Introduction
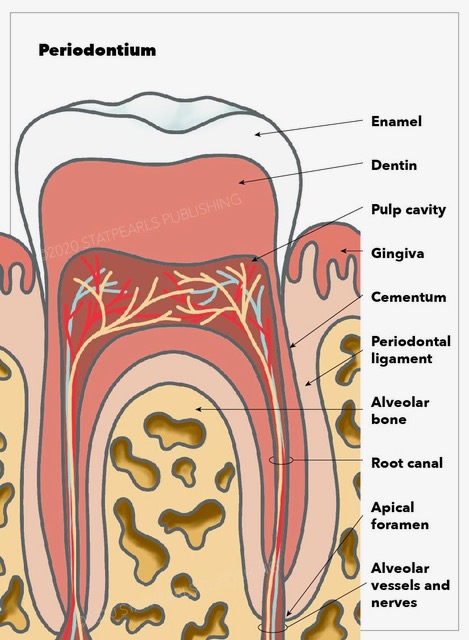
The periodontium is a connective tissue consisting of four components: cementum, the periodontal ligament (PDL), alveolar bone, and gingival tissue (see Image. Periodontium Anatomy).[1] This topic details the histological and embryological structure and origin of this tissue, as well as the function, clinical significance, and pathophysiology of some of the periodontal diseases.
Structure
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure
Embryologically, PDL, cementum, and alveolar bone development are accompanied by the formation of the root of the tooth and its eruption.[2] However, the gingival component of the periodontium is derived from the ectoderm of the pharyngeal arches. It contains keratinized stratified squamous epithelium on its masticatory surface and non-keratinized on its crevicular and junctional surface.[1] As mentioned, PDL, cementum, and alveolar bone are derived from the dental sac's neuro-mesenchymal stem cells.[3] To better understand the gingival epithelium, it can be studied in three different sections: the oral epithelium, sulcular epithelium, and junctional epithelium, which is the closest to the tooth surface and attaches to it with hemidesmosomes. Histologically, cementum is comprised of two parts: cellular and acellular. The gingival epithelium is composed of stratified squamous epithelium.
Function
The periodontium functions include supporting the tooth, protecting it against oral microflora, and making the attachment of the tooth to the bone possible.[1] To understand the function of the periodontium even further, we can look at its components function individually. The PDL is a connective tissue that consists mostly of type I collagen bands and fibroblasts. Using collagen bands, PDL connects the tooth's cementum to the gingivae in the alveolar socket. On the other hand, fibroblasts have a role in forming and repairing the alveolar bone and cementum.[4] Besides attaching the tooth roots to the bone and gingival tissue, PDL is also involved in proprioception by relaying information to the somatosensory cortex. This is important for avoiding occlusal forces during mastication and speech.[5] Cementum is the mineralized tissue covering the dentin surfaces and is known to be the attachment site for PDL.
Tissue Preparation
Various processing techniques have been suggested and studied in the literature, exploring different fixing and demineralizing solutions for tooth and periodontium. In light microscopy analysis done on histological processing of teeth and periodontium, it was demonstrated that 10% buffered neutral formalin was used as a fixative solution and 10% pH 7.3 EDTA, as a demineralizing solution, has shown satisfactory results. Paraffin-embedded sections of the sample are obtained to obtain morphometric and morphological satisfaction, each with a thickness of 6 μm. These slides were then stained with hematoxylin and eosin. This routine protocol is also beneficial since it allows serial sectioning for more specific techniques. For instance, this routine protocol can use techniques such as histochemical and immunohistochemical analyses that are more fitting for evaluating cellular components and the extracellular matrix of the periodontium.[6] The biologically oriented preparation technique is a reliable alternative to conventional horizontal finish lines.[7] In a cross-sectional study conducted in Turkey by Alkan et al, the effects of antibiotic therapy in oral histology were studied. The results showed that in healthy participants in oral histology trials, a minimum of 3 months should pass after the final amoxicillin dose to avoid the negative effect of gingival tissue smear samples.[8]
Pathophysiology
Understanding the structure and histology of the periodontium is clinically significant to the discussion of wound healing, drugs and environmental factors affecting it, periodontal diseases, and overall health risk factors. Lamina propria of gingiva regenerates more readily due to fiber differentiation after wounding, compared to the alveolar bone, which relies on osteocyte, bone marrow cells, endosteum cells, and osteogenic cells of periosteum, which occupy different compartments.[1]
Histopathology of periodontal diseases is described in 4 stages:
- Initially, the gingival crevicular fluid amount is increased due to vascular changes in response to the initial insult. At this benign stage, polymorphonuclear neutrophils are attracted to the lesion site, and T lymphocytes are responsible for fibroblasts.
