Introduction
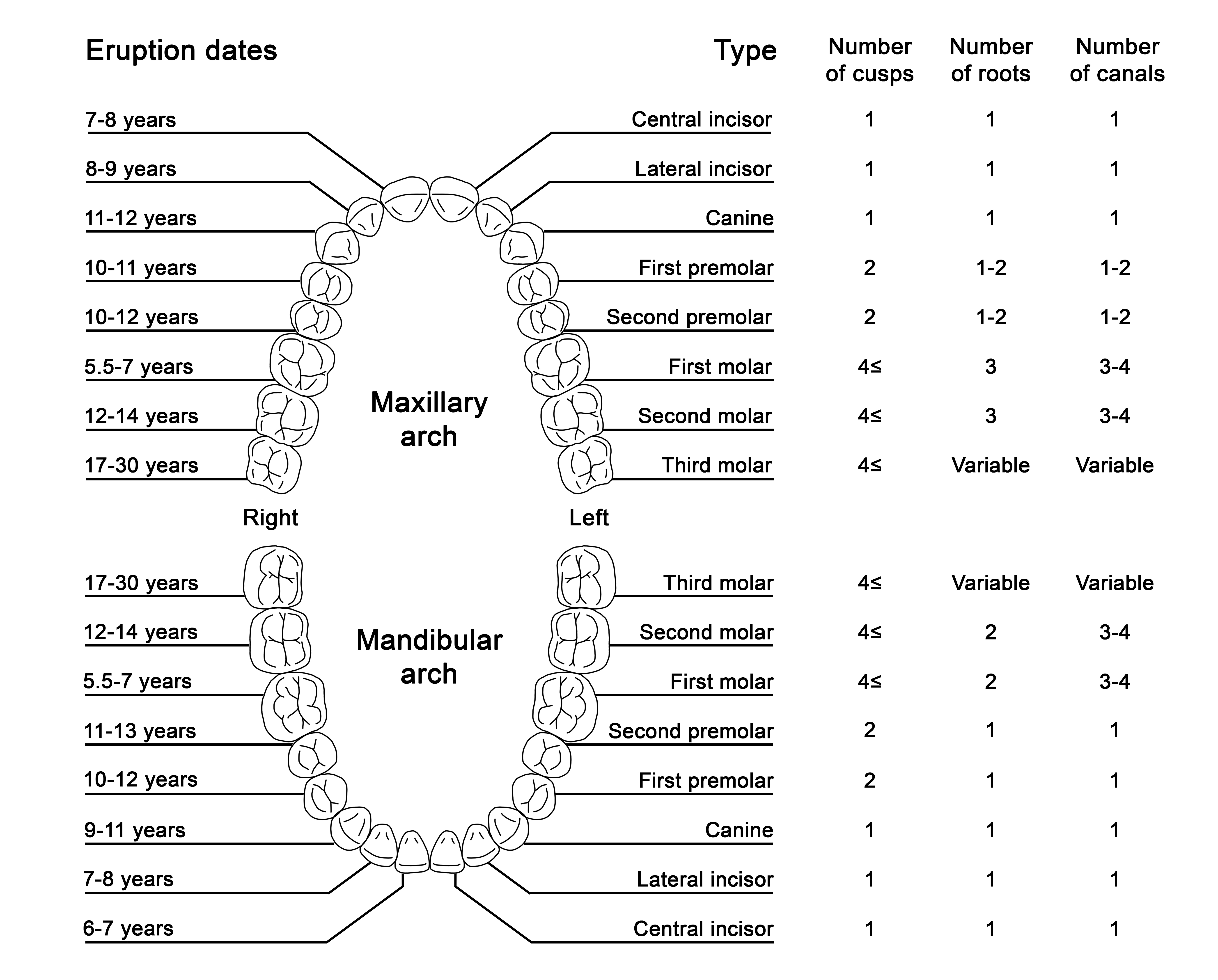
Humans have two sets of teeth during their lifetime: the initial deciduous (primary) teeth and the successive permanent (secondary) teeth.[1] There are typically 20 deciduous teeth divided evenly across the maxilla and mandible. The deciduous teeth eventually exfoliate and are replaced by 32 permanent teeth: 16 in the maxilla and 16 in the mandible. Permanent teeth are classified as incisors, canines, premolars, and molars, while primary teeth do not include premolars.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
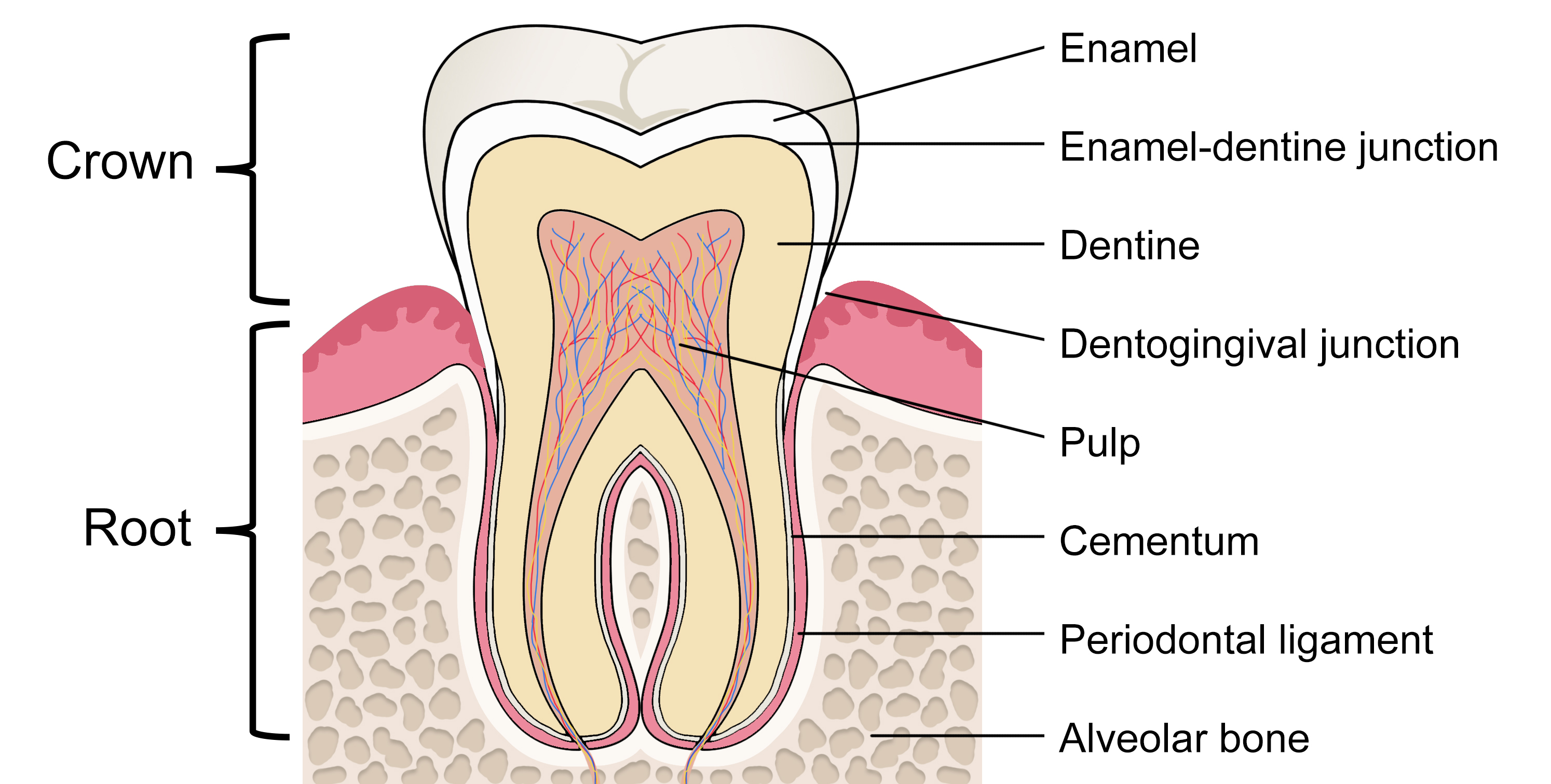
The portion of the tooth that is visible in the oral cavity is termed the crown. The crown is connected to the tooth root, situated within the periodontium, and is not clinically visible to evaluate health without imaging technology (Figure 1).[2] The permanent dentition usually erupts in a predictable sequence, and the number of cusps, roots, and root canals varies by the individual tooth (Figure 2). Dental enamel forms the outer surface of the crown and is the hardest and most highly mineralized tissue within the human body. Carbonated hydroxyapatite crystals form the mineral component of dental enamel and contribute to approximately 96% of its weight. In addition, water contributes 3% of the weight of dental enamel, and the remaining 1% is composed of non-collagenous protein.[3]
Enamel has an organized and complex structure, fundamental to its mechanical performance. The hydroxyapatite crystals align to form enamel rods (previously known as prisms) enclosed in a sheath of the organic matrix.[4] The rods follow an undulating path from the enamel-dentine junction (EDJ) towards the outer occlusal surface, where their arrangement is more parallel.[5] The inner enamel layer's decussating pattern creates Hunter-Schreger bands (viewable under reflected light), accounting for its resistance to crack formation. Within the oral cavity, dental enamel can repeatedly withstand masticatory forces of up to 770 newtons, owing to its resilience.[4]
