Introduction
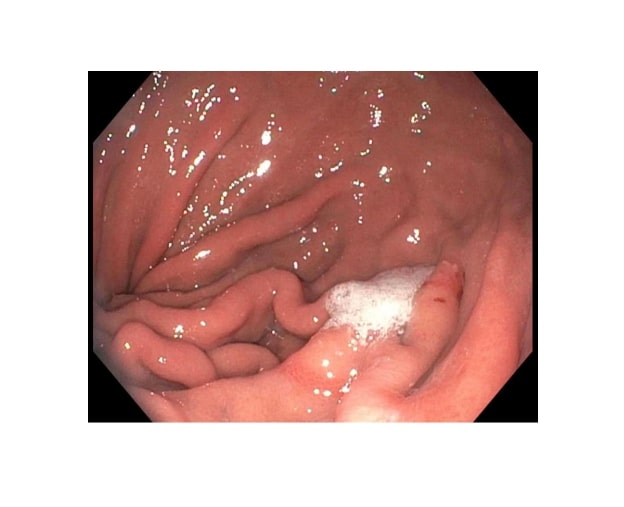
Gastrointestinal (GI) bleeding is the most common diagnosis among GI-related hospital admissions. Of all the GI hemorrhages, nearly 50% are due to upper GI bleeding. The most common causes of upper GI bleed include peptic ulcer disease, gastroesophageal varices, esophagitis, angioectasia, and vascular lesions. The etiology is unknown in about 8% of the cases.[1] Cameron lesion is a rare cause of occult upper GI bleed (see Image. Cameron Lesion). Cameron lesions are linear gastric ulcers or erosions on the mucosal folds at the diaphragmatic impression in patients with a large hiatal hernia. Cameron lesions were first described in 1986 by Cameron and Higgins. The lesions were seen in people who had the chest x-ray showing one-third or more of the stomach above the diaphragm. Of the total cases, 50% were found to be anemic.[2][3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Various theories have been proposed for the possible pathogenesis of Cameron lesions, with mechanical trauma caused by constant rubbing of mucosal folds at the constriction level of the diaphragm being the most likely cause. It has been postulated that different forces act at the neck of the gastric sac during breathing, including the upward sliding movement of the stomach, negative intrathoracic pressure causing outward and upward force, and impinging force by the crura during gastric sliding through the diaphragm.[4] Other mechanisms include gastric acid injury, focal ischemia due to diaphragmatic pressure on the herniated sac, and gut wall edema due to venous obstruction leading to vascular stasis.[5] Cameron lesions have not been shown to have a positive correlation with nonsteroidal anti-inflammatory drug use, Helicobacter pylori infection, or the absence of protein pup inhibitor usage. However, binge drinking in some studies has been related to the Cameron lesions.[6]
Epidemiology
The prevalence rates of hiatal hernia range from 0.8 to 2.9 in all patients undergoing upper gastrointestinal endoscopy. Cameron lesions are seen in 5% of patients with known hiatal hernia discovered in upper endoscopic studies.[7][8] Cameron lesions may heal spontaneously within a couple of days, contributing to the underreporting of these lesions. The size of the hiatal hernia correlates directly with the prevalence of these lesions. Since the frequency of hiatal hernia increases with age, most cases are reported in the elderly population.[9] It is rare in the pediatric subgroup of patients, with the youngest patient being 3. The prevalence is higher in women as compared to men.[10]
Most of the cases have been reported from developed countries, including North America and Western Europe. There are few cases reported from Africa, but this could be attributed to underreporting. There has been no correlation with any environmental factors or genetics. Studies have shown that inadequate fiber intake and high sitting position during defecation could be possible risk factors for developing Cameron lesions.[11]
Histopathology
Macroscopically, the lesions appear as white, linear, superficial erosions on the crests of inflamed mucosal folds at the site of diaphragmatic indentation. A biopsy is not recommended to make the diagnosis of Cameron lesions. Histopathological examination in the past has revealed gastric atrophy, fibrosis, necrosis, and infiltration by immune-related cells. Microscopic characteristics have shown changes in vascular obstruction indicative of ischemic gastropathy. These changes include inflammatory markers, hemorrhagic infiltrates, fibrin deposition, sloughing of the epithelium, cryptic atrophy, and coagulative necrosis.[12]
History and Physical
Cameron lesions can present with a broad range of clinical features. The patients can be completely asymptomatic in the beginning. Cameron lesions have been identified as an etiology for occult upper gastrointestinal blood loss, at times leading to iron deficiency anemia.[13] The usual presentation in these cases includes anemia symptoms like fatigue, pallor, melena, palpitations, or dyspnea on exertion.[14] The larger hernias (>3 cm) are associated more frequently with iron deficiency anemia than the small hernias (<3 cm).[9] This is because the size of the lesions increases with an increase in the hiatal hernia size. The patients may also present with gastroesophageal reflux disease-like symptoms with epigastric discomfort. Rarely, patients may experience life-threatening overt GI bleed.[5]
Evaluation