- The early lesion is characterized by redness. At this stage, PMNs clear and breakdown the collagen fibers, which increases the previously made space for infiltrates.
- At this stage, the established lesion is dominated by B cells and leukocyte aggregation. This initiates the lesion side transformation by changing the junctional and sulcular epithelium into an extremely vulnerable epithelium called the pocket epithelium. This is apparent as bleeding upon gentle gingival manipulation.
- An advanced lesion characterized as loss of gingival fibers and alveolar bone is caused by the migration of biofilm into the pocket and creating an environment for anaerobic bacterias' proliferation.[9]
- Gingivitis is an initial response to combat the first insult to the periodontium. As the name suggests, it is an inflammation of the gingiva, a component of the periodontium resulting from the accumulation of microbial plaque near the gingival sulcus. Gingivitis has a delayed appearance in children with a predominance of T lymphocyte infiltrates.[10] Most cases are transient, non-progressive, and reversible. However, it can progress to Periodontitis.
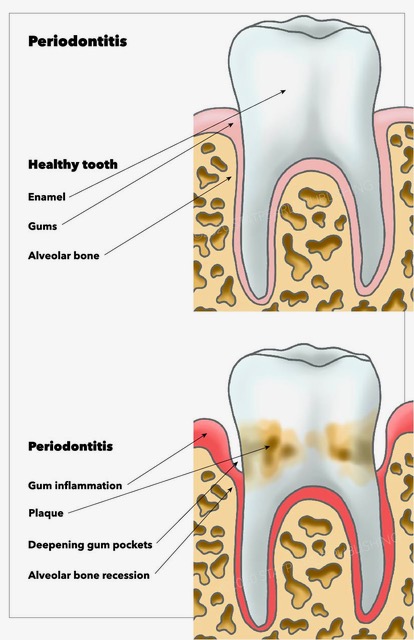
- Periodontitis is the inflammation of the periodontium, which extends to the jawbone and is irreversible (see Image. Periodontitis). The pathophysiology of periodontitis is explained by the replacement and remodeling of previously normal alveolar bone, especially the spongy bone, which can happen in healthy individuals due to chronic inflammation. White blood cells are active during inflammation, and osteoclast lineage is related to WBC. As a result, osteoblast is inhibited without reducing the rate of osteoclasts. It is observed on a radiograph as a reduced opacity of the alveolar crest.[11]
- Hyper-cementosis is thought to be due to growth factor disturbances in conditions such as acromegaly, gigantism, or Paget’s disease. Cementoblasts are located within the PDL and can undergo remodeling and repair. Excessive cementoblast deposition can cause hyper-cementosis. This can cause concrescence, which is the adherence of two teeth together by the roots (hence, a radiograph is needed before tooth extraction). This rare finding has been seen in patients with systemic lupus erythematosus.[12]
- Cementicle is a condition in which masses of acellular cementum appear in abnormal locations due to the disruption of the oppositional growth of cementum. This condition happens mostly in the elderly and appears as psammoma body-like calcifications. The process is thought to develop due to the encounter of cementoblasts with thrombus lodged in a nearby capillary.[13][14]
- Mesial drift: Also known as physiological drift, this refers to the migration of teeth in a mesial direction caused by the asymmetrical remodeling of alveolar bone tissue. Two major concepts are believed to cause this drift: First, unbalanced bone remodeling of 2 adjacent teeth. Second, remodeling due to occlusal forces leads to an imbalance of acellular cementum. This asymmetrical remodeling causes tooth movement.[15]
- Peri-implantitis is due to the failure to create the junction between the implant and the oral mucosa, leaving the underlying connective tissue exposed to the permanent oral microbiome and creating chronic inflammation of gingival and bone tissue.[11] Knowledge of histology helped to demonstrate that coating the peri-implant pocket with hyaluronic acid gel reduces inflammation in peri-implantitis by improving the connection between the core and recipients’ connective tissue through reducing inflammation.[16]
- Widening of PDL space: with occlusal/orthodontic trauma, the fibroblast in PDL responds by increasing its activity and leads to the widening of PDL, as the neighboring tissue is lost. This can be seen on a radiograph and can be accompanied by bone loss or hyper-cementosis. Certain medications, such as bisphosphonate, are used to manage rheumatoid arthritis, osteoporosis, osteogenesis imperfecta, and multiple myeloma.[4] can cause PDL widening. In contrast, conditions that affect bone health, such as radiation-induced bone defects, reduce the force and also cause a widening of the PDL space. Infection, inflammation, and malignancy can also cause PDL widening.