Dentine constitutes the main bulk of the tooth. Its global distribution can be broadly separated into three categories: a mineral phase, an organic matrix, and water, forming 70%, 20%, and 10%, respectively, of its weight.[6] The most distinctive dentine feature is its honeycomb-like network of tubules, which extend outwards from the pulp towards the EDJ. At the dental pulp and dentine interface, the density and diameter of the tubules are highest, and this decreases with proximity to the EDJ. The dentinal tubules allow for the pulp's communication with the mineralized tissues and permit the transduction of physical signals to sensory responses. Their structure is used for bonding adhesive materials in some restorative dental treatment procedures.[5][7]
Within the central portion of the tooth lies the dental pulp. The pulp chamber provides mechanical support and functions as a barrier to external stimuli and the oral microbiome. The dental pulp is a unique tissue that is richly innervated and has an extensive microvascular network.[8] Maintaining its vitality increases both the mechanical resistance of the tooth and its long-term survival.[9] The junctional epithelium forms a band around the tooth at the base of the gingival sulcus, sealing off the periodontal tissues from the oral cavity.[10]
The cell bodies of odontoblasts are present on the surface of the pulp, where they form a natural barrier between the pulp and the dentine. Their long processes extend through the dentinal tubules. Secondary dentin is deposited slowly throughout life once the tooth has erupted, resulting in a gradual reduction in the pulp chamber's size with age (pulpal recession). In the presence of external stimuli, such as with early dental caries, the odontoblasts respond by secreting reactionary (tertiary) dentin to protect the pulp.[11] However, in cases where dental caries extend deep within the dentine, pulpal inflammation may persist despite restoration of the cavity, necessitating endodontic (root canal) treatment.[12]
The root surface is completely covered by a mineralized tissue called cementum, which provides attachment for the collagen fibers of the periodontal ligament. Thus, one end of the periodontal ligament attaches to the root cementum, and the other end attaches to the alveolar bone. The ends of the periodontal ligament's principal fibers are known as Sharpey fibers, a perforating connective tissue matrix where the periodontal ligaments attach to the cementum and bone. The dento-gingival junction, cementum, and periodontal ligament collectively form the periodontium, a support structure that anchors teeth within the surrounding alveolar bone.[10][13] The resulting fibrous joint between tooth and bone is called gomphosis (or dentoalveolar syndesmosis).