Cameron lesions pose a diagnostic challenge to physicians. The laboratory tests show iron deficiency with low hemoglobin levels and microcytic hypochromic anemia. Iron studies reveal low iron and ferritin levels with high total iron-binding capacity.[15] The chest x-ray may help visualize the hiatal hernia, showing a large posterior mediastinum structure. A barium swallow is also a non-invasive modality to diagnose hiatal hernia. The hernias are usually seen on radiographic films but not the Cameron lesions.[8]
Endoscopy is the gold standard for diagnosis, although it is not uncommon to overlook these lesions due to their unique location. They are often missed on the initial endoscopy and are usually discovered on subsequent endoscopies.[16] The endoscopic evaluation involves careful antegrade and retrograde visualization of the region of the hernia sac and adjacent mucosa. Endoscopic findings include mucosal erythema, edema, and ecchymotic bleeding, along with the Cameron lesions in the gastric mucosal folds. Cameron lesions usually present as linear erosions of the gastric mucosa, frequently with erythematous borders.[17]
Newer diagnostic studies like magnification chromoendoscopy may be useful to demonstrate the mucosal defects. They have also been shown to be useful in situations where there are no well-defined lesions. The usefulness of capsule endoscopy has not been fully documented. Given the short transit time and the cost involved, it is unlikely to be a diagnostic modality of choice.[18]
Treatment / Management
The treatment options include medical management, surgery, and endoscopic intervention.[2] The medical management consists of acid suppression with a proton pump inhibitor to help hasten mucosal healing and iron supplementation for concurrent iron deficiency anemia. Most patients have some acid-related problems (reflux disease, esophagitis) and are usually on acid inhibitors. This combination therapy has shown good results, with a noticeable improvement in the ulcer’s healing and the correction of the hemoglobin levels.[6] Endoscopic band ligation of actively bleeding Cameron lesions has shown good results in a limited number of cases. Endoscopic hemostasis is difficult in such scenarios due to technical and anatomical reasons. Cauterization and Epinephrine injection have been tried in some cases with temporary improvement.[19] The surgical options include fundoplication. It is recommended in patients with uncontrolled bleeding from these lesions, medically refractory disease, and complicated hernia with incarceration, volvulus, or perforation. Considering the close relation of hiatal hernia and Cameron lesions, fundoplication has been considered to be a reasonable treatment.[20][21](A1)
Differential Diagnosis
Cameron lesion has several differential diagnoses based on the patient’s age, comorbidities, and overall health. Some conditions have similar clinical features and need to be differentiated from Cameron lesions based on the presenting signs and symptoms and endoscopy. These include telangiectasias, ulcers, esophagitis, Mallory-Weiss tear, diverticulosis, Dieulafoy lesion, and neoplasms (see Image. Cameron Lesion and Ulcer).[22]
Prognosis
The outcomes of medical therapy are excellent. There has been good healing with acid inhibitors and iron supplementation at 6 weeks. Some studies have shown complications in the form of persistent anemia and rebleeding. The majority of patients who have been unresponsive to medical therapy continue to lose blood and require multiple transfusions.[23] Surgical treatment with fundoplication remains the mainstay of treatment in non-responsive cases. The surgical treatment has shown good results, with no recurrent ulcer or hemorrhage seen in the follow-up period.[24]
Complications
The diagnosis is usually missed due to the non-specific findings. It may result in delayed treatment, eventually resulting in adverse complications. The fatal complication is spontaneous hemorrhage if left untreated. The other complication includes worsening iron deficiency anemia, which in severe cases may cause hemodynamic instability and worsening of pre-existing comorbidities.[13]
Deterrence and Patient Education
Patients should minimize the risk factors by lifestyle modifications including, avoiding alcohol use, avoiding fried and fatty foods, and avoiding the exercises that increase the intraabdominal pressure. Regular follow-up with the primary physician for monitoring the labs and with the gastroenterologist for monitoring the progression is necessary.