Clinical Significance
The periodontium is also significant because of its effect on systemic health and environmental risk factors affecting it. Some periodontium medications include anti-convulsants (diphenylhydantoin), immunosuppressants, corticosteroids, NSAIDs, and CCBs.[17] These drugs could directly affect the periodontium by increasing matrix production or decreasing its degradation and, as a result, cause gingival hyperplasia.[18] Smoking and bacterial biofilms within periodontal pockets are common causes of attachment loss between cementum and alveolar bone, promoting tooth loss.[19] Periodontal diseases have also been studied as a risk for dental implants over time.[20] This study has shown that periodontal status and smoking are important contributors to long-term implant failure.
Epidemiological studies have shown that periodontal diseases are associated with an increased risk of preterm birth and low birth weight (LBW). This can happen by direct vertical transfer of maternal bacteria that cause inflammation (Gram-negative bacteria in the dental plaque biofilm, such as Porphyromonas gingivalis and Fusobacterium nucleatum.[11] It is also believed that the release of inflammatory cytokines, specifically IL-lα, IL-1β, IL-6, and IL-8 and systemic inflammation, contributes to preterm birth and LBW. This emphasizes the importance of oral hygiene measurements and preventions during pregnancy.[21]
Media
(Click Image to Enlarge)
References
Melcher AH. On the repair potential of periodontal tissues. Journal of periodontology. 1976 May:47(5):256-60 [PubMed PMID: 775048]
Rathee M, Jain P. Embryology, Teeth. StatPearls. 2025 Jan:(): [PubMed PMID: 32809350]
Cho MI, Garant PR. Development and general structure of the periodontium. Periodontology 2000. 2000 Oct:24():9-27 [PubMed PMID: 11276876]
Level 3 (low-level) evidenceMortazavi H, Baharvand M. Review of common conditions associated with periodontal ligament widening. Imaging science in dentistry. 2016 Dec:46(4):229-237. doi: 10.5624/isd.2016.46.4.229. Epub 2016 Dec 20 [PubMed PMID: 28035300]
Willis RD, DiCosimo CJ. The absence of proprioceptive nerve endings in the human periodontal ligament: the role of periodontal mechanoreceptors in the reflex control of mastication. Oral surgery, oral medicine, and oral pathology. 1979 Aug:48(2):108-15 [PubMed PMID: 157454]
Silva GA, Moreira A, Alves JB. Histological processing of teeth and periodontal tissues for light microscopy analysis. Methods in molecular biology (Clifton, N.J.). 2011:689():19-36. doi: 10.1007/978-1-60761-950-5_2. Epub [PubMed PMID: 21153784]
Agustín-Panadero R, Martín-de Llano JJ, Fons-Font A, Carda C. Histological study of human periodontal tissue following biologically oriented preparation technique (BOPT). Journal of clinical and experimental dentistry. 2020 Jun:12(6):e597-e602. doi: 10.4317/jced.56290. Epub 2020 Jun 1 [PubMed PMID: 32665820]
Alkan B, Koroglu P. Effects of amoxicillin on gingival biopsies and oral smears: A cross-sectional study. Nigerian journal of clinical practice. 2021 Feb:24(2):233-239. doi: 10.4103/njcp.njcp_660_19. Epub [PubMed PMID: 33605914]