Embryology
Neural crest cells (NCCs) are a vertebrate-specific multipotent population of migratory cells that have a fundamental role in craniofacial development. They arise secondary to interactions between the neuroectoderm of the fusing neural tube and the surface ectoderm in the early stages of embryogenesis. These transient cells detach from the ectoderm through a delamination process, during which NCCs undergo an epithelial-to-mesenchymal transition. They migrate throughout the entire embryo, where they accumulate and proliferate. NCCs gather in the cranial region, forming characteristic swellings known as the pharyngeal arches and the frontonasal process. The maxilla and mandible are derived from the first pharyngeal arch, and the pre-maxilla arises from the frontonasal process.[14]
The formation of the dental lamina along the maxilla and the mandible occur during the six weeks of prenatal development and represents the first tooth development evidence. This horseshoe-shaped epithelial structure is externally bound by the similarly shaped vestibular lamina, which later gives rise to the oral vestibule.[15] Incisors, canines, premolars, and molars, despite their different characteristics, essentially follow the same developmental process. Odontogenesis occurs in three progressive morphological stages - bud, cap, and bell stage.[16]
This process is initiated through the proliferation of the dental lamina, which then invaginates to form an epithelial bud, extending inferiorly into the underlying mesenchyme during the bud stage. These swellings are termed enamel organs and eventually lead to the development of each tooth.[17] The enamel organ enlarges and acquires the shape of a dental crown as the tip folds inwards, representing the cap stage.
During the later stages, this entity develops to form an inner and outer enamel epithelium. The outer enamel epithelium maintains the shape of the enamel organ, and the inner enamel epithelium is responsible for the crown's shape. Further proliferation of these two layers creates a cervical loop where they later meet to produce Hertwig's epithelial root sheath, which determines the eventual form of the root. The underlying mesenchyme forms the dental papilla, giving rise to the pulp.