Enhancing Healthcare Team Outcomes
The majority of Cameron lesion cases go undiagnosed due to the absence of acute signs and symptoms, an occult bleeding pattern, and difficulty in visualization on endoscopy. The lesions are difficult to diagnose due to their location and small size. They often require multiple endoscopies before the diagnosis is made. Clinicians, including gastroenterologists, should consider this condition in the differentials for intermittent or massive GI bleed. Once diagnosed, the patient should be started on appropriate medical therapy and have regular follow-ups to ensure resolution. If the lesions and the symptoms persist, the physician should refer the patient for endoscopic and surgical management.[1][23] An interprofessional healthcare team consisting of clinicians, nursing staff, and pharmacists operating as a collaborative unit is the best means to achieve optimal results in cases of Cameron lesions.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Maganty K, Smith RL. Cameron lesions: unusual cause of gastrointestinal bleeding and anemia. Digestion. 2008:77(3-4):214-7. doi: 10.1159/000144281. Epub 2008 Jul 12 [PubMed PMID: 18622137]
Level 3 (low-level) evidenceCameron AJ, Higgins JA. Linear gastric erosion. A lesion associated with large diaphragmatic hernia and chronic blood loss anemia. Gastroenterology. 1986 Aug:91(2):338-42 [PubMed PMID: 3487479]
Moskovitz M, Fadden R, Min T, Jansma D, Gavaler J. Large hiatal hernias, anemia, and linear gastric erosion: studies of etiology and medical therapy. The American journal of gastroenterology. 1992 May:87(5):622-6 [PubMed PMID: 1595651]
Richter IA, Rabin MS. The 'riding' ulcer: A report of 3 cases. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1979 Oct 6:56(15):612-4 [PubMed PMID: 550413]
Level 3 (low-level) evidenceWindsor CW, Collis JL. Anaemia and hiatus hernia: experience in 450 patients. Thorax. 1967 Jan:22(1):73-8 [PubMed PMID: 6031918]
Weston AP. Hiatal hernia with cameron ulcers and erosions. Gastrointestinal endoscopy clinics of North America. 1996 Oct:6(4):671-9 [PubMed PMID: 8899401]
Johnson DA, Ruffin WK. Hiatal hernia. Gastrointestinal endoscopy clinics of North America. 1996 Jul:6(3):641-66 [PubMed PMID: 8803572]
Gray DM, Kushnir V, Kalra G, Rosenstock A, Alsakka MA, Patel A, Sayuk G, Gyawali CP. Cameron lesions in patients with hiatal hernias: prevalence, presentation, and treatment outcome. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus. 2015 Jul:28(5):448-52. doi: 10.1111/dote.12223. Epub 2014 Apr 24 [PubMed PMID: 24758713]
Level 2 (mid-level) evidenceNguyen N, Tam W, Kimber R, Roberts-Thomson IC. Gastrointestinal: Cameron's erosions. Journal of gastroenterology and hepatology. 2002 Mar:17(3):343 [PubMed PMID: 11982707]
Fakhre Yaseri H. Gender is a risk factor in patients with gastroesophageal reflux disease. Medical journal of the Islamic Republic of Iran. 2017:31():58. doi: 10.14196/mjiri.31.58. Epub 2017 Sep 8 [PubMed PMID: 29445687]
Smith RE, Sharma S, Shahjehan RD. Hiatal Hernia. StatPearls. 2025 Jan:(): [PubMed PMID: 32965871]
