti MS, Schmid J. Pathogenesis of implant failures. Periodontology 2000. 1994 Feb:4():127-38 [PubMed PMID: 9673201]
Esposito M, Grusovin MG, Worthington HV. Interventions for replacing missing teeth: antibiotics at dental implant placement to prevent complications. The Cochrane database of systematic reviews. 2013 Jul 31:2013(7):CD004152. doi: 10.1002/14651858.CD004152.pub4. Epub 2013 Jul 31 [PubMed PMID: 23904048]
Level 1 (high-level) evidenceBraun RS, Chambrone L, Khouly I. Prophylactic antibiotic regimens in dental implant failure: A systematic review and meta-analysis. Journal of the American Dental Association (1939). 2019 Jun:150(6):e61-e91. doi: 10.1016/j.adaj.2018.10.015. Epub 2019 Apr 20 [PubMed PMID: 31010572]
Level 1 (high-level) evidenceSingh Gill A, Morrissey H, Rahman A. A Systematic Review and Meta-Analysis Evaluating Antibiotic Prophylaxis in Dental Implants and Extraction Procedures. Medicina (Kaunas, Lithuania). 2018 Dec 1:54(6):. doi: 10.3390/medicina54060095. Epub 2018 Dec 1 [PubMed PMID: 30513764]
Level 1 (high-level) evidenceLindeboom JA, Frenken JW, Tuk JG, Kroon FH. A randomized prospective controlled trial of antibiotic prophylaxis in intraoral bone-grafting procedures: preoperative single-dose penicillin versus preoperative single-dose clindamycin. International journal of oral and maxillofacial surgery. 2006 May:35(5):433-6 [PubMed PMID: 16472987]
Level 1 (high-level) evidenceGoormans F, Coropciuc R, Vercruysse M, Spriet I, Willaert R, Politis C. Systemic Antibiotic Prophylaxis in Maxillofacial Trauma: A Scoping Review and Critical Appraisal. Antibiotics (Basel, Switzerland). 2022 Apr 5:11(4):. doi: 10.3390/antibiotics11040483. Epub 2022 Apr 5 [PubMed PMID: 35453234]
Level 2 (mid-level) evidenceChoi SH, Lee JH. Absorbable Plate-Related Infection after Facial Bone Fracture Reduction. Archives of craniofacial surgery. 2016 Mar:17(1):1-4. doi: 10.7181/acfs.2016.17.1.1. Epub 2016 Mar 21 [PubMed PMID: 28913243]
Elad S, Raber-Durlacher JE, Brennan MT, Saunders DP, Mank AP, Zadik Y, Quinn B, Epstein JB, Blijlevens NM, Waltimo T, Passweg JR, Correa ME, Dahllöf G, Garming-Legert KU, Logan RM, Potting CM, Shapira MY, Soga Y, Stringer J, Stokman MA, Vokurka S, Wallhult E, Yarom N, Jensen SB. Basic oral care for hematology-oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT). Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2015 Jan:23(1):223-36. doi: 10.1007/s00520-014-2378-x. Epub 2014 Sep 5 [PubMed PMID: 25189149]
de Leeuw K, Bootsma H, Middel A, Vissink A. [Antibiotic prophylaxis and immune-compromised patients]. Nederlands tijdschrift voor tandheelkunde. 2019 Oct:126(10):521-525. doi: 10.5177/ntvt.2019.10.19065. Epub [PubMed PMID: 31613281]
Gutiérrez JL, Bagán JV, Bascones A, Llamas R, Llena J, Morales A, Noguerol B, Planells P, Prieto J, Salmerón JI. Consensus document on the use of antibiotic prophylaxis in dental surgery and procedures. Medicina oral, patologia oral y cirugia bucal. 2006 Mar 1:11(2):E188-205 [PubMed PMID: 16505802]
Level 3 (low-level) evidenceSykara M, Maniatakos P, Tentolouris A, Karoussis IK, Tentolouris N. The necessity of administrating antibiotic prophylaxis to patients with diabetes mellitus prior to oral surgical procedures-a systematic review. Diabetes & metabolic syndrome. 2022 Oct:16(10):102621. doi: 10.1016/j.dsx.2022.102621. Epub 2022 Sep 24 [PubMed PMID: 36183455]
Level 1 (high-level) evidenceDi Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet (London, England). 2011 Jul 2:378(9785):86-97. doi: 10.1016/S0140-6736(10)61493-6. Epub 2011 Apr 5 [PubMed PMID: 21474172]