The enamel organ is surrounded by a fibrous capsule that develops into the periodontal ligament. Secondary folds occur during the bell stage, accounting for cusp formation in multicusped teeth. The inner enamel epithelium differentiates into ameloblasts during the late bell stage, responsible for enamel formation. Neural crest-derived odontoblasts also differentiate and secrete dentine. The formation of dental hard tissues commences, and the dental lamina disintegrates.[15][16][17]
Blood Supply and Lymphatics
The maxillary artery is the largest terminal division of the external carotid artery and distributes blood to the maxillofacial region, supplying the dental pulp's microcirculation.[18] It originates within the parotid gland and courses anteriorly between the sphenomandibular ligament and the mandibular ramus. The maxillary is situated in the infratemporal fossa and has a tortuous and highly variable route. Nonetheless, it often lies lateral to the lateral pterygoid muscle. It continues through the pterygomaxillary fissure and enters the pterygopalatine fossa.[19] It is divided into three segments, each with multiple arterial branches serving craniofacial structures: the first mandibular division lying medial to the condylar neck, the second pterygoid division that is closely related to the lateral pterygoid muscle, and the third pterygopalatine division where the artery traverses the pterygopalatine fossa.[20]
The inferior alveolar artery is a continuation of the maxillary artery's mandibular division, which enters the mandibular foramen together with the inferior alveolar vein and nerve. It proceeds to supply the teeth through the apical foramen via arterioles.[21] The pterygopalatine division gives rise to the posterior superior alveolar artery supplying the maxillary molars and premolars. The infraorbital artery branches to form the anterior superior alveolar artery, supplying the maxillary canines and incisors.[19]
The arterioles travel through the pulp towards the center of the tooth, where they branch to form a capillary network at the periphery. The circulating blood delivers oxygen and nutrients to the pulp's constituent cells, particularly the odontoblasts, through exchange diffusion. Metabolic waste products from the pulp are drained into central venules. The pterygoid plexus located within the infratemporal fossa is a complex venous network that provides venous drainage for tissues supplied by the maxillary artery.[8][19]
This highly vascular network has an estimated blood flow of 40 to 50 ml/min/100g of pulpal tissue, which is relatively high compared to other oral systems. Shunt vessels within the pulp allow direct communication between the venules and arterioles. They can regulate intrapulpal pressure during inflammation by opening to decrease the pressure and maintain normal blood flow. Persistent inflammation can generate an overwhelming increase in intrapulpal pressure, resulting in compression of the apical blood vessels and subsequent tissue necrosis.[8]
It is important for the dental pulp to sufficiently clear tissue fluid and cell components within its microcirculation through the presence of an effective lymphatic drainage system, although the exact functioning remains unclear. It has been postulated that lymph is collected in interstitial tissue clefts within the coronal pulp and is transported to lymphatic capillaries via the apical pulp.[22] The capillary walls are composed of a single layer of endothelial cells, which allows for the absorption of lymph from tissue fluid. Once transported to lymph nodes, the lymph is filtered and exposed to lymphocytes. All teeth drain to the submandibular lymph nodes, except the mandibular incisors, which drain the submental lymph nodes.[23][24]
Nerves
Three terminal divisions of the trigeminal nerve arise from the trigeminal ganglion; the ophthalmic (V1), maxillary (V2), and mandibular nerves (V3). As V3 exits the foramen ovale, it runs inferiorly towards the mandible and approaches the infratemporal fossa, where it branches extensively. It contains motor fibers serving the muscles of mastication, mylohyoid, the anterior belly of the digastric, and sensory fibers. Two particularly important branches of the mandibular nerve (V3) are the inferior alveolar nerve and the lingual nerve. Before entering the mandible, the inferior alveolar nerve supplies the mandibular posterior teeth and divides to form the mylohyoid nerve. The inferior alveolar nerve traverses medial to the mandibular ramus and enters the mandible via the mandibular foramen to serve the rest of the mandibular teeth. It then divides into its terminal branches: the incisive nerve innervating the incisors, canines, premolars, and their associated gingiva, and the mental nerve returning sensation from the chin and lip.[24]
The maxillary nerve (V2) is purely sensory. It passes through the foramen rotundum to enter the pterygopalatine fossa, where it divides into several branches. The V2 branch innervating the maxillary teeth is the superior alveolar nerve. It exits the infraorbital canal and gives off an anterior branch that innervates the incisors, canines, premolars, and a posterior superior alveolar nerve, innervating the molar teeth. The middle superior alveolar branch of V2 provides additional innervation to the premolars and maxillary sinuses.[24][25]
Pain perception and transduction from the teeth are achieved through sensory nerves branching from V2 and V3, which enter the apical foramen of the teeth. They extend coronally alongside the blood vessels and branch extensively at the periphery to form the plexus of Raschkow, which contains unmyelinated C fibers (0.3 to 1.2 µm in diameter) and the larger myelinated A-fibers (2 to 5 µm in diameter). These fibers demyelinate close to the level of the cell-rich zone and proceed to form an abundant network of free nerve fibers that are nociception-specific receptors. These free nerve endings may then penetrate through the dentinal tubules of the inner dentine.