Katz J, Brar S, Sidhu JS. Histopathological characterization of a Cameron lesion. International journal of surgical pathology. 2012 Oct:20(5):528-30 [PubMed PMID: 22614163]
Zullo A, Manta R, De Francesco V, Fiorini G, Lahner E, Vaira D, Annibale B. Cameron lesions: A still overlooked diagnosis. Case report and systematic review of literature. Clinics and research in hepatology and gastroenterology. 2018 Dec:42(6):604-609. doi: 10.1016/j.clinre.2018.05.002. Epub 2018 Jun 14 [PubMed PMID: 29910147]
Level 3 (low-level) evidenceCamus M, Jensen DM, Ohning GV, Kovacs TO, Ghassemi KA, Jutabha R, Machicado GA, Dulai GS, Hines OJ. Severe upper gastrointestinal hemorrhage from linear gastric ulcers in large hiatal hernias: a large prospective case series of Cameron ulcers. Endoscopy. 2013:45(5):397-400. doi: 10.1055/s-0032-1326294. Epub 2013 Apr 24 [PubMed PMID: 23616128]
Level 2 (mid-level) evidenceHolt JM, Mayet FG, Warner GT, Callender ST, Gunning AJ. Iron absorption and blood loss in patients with hiatus hernia. British medical journal. 1968 Jul 6:3(5609):22-5 [PubMed PMID: 5301883]
Zaman A, Katon RM. Push enteroscopy for obscure gastrointestinal bleeding yields a high incidence of proximal lesions within reach of a standard endoscope. Gastrointestinal endoscopy. 1998 May:47(5):372-6 [PubMed PMID: 9609429]
Miller G. Linear gastric erosion associated with diaphragmatic hernia. Gastroenterology. 1987 Jan:92(1):271 [PubMed PMID: 3781200]
Level 3 (low-level) evidenceBornstein JD, Brazer SR. Cameron erosions. Gastrointestinal endoscopy. 1999 Apr:49(4 Pt 1):514 [PubMed PMID: 10202069]
Level 3 (low-level) evidenceLin CC, Chen TH, Ho WC, Chen TY. Endoscopic treatment of a Cameron lesion presenting as life-threatening gastrointestinal hemorrhage. Journal of clinical gastroenterology. 2001 Nov-Dec:33(5):423-4 [PubMed PMID: 11606864]
Level 3 (low-level) evidencePauwelyn KA, Verhamme M. Large hiatal hernia and iron deficiency anaemia: clinico-endoscopical findings. Acta clinica Belgica. 2005 Sep-Oct:60(4):166-72 [PubMed PMID: 16279396]
Level 2 (mid-level) evidenceRothstein R, Filipi C, Caca K, Pruitt R, Mergener K, Torquati A, Haber G, Chen Y, Chang K, Wong D, Deviere J, Pleskow D, Lightdale C, Ades A, Kozarek R, Richards W, Lembo A. Endoscopic full-thickness plication for the treatment of gastroesophageal reflux disease: A randomized, sham-controlled trial. Gastroenterology. 2006 Sep:131(3):704-12 [PubMed PMID: 16952539]
Level 1 (high-level) evidenceMalik TF, Anjum F. Dieulafoys Lesion Causing Gastrointestinal Bleeding. StatPearls. 2025 Jan:(): [PubMed PMID: 32965938]
Mehershahi S, Jog A, Ronderos DM, Shaikh D, Ihimoyan A. Cameron Ulcers: Rare Case of Overt Upper Gastrointestinal Bleed in a Patient with Alcohol Use Disorder. Cureus. 2020 Apr 12:12(4):e7644. doi: 10.7759/cureus.7644. Epub 2020 Apr 12 [PubMed PMID: 32411546]
Level 3 (low-level) evidenceCougard P, Sala JJ, Favre JP, Viard H. [Ulcers of the neck of hiatal hernias. 15 cases]. Journal de chirurgie. 1985 Jun-Jul:122(6-7):399-402 [PubMed PMID: 3876349]
Level 3 (low-level) evidence