Introduction
Hydroxyapatite crystal deposition disease (HADD) is characterized by the deposition of hydroxyapatite (HA) crystals within the intra-articular and periarticular regions of the joint.[1][2] One of the most commonly involved sites of disease includes the rotator cuff muscle tendons. When involving the tendons and symptomatic, HADD is recognized as the clinical entity of calcific tendinitis. Shoulder barbotage of hydroxyapatite deposition disease is a treatment option for managing pain in patients affected by calcific tendinitis. [3] It is often performed with subacromial/subdeltoid bursal steroid injection. The important aspects of ultrasound-guided shoulder barbotage include the following:
- Maintaining proper needle technique with constant visualization of the affected tendon during the procedure to avoid injury of adjacent structures.
- Minimizing patient pain during the procedure
- Maintaining sterile technique to limit the risk of post-procedural infection.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Hydroxyapatite crystal deposition disease is a relatively common pathologic entity resulting from the formation of calcium crystal deposits within and around tendons and other connective tissue structures. [4] Often affecting middle-aged adults, the majority of cases affect the supraspinatus and the infraspinatus tendons, followed by the hip and spine. [5][6] While the cause of HADD is not clearly understood, various sources have suggested a degenerative process. [5] HADD can be divided into four phases: the pre-calcific, formative, resorptive, and post-calcific phases. [5] The pre-calcific phase is characterized by the metaplasia of collagen fibers of a tendon into fibrocartilage tissue. The formative phase is characterized by chondrocyte development and HADD crystal formation. The formative phase is also characterized by a resting state of the crystalline deposits. The resorptive phase is characterized by inflammation, while the post-calcific phase is characterized by capillary and collagen formation. Oftentimes underlying bone is affected, resulting in cortical erosion and periosteal reaction both in a solid and lamellated appearance. Underlying marrow involvement can also be seen. In addition, HA crystals can rupture into the subacromial/subdeltoid bursa resulting in painful bursitis, or rupture into the glenohumeral joint resulting in a destructive, inflammatory arthropathy termed Milwaukee shoulder, which can mimic septic arthritis.
HADD is most often diagnosed on radiographs. Frequently HADD is noted involving the rotator cuff tendons on chest radiographs. On radiography, hydroxyapatite crystals are found to be "fluffy" and "globular" in appearance. These crystals can either be well-defined or ill-defined based on their chronicity, with well-defined crystals suggesting a chronic deposit. CT can also be used to diagnose calcific tendinitis of the longus colli muscle, especially when evaluating for deep neck soft tissue infection and trauma. [7] CT also has the added ability to distinguish HADD from monosodium urate crystallopathy. [8] Magnetic resonance imaging(MRI) is also often performed to assess for the presence of inflammation. MRI can also assist in evaluating other pathologies, especially if HADD is suspected to be an incidental finding in the setting of a suspected alternative diagnosis. [9] Soft tissue edema is often seen in the acute inflammatory setting adjacent to the crystalline deposits. MRI sequences often show signal voids at the site of mineralization but can also present as an intermediate signal on T1 sequences. HA deposits can be difficult to identify on MRI as they often appear T1/T2 hypointense, blending with the hypointense tendons. This is just one reason why obtaining and reviewing radiographs prior to MRI is essential.
The rotator cuff tendons constitute the most common site of HADD. The supraspinatus, subscapularis, infraspinatus, and the teres minor muscle tendons form a covering over the humeral head. Deposition of HA at these tendons is often noted on radiographs at the respective tendon sites at or adjacent to the humeral head. Considering the tendon fibers are not visualized on radiography, HA is often noted as floating radiopaque densities close to or at the insertion of these tendons. The supraspinatus tendon constitutes one of the most common sites of HADD involvement. The supraspinatus muscle originates from the supraspinatus fossa of the scapula and inserts onto the greater tuberosity on the superior (most anterior adjacent to the bicipital groove) and most anterior aspect of the middle facet of the humerus as the supraspinatus tendon. [10] The infraspinatus tendon inserts on the middle facet, which is sloped posteriorly on a sagittal view. It should be noted that the posterior fibers of the supraspinatus tendon blend with the anterior fibers of the infraspinatus tendon for a conjoined attachment at the anterior aspect of the middle facet. Therefore it is possible for HADD and rotator cuff tears to involve both tendons simultaneously. Several bursae also surround the shoulder, with the largest being the subacromial-subdeltoid bursa. The subacromial-subdeltoid bursa assists in shoulder motion and plays an important role in the ultrasound-guided barbotage procedure detailed further below.