Fibers have been attributed to sharp pulpal pain in the absence of tissue damage due to their peripheral location, rapid conduction, and low excitability threshold. C fibers have a higher threshold of excitability and slow conduction, which produces dull pain with pulp tissue injury. The hydrodynamic theory is the most widely accepted theory explaining the mechanism of dentine hypersensitivity. It proposes that fluid movement and pressure changes within the dentinal tubules deform the low threshold nerve fibers at the periphery of the pulp, which increases its permeability to sodium ions, causing depolarization and initiation of an action potential or pain impulse.[8]
The pulp also has sympathetic adrenergic vasomotor control. The cervical sympathetic ganglion conveys sympathetic nerve fibers, which join the trigeminal nerve ganglion and branch to either follow the path of the sensory nerve fibers from the teeth or travel alongside the blood vessels. Painful stimuli cause a release of noradrenaline or neuropeptide from sympathetic nerve endings, which promotes vasoconstriction and may modulate the excitability of sensory nerves. This effect is lessened in pulpal inflammation, where local sensory vasodilation becomes predominant and, as a result, can contribute to further pulpal inflammation.[8]
Physiologic Variants
An additional cusp called the cusp of Carabelli is sometimes located on the mesio-palatal surface of permanent maxillary first molars and is rarely present on the other permanent molars. Although less common, an accessory cusp can protrude from the cingulum of the primary or permanent anterior teeth, referred to as the talon cusp.[26] Macrodontia and microdontia are rare shape anomalies of dentition, where teeth are larger or smaller than average.[27][28] Taurodontism is an abnormality of tooth morphology that often occurs in multi-rooted teeth and is characterized by a short root and an enlarged body containing an equally enlarged pulp chamber.[29]
Dilaceration describes an unusually abrupt angle between tooth crown and root or within the root itself, resulting from trauma during the development of the tooth. Further anomalies of the dental hard tissues include gemination, where a single tooth forms two crowns. This is a consequence of the tooth bud's partial division, resulting in a bifid crown but retaining the normal number of expected teeth within the dental arch.[30] However, fusion leads to one less tooth within the dental arch as it comprises two separate developing tooth germs which join together.[31] Concrescence is a rare phenomenon where adjacent teeth are united by cementum.[32]
Hyperdontia is a condition characterized by additional teeth within the normal dentition, termed supernumerary teeth. Supernumerary teeth are frequently located in the anterior maxilla, between the central incisors, termed mesiodens.[33] In permanent dentition, the prevalence of supernumerary teeth ranges between 0.5% to 5.3%.[34][35] It affects males more frequently than females, with a ratio of 2 to 1. This phenomenon is often associated with syndromes such as Ehlers-Danlos, cleidocranial dysplasia, and Gardner syndrome. However, it may also be seen in non-syndromic patients. It is also more commonly seen in males than females. As a result of their formation in any part of the dental arch, this can lead to complications, including delayed eruption, malposition, impaction, diastemas, crowding, and poor aesthetics.[33][34][35] True anodontia is the absence of primary teeth, and total anodontia is complete tooth agenesis in both the primary and permanent dentition, both of which are extremely rare.[36]
Tooth agenesis may involve the primary or permanent dentition and is a developmental anomaly where at least one tooth is absent. It can be further classified as hypodontia, involving 1 to 5 absent teeth, and oligodontia involving six or more missing teeth. When excluding third molars (wisdom teeth), tooth agenesis has a prevalence between 3% and 10% in the permanent dentition. Third molars are the most commonly congenitally missing permanent teeth, with a reported prevalence of 23%. This is followed by mandibular second premolars, maxillary lateral incisors, and, less frequently, the maxillary second premolars.[37]
It is argued that the use of fire to cook and soften food during human evolution resulted in a reduced dependency on teeth for survival, which has been linked to a decreased reliance on all tooth types, especially the third molars. This, combined with a decrease in mandibular size, is hypothesized to have increased the prevalence of agenesis and impaction of the third molars in human populations[16].