Sollecito TP, Abt E, Lockhart PB, Truelove E, Paumier TM, Tracy SL, Tampi M, Beltrán-Aguilar ED, Frantsve-Hawley J. The use of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints: Evidence-based clinical practice guideline for dental practitioners--a report of the American Dental Association Council on Scientific Affairs. Journal of the American Dental Association (1939). 2015 Jan:146(1):11-16.e8. doi: 10.1016/j.adaj.2014.11.012. Epub 2014 Dec 18 [PubMed PMID: 25569493]
Level 1 (high-level) evidenceWilson WR, Gewitz M, Lockhart PB, Bolger AF, DeSimone DC, Kazi DS, Couper DJ, Beaton A, Kilmartin C, Miro JM, Sable C, Jackson MA, Baddour LM, American Heart Association Young Hearts Rheumatic Fever, Endocarditis and Kawasaki Disease Committee of the Council on Lifelong Congenital Heart Disease and Heart Health in the Young; Council on Cardiovascular and Stroke Nursing; and the Council on Quality of Care and Outcomes Research. Prevention of Viridans Group Streptococcal Infective Endocarditis: A Scientific Statement From the American Heart Association. Circulation. 2021 May 18:143(20):e963-e978. doi: 10.1161/CIR.0000000000000969. Epub 2021 Apr 15 [PubMed PMID: 33853363]
Level 2 (mid-level) evidenceSung S, Kim EH, Kwon JW, Lee JS, Lee SB, Moon SH, Lee HM, Jung I, Lee BH. Invasive dental procedures as risk factors for postoperative spinal infection and the effect of antibiotic prophylaxis. Journal of clinical periodontology. 2021 Sep:48(9):1270-1280. doi: 10.1111/jcpe.13514. Epub 2021 Jul 8 [PubMed PMID: 34189757]
Ito HO. Infective endocarditis and dental procedures: evidence, pathogenesis, and prevention. The journal of medical investigation : JMI. 2006 Aug:53(3-4):189-98 [PubMed PMID: 16953053]
Czepiel J, Dróżdż M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A, Goldman S, Wultańska D, Garlicki A, Biesiada G. Clostridium difficile infection: review. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2019 Jul:38(7):1211-1221. doi: 10.1007/s10096-019-03539-6. Epub 2019 Apr 3 [PubMed PMID: 30945014]
Hicks LA, Bartoces MG, Roberts RM, Suda KJ, Hunkler RJ, Taylor TH Jr, Schrag SJ. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015 May 1:60(9):1308-16. doi: 10.1093/cid/civ076. Epub 2015 Mar 5 [PubMed PMID: 25747410]
Suda KJ, Calip GS, Zhou J, Rowan S, Gross AE, Hershow RC, Perez RI, McGregor JC, Evans CT. Assessment of the Appropriateness of Antibiotic Prescriptions for Infection Prophylaxis Before Dental Procedures, 2011 to 2015. JAMA network open. 2019 May 3:2(5):e193909. doi: 10.1001/jamanetworkopen.2019.3909. Epub 2019 May 3 [PubMed PMID: 31150071]
Gross AE, Suda KJ, Zhou J, Calip GS, Rowan SA, Hershow RC, Perez R, Evans CT, McGregor JC. Serious antibiotic-related adverse effects following unnecessary dental prophylaxis in the United States. Infection control and hospital epidemiology. 2021 Jan:42(1):110-112. doi: 10.1017/ice.2020.1261. Epub 2020 Nov 11 [PubMed PMID: 33172505]
Brinkac L, Voorhies A, Gomez A, Nelson KE. The Threat of Antimicrobial Resistance on the Human Microbiome. Microbial ecology. 2017 Nov:74(4):1001-1008. doi: 10.1007/s00248-017-0985-z. Epub 2017 May 11 [PubMed PMID: 28492988]
Šutej I, Par M, Lepur D, Peroš K, Pintarić H, Alajbeg I, Vuger L. Dentists' practice and compliance with current guidelines of infective endocarditis prophylaxis- National survey study. Journal of clinical and experimental dentistry. 2021 Jul:13(7):e648-e652. doi: 10.4317/jced.58054. Epub 2021 Jul 1 [PubMed PMID: 34306527]
Level 3 (low-level) evidenceGoff DA, Mangino JE, Trolli E, Scheetz R, Goff D. Private Practice Dentists Improve Antibiotic Use After Dental Antibiotic Stewardship Education From Infectious Diseases Experts. Open forum infectious diseases. 2022 Aug:9(8):ofac361. doi: 10.1093/ofid/ofac361. Epub 2022 Jul 25 [PubMed PMID: 35959211]