Level 2 (mid-level) evidenceMehrotra N, Singh S. Periodontitis. StatPearls. 2025 Jan:(): [PubMed PMID: 31082170]
Page RC. Gingivitis. Journal of clinical periodontology. 1986 May:13(5):345-59 [PubMed PMID: 3522644]
Level 3 (low-level) evidenceAli M, Yang F, Plachokova AS, Jansen JA, Walboomers XF. Application of specialized pro-resolving mediators in periodontitis and peri-implantitis: a review. European journal of oral sciences. 2021 Feb:129(1):e12759. doi: 10.1111/eos.12759. Epub 2021 Feb 9 [PubMed PMID: 33565133]
Shoor H, Sujir N, Mutalik S, Pai KM. Hypercementosis: a rare finding in a patient with systemic lupus erythematosus. BMJ case reports. 2014 Nov 26:2014():. doi: 10.1136/bcr-2013-202370. Epub 2014 Nov 26 [PubMed PMID: 25427926]
Level 3 (low-level) evidenceHolton WL, Hancock EB, Pelleu GB Jr. Prevalence and distribution of attached cementicles on human root surfaces. Journal of periodontology. 1986 May:57(5):321-4 [PubMed PMID: 3457946]
MIKOLA OJ, BAUER WH. Cementicles and fragments of cementum in the periodontal membrane. Oral surgery, oral medicine, and oral pathology. 1949 Aug:2(8):1063-74 [PubMed PMID: 18146240]
Tsuchiya S, Tsuchiya M, Nishioka T, Suzuki O, Sasano Y, Igarashi K. Physiological distal drift in rat molars contributes to acellular cementum formation. Anatomical record (Hoboken, N.J. : 2007). 2013 Aug:296(8):1255-63. doi: 10.1002/ar.22731. Epub 2013 Jun 17 [PubMed PMID: 23775928]
Level 3 (low-level) evidenceSánchez-Fernández E, Magán-Fernández A, O'Valle F, Bravo M, Mesa F. Hyaluronic acid reduces inflammation and crevicular fluid IL-1β concentrations in peri-implantitis: a randomized controlled clinical trial. Journal of periodontal & implant science. 2021 Feb:51(1):63-74. doi: 10.5051/jpis.1903660183. Epub [PubMed PMID: 33634616]
Level 1 (high-level) evidenceSeymour RA, Heasman PA. Drugs and the periodontium. Journal of clinical periodontology. 1988 Jan:15(1):1-16 [PubMed PMID: 3276739]
Level 3 (low-level) evidenceLauritano D, Moreo G, Limongelli L, Tregambi E, Palmieri A, Carinci F. Drug-Induced Gingival Overgrowth: A Pilot Study on the Effect of Diphenylhydantoin and Gabapentin on Human Gingival Fibroblasts. International journal of environmental research and public health. 2020 Nov 7:17(21):. doi: 10.3390/ijerph17218229. Epub 2020 Nov 7 [PubMed PMID: 33171749]
Level 3 (low-level) evidenceGamal AY, Bayomy MM. Effect of cigarette smoking on human PDL fibroblasts attachment to periodontally involved root surfaces in vitro. Journal of clinical periodontology. 2002 Aug:29(8):763-70 [PubMed PMID: 12390574]
Level 2 (mid-level) evidenceLevin L, Ofec R, Grossmann Y, Anner R. Periodontal disease as a risk for dental implant failure over time: a long-term historical cohort study. Journal of clinical periodontology. 2011 Aug:38(8):732-7. doi: 10.1111/j.1600-051X.2011.01745.x. Epub 2011 Jun 2 [PubMed PMID: 21635280]
Level 2 (mid-level) evidenceSaini R, Saini S, Saini SR. Periodontitis: A risk for delivery of premature labor and low-birth-weight infants. Journal of natural science, biology, and medicine. 2010 Jul:1(1):40-2. doi: 10.4103/0976-9668.71672. Epub [PubMed PMID: 22096335]