Syndromes such as ectodermal dysplasia or cleft lip and palate are frequently associated with tooth agenesis and factors such as trauma, infection, or drugs. Congenitally missing teeth can also occur in isolation. Complications of tooth agenesis include malocclusion, poor aesthetics, reduced masticatory function, and speech difficulties.[38] Clinical and radiographic examination guides the treatment options, including routine surveillance or a combination of surgery and orthodontics before prosthetic dental rehabilitation. The best course of treatment depends on the morphology, location, and potential complications specific to the particular case.[35][39]
Surgical Considerations
One of the most common dentoalveolar surgical procedures is removing mandibular third molars, which have a close anatomical relationship to the inferior alveolar nerve that returns sensation from the lower lip and chin.[40] The lingual nerve is also in close proximity. It returns sensation from the anterior two-thirds of the tongue and traverses with the chorda tympani of the facial nerve, which conveys special taste sensations. Both nerves pass through the neighboring lingual soft tissue and, thus, are at risk of injury during mandibular third molar surgery.[41]
Panoramic imaging is often the primary modality of choice to investigate the proximity of mandibular third molars to the inferior alveolar canal. If high-risk features are identified, such as loss of cortication, diversion or narrowing of the canal, deflection, darkening and narrowing of the roots, or a bifid root apex, then cone-beam computed tomography (CBCT) may be indicated to evaluate the risk and aid treatment planning.[42] Coronectomy may be employed in high-risk cases to decrease the risk of injury to the inferior alveolar nerve by removing the crown to prevent the symptoms of pericoronitis and the risk of caries.[43]
The maxillary posterior teeth lie near the antrum, and several complications can result from this relationship. Oro-antral communication formation is a recognized complication of extraction of maxillary posterior teeth, which requires prompt closure to prevent sinus contamination, formation of an epithelialized fistulous tract, and development of chronic sinusitis.[44] Recognition of root morphology will guide the optimal extraction technique and ensure the clinician can determine complete removal. There is a risk of displacing these fragments into the maxillary antrum or adjacent anatomical structures in cases of root fracture.[45] Extrusion of sodium hypochlorite into the maxillary antrum is a potential risk of endodontic treatment of maxillary posterior teeth.[46]
Wrong tooth removal is a preventable complication of exodontia, and precautions should be taken to reduce the risk of its occurrence by implementing protocols such as the World Health Organisation surgical safety checklist.[47][48]
Clinical Significance
A comprehensive understanding of the normal anatomy of the permanent dentition, variant anatomy, and general head and neck anatomy is essential for dental practitioners, particularly when considering surgical treatment and routine clinical examination of patients.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
ADA Division of Communications, Journal of the American Dental Association, ADA Council on Scientific Affairs. For the dental patient. Tooth eruption: the permanent teeth. Journal of the American Dental Association (1939). 2006 Jan:137(1):127 [PubMed PMID: 16457009]
Koussoulakou DS, Margaritis LH, Koussoulakos SL. A curriculum vitae of teeth: evolution, generation, regeneration. International journal of biological sciences. 2009:5(3):226-43 [PubMed PMID: 19266065]
Level 3 (low-level) evidenceAl-Jawad M, Steuwer A, Kilcoyne SH, Shore RC, Cywinski R, Wood DJ. 2D mapping of texture and lattice parameters of dental enamel. Biomaterials. 2007 Jun:28(18):2908-14 [PubMed PMID: 17367851]
Beniash E, Stifler CA, Sun CY, Jung GS, Qin Z, Buehler MJ, Gilbert PUPA. The hidden structure of human enamel. Nature communications. 2019 Sep 26:10(1):4383. doi: 10.1038/s41467-019-12185-7. Epub 2019 Sep 26 [PubMed PMID: 31558712]
Level 2 (mid-level) evidenceArola DD, Gao S, Zhang H, Masri R. The Tooth: Its Structure and Properties. Dental clinics of North America. 2017 Oct:61(4):651-668. doi: 10.1016/j.cden.2017.05.001. Epub [PubMed PMID: 28886762]
Goldberg M, Kulkarni AB, Young M, Boskey A. Dentin: structure, composition and mineralization. Frontiers in bioscience (Elite edition). 2011 Jan 1:3(2):711-35 [PubMed PMID: 21196346]
Level 3 (low-level) evidenceIvancik J, Arola DD. The importance of microstructural variations on the fracture toughness of human dentin. Biomaterials. 2013 Jan:34(4):864-74. doi: 10.1016/j.biomaterials.2012.10.032. Epub 2012 Nov 3 [PubMed PMID: 23131531]
Yu C, Abbott PV. An overview of the dental pulp: its functions and responses to injury. Australian dental journal. 2007 Mar:52(1 Suppl):S4-16 [PubMed PMID: 17546858]
Level 3 (low-level) evidenceZanini M, Meyer E, Simon S. Pulp Inflammation Diagnosis from Clinical to Inflammatory Mediators: A Systematic Review. Journal of endodontics. 2017 Jul:43(7):1033-1051. doi: 10.1016/j.joen.2017.02.009. Epub 2017 May 17 [PubMed PMID: 28527838]
Level 1 (high-level) evidenceNanci A, Bosshardt DD. Structure of periodontal tissues in health and disease. Periodontology 2000. 2006:40():11-28 [PubMed PMID: 16398683]
Yumoto H, Hirao K, Hosokawa Y, Kuramoto H, Takegawa D, Nakanishi T, Matsuo T. The roles of odontoblasts in dental pulp innate immunity. The Japanese dental science review. 2018 Aug:54(3):105-117. doi: 10.1016/j.jdsr.2018.03.001. Epub 2018 Mar 27 [PubMed PMID: 30128058]
Farges JC, Alliot-Licht B, Renard E, Ducret M, Gaudin A, Smith AJ, Cooper PR. Dental Pulp Defence and Repair Mechanisms in Dental Caries. Mediators of inflammation. 2015:2015():230251. doi: 10.1155/2015/230251. Epub 2015 Oct 11 [PubMed PMID: 26538821]
Yamamoto T, Hasegawa T, Yamamoto T, Hongo H, Amizuka N. Histology of human cementum: Its structure, function, and development. The Japanese dental science review. 2016 Aug:52(3):63-74. doi: 10.1016/j.jdsr.2016.04.002. Epub 2016 Apr 27 [PubMed PMID: 28408958]
Miletich I, Sharpe PT. Neural crest contribution to mammalian tooth formation. Birth defects research. Part C, Embryo today : reviews. 2004 Jun:72(2):200-12 [PubMed PMID: 15269893]
Level 3 (low-level) evidenceHovorakova M, Lesot H, Peterka M, Peterkova R. The developmental relationship between the deciduous dentition and the oral vestibule in human embryos. Anatomy and embryology. 2005 Apr:209(4):303-13 [PubMed PMID: 15666156]
Zou D, Zhao J, Ding W, Xia L, Jang X, Huang Y. Wisdom teeth: mankind's future third vice-teeth? Medical hypotheses. 2010 Jan:74(1):52-5. doi: 10.1016/j.mehy.2009.08.004. Epub 2009 Sep 17 [PubMed PMID: 19765911]
Level 3 (low-level) evidenceRathee M, Jain P. Embryology, Teeth. StatPearls. 2023 Jan:(): [PubMed PMID: 32809350]
Maia FPA, de Sousa Filho GC, Pacífico FA, Albuquerque LCA, de Melo Vasconcelos AF, do Egito Vasconcelos BC. Proximity of the maxillary artery to the neck of the mandibular condyle: anatomical study. Oral and maxillofacial surgery. 2019 Dec:23(4):423-427. doi: 10.1007/s10006-019-00788-9. Epub 2019 Jul 5 [PubMed PMID: 31278592]
Gofur EM, Al Khalili Y. Anatomy, Head and Neck: Internal Maxillary Arteries. StatPearls. 2023 Jan:(): [PubMed PMID: 31194441]
Claire PG, Gibbs K, Hwang SH, Hill RV. Divided and reunited maxillary artery: developmental and clinical considerations. Anatomical science international. 2011 Dec:86(4):232-6. doi: 10.1007/s12565-011-0106-x. Epub 2011 Apr 19 [PubMed PMID: 21503610]
Level 3 (low-level) evidenceJergenson MA, Norton NS, Opack JM, Barritt LC. Unique origin of the inferior alveolar artery. Clinical anatomy (New York, N.Y.). 2005 Nov:18(8):597-601 [PubMed PMID: 16187317]
Level 3 (low-level) evidenceOehmke MJ, Knolle E, Oehmke HJ. Lymph drainage in the human dental pulp. Microscopy research and technique. 2003 Oct 15:62(3):187-91 [PubMed PMID: 14506683]
Amodini Rajakaruna G, Umeda M, Uchida K, Furukawa A, Yuan B, Suzuki Y, Noriko E, Izumi Y, Eishi Y. Possible translocation of periodontal pathogens into the lymph nodes draining the oral cavity. Journal of microbiology (Seoul, Korea). 2012 Oct:50(5):827-36. doi: 10.1007/s12275-012-2030-8. Epub 2012 Nov 4 [PubMed PMID: 23124752]
Rodella LF, Buffoli B, Labanca M, Rezzani R. A review of the mandibular and maxillary nerve supplies and their clinical relevance. Archives of oral biology. 2012 Apr:57(4):323-34. doi: 10.1016/j.archoralbio.2011.09.007. Epub 2011 Oct 11 [PubMed PMID: 21996489]
Nguyen JD, Duong H. Anatomy, Head and Neck: Alveolar Nerve. StatPearls. 2023 Jan:(): [PubMed PMID: 31536318]
Mavrodisz K, Rózsa N, Budai M, Soós A, Pap I, Tarján I. Prevalence of accessory tooth cusps in a contemporary and ancestral Hungarian population. European journal of orthodontics. 2007 Apr:29(2):166-9 [PubMed PMID: 17317866]
Canoglu E, Canoglu H, Aktas A, Cehreli ZC. Isolated bilateral macrodontia of mandibular second premolars: A case report. European journal of dentistry. 2012 Jul:6(3):330-4 [PubMed PMID: 22904663]
Level 3 (low-level) evidenceChen Y, Zhou F, Peng Y, Chen L, Wang Y. Non-syndromic occurrence of true generalized microdontia with hypodontia: A case report. Medicine. 2019 Jun:98(26):e16283. doi: 10.1097/MD.0000000000016283. Epub [PubMed PMID: 31261601]
Level 3 (low-level) evidenceJayashankara C, Shivanna AK, Sridhara K, Kumar PS. Taurodontism: A dental rarity. Journal of oral and maxillofacial pathology : JOMFP. 2013 Sep:17(3):478. doi: 10.4103/0973-029X.125227. Epub [PubMed PMID: 24574680]
Level 3 (low-level) evidenceSharada HL, Deo B, Briget B. Gemination of a permanent lateral incisor- a case report with special emphasis on management. Journal of international oral health : JIOH. 2013 Apr:5(2):49-53 [PubMed PMID: 24155591]
Level 3 (low-level) evidenceLey AM, Viana FLP, Cruz SML, Vasconcelos BC. Fused tooth: clinical approach to endodontic treatment. General dentistry. 2019 Nov-Dec:67(6):59-61 [PubMed PMID: 31658027]
Palermo D, Davies-House A. Unusual finding of concrescence. BMJ case reports. 2016 Mar 23:2016():. doi: 10.1136/bcr-2016-214597. Epub 2016 Mar 23 [PubMed PMID: 27009195]
Level 3 (low-level) evidenceMeighani G, Pakdaman A. Diagnosis and management of supernumerary (mesiodens): a review of the literature. Journal of dentistry (Tehran, Iran). 2010 Winter:7(1):41-9 [PubMed PMID: 21998774]
Demiriz L, Durmuşlar MC, Mısır AF. Prevalence and characteristics of supernumerary teeth: A survey on 7348 people. Journal of International Society of Preventive & Community Dentistry. 2015 May:5(Suppl 1):S39-43. doi: 10.4103/2231-0762.156151. Epub [PubMed PMID: 25984466]
Level 3 (low-level) evidenceAta-Ali F, Ata-Ali J, Peñarrocha-Oltra D, Peñarrocha-Diago M. Prevalence, etiology, diagnosis, treatment and complications of supernumerary teeth. Journal of clinical and experimental dentistry. 2014 Oct:6(4):e414-8. doi: 10.4317/jced.51499. Epub 2014 Oct 1 [PubMed PMID: 25593666]
Bala M, Pathak A. Ectodermal dysplasia with true anodontia. Journal of oral and maxillofacial pathology : JOMFP. 2011 May:15(2):244-6. doi: 10.4103/0973-029X.84515. Epub [PubMed PMID: 22529591]
Level 3 (low-level) evidenceJonsson L, Magnusson TE, Thordarson A, Jonsson T, Geller F, Feenstra B, Melbye M, Nohr EA, Vucic S, Dhamo B, Rivadeneira F, Ongkosuwito EM, Wolvius EB, Leslie EJ, Marazita ML, Howe BJ, Moreno Uribe LM, Alonso I, Santos M, Pinho T, Jonsson R, Audolfsson G, Gudmundsson L, Nawaz MS, Olafsson S, Gustafsson O, Ingason A, Unnsteinsdottir U, Bjornsdottir G, Walters GB, Zervas M, Oddsson A, Gudbjartsson DF, Steinberg S, Stefansson H, Stefansson K. Rare and Common Variants Conferring Risk of Tooth Agenesis. Journal of dental research. 2018 May:97(5):515-522. doi: 10.1177/0022034517750109. Epub 2018 Jan 24 [PubMed PMID: 29364747]
Rakhshan V. Congenitally missing teeth (hypodontia): A review of the literature concerning the etiology, prevalence, risk factors, patterns and treatment. Dental research journal. 2015 Jan-Feb:12(1):1-13 [PubMed PMID: 25709668]
Bural C, Oztas E, Ozturk S, Bayraktar G. Multidisciplinary treatment of non-syndromic oligodontia. European journal of dentistry. 2012 Apr:6(2):218-26 [PubMed PMID: 22509127]
Level 3 (low-level) evidenceSarikov R, Juodzbalys G. Inferior alveolar nerve injury after mandibular third molar extraction: a literature review. Journal of oral & maxillofacial research. 2014 Oct-Dec:5(4):e1. doi: 10.5037/jomr.2014.5401. Epub 2014 Dec 29 [PubMed PMID: 25635208]
Leung YY. Management and prevention of third molar surgery-related trigeminal nerve injury: time for a rethink. Journal of the Korean Association of Oral and Maxillofacial Surgeons. 2019 Oct:45(5):233-240. doi: 10.5125/jkaoms.2019.45.5.233. Epub 2019 Oct 30 [PubMed PMID: 31728330]
Saha N, Kedarnath NS, Singh M. Orthopantomography and Cone-Beam Computed Tomography for the Relation of Inferior Alveolar Nerve to the Impacted Mandibular Third Molars. Annals of maxillofacial surgery. 2019 Jan-Jun:9(1):4-9. doi: 10.4103/ams.ams_138_18. Epub [PubMed PMID: 31293923]
Pitros P, O'Connor N, Tryfonos A, Lopes V. A systematic review of the complications of high-risk third molar removal and coronectomy: development of a decision tree model and preliminary health economic analysis to assist in treatment planning. The British journal of oral & maxillofacial surgery. 2020 Nov:58(9):e16-e24. doi: 10.1016/j.bjoms.2020.07.015. Epub 2020 Aug 14 [PubMed PMID: 32800608]
Level 1 (high-level) evidenceKhandelwal P, Hajira N. Management of Oro-antral Communication and Fistula: Various Surgical Options. World journal of plastic surgery. 2017 Jan:6(1):3-8 [PubMed PMID: 28289607]
Nayyar J, Clarke M, O'Sullivan M, Stassen LF. Fractured root tips during dental extractions and retained root fragments. A clinical dilemma? British dental journal. 2015 Mar 13:218(5):285-90. doi: 10.1038/sj.bdj.2015.147. Epub [PubMed PMID: 25766165]
Zairi A, Lambrianidis T. Accidental extrusion of sodium hypochlorite into the maxillary sinus. Quintessence international (Berlin, Germany : 1985). 2008 Oct:39(9):745-8 [PubMed PMID: 19093046]
Level 3 (low-level) evidenceWali R, Halai T, Koshal S. WHO surgical safety checklist training: An alternative approach to training in local safety standards for invasive procedures. European journal of dental education : official journal of the Association for Dental Education in Europe. 2020 Feb:24(1):71-78. doi: 10.1111/eje.12469. Epub 2019 Sep 29 [PubMed PMID: 31518469]
Jan AM, Albenayan R, Alsharkawi D, Jadu FM. The prevalence and causes of wrong tooth extraction. Nigerian journal of clinical practice. 2019 Dec:22(12):1706-1714. doi: 10.4103/njcp.njcp_206_19. Epub [PubMed PMID: 31793478]