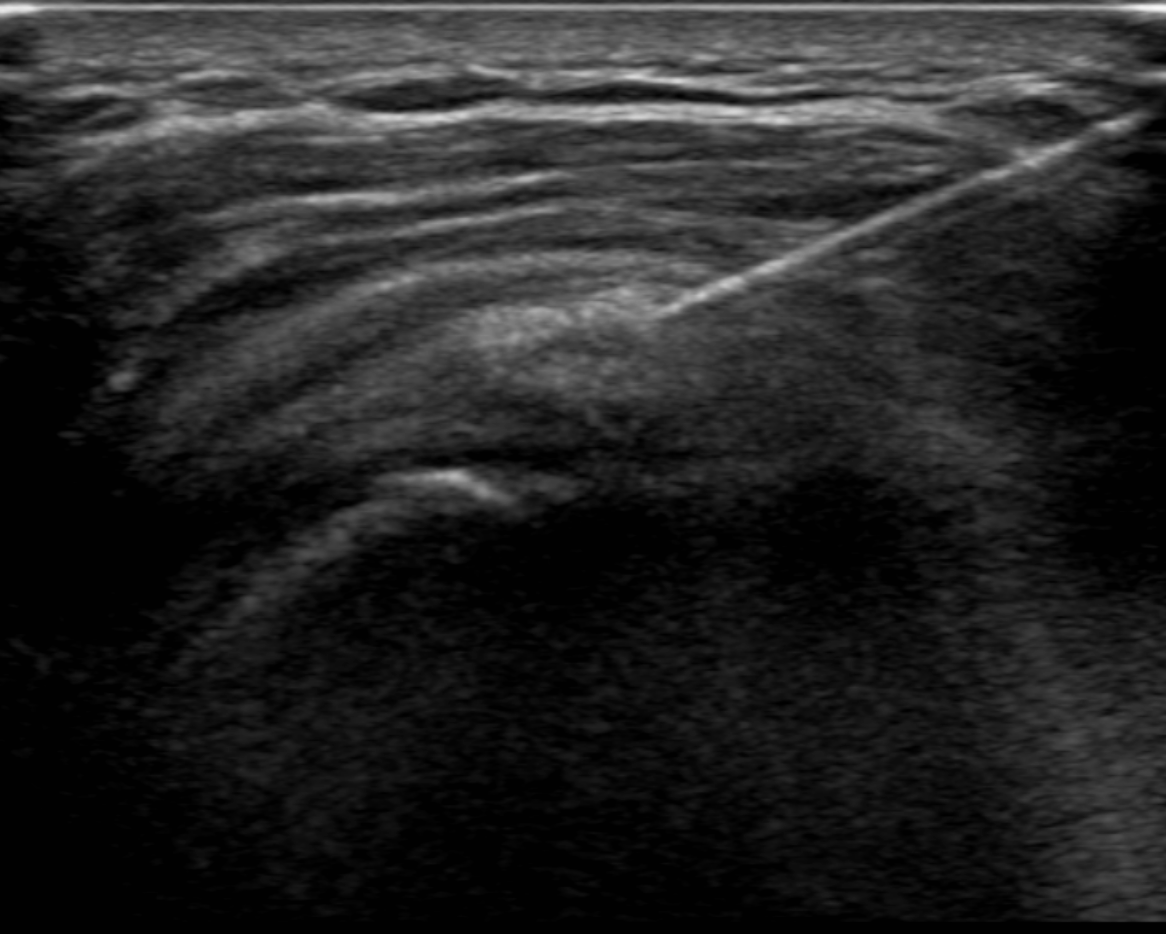
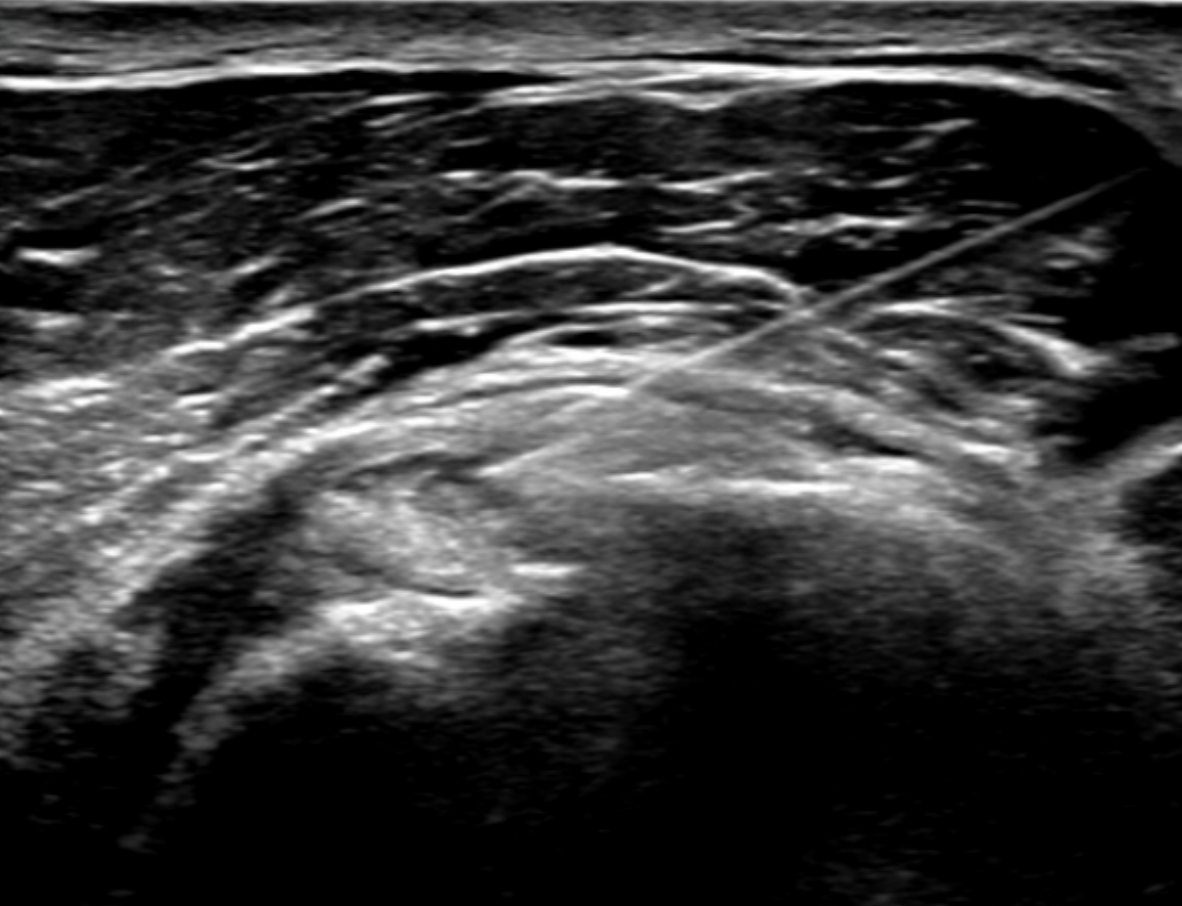
Ultrasound plays a primary role in image-guided treatment, such as in the setting of barbotage and steroid injection for pain relief, and is safe and effective. [11][12][13] HADD can present as echogenic calcifications with posterior acoustic shadowing or present as nodular echogenic foci of calcification without posterior acoustic shadowing. Often crystals can be multiple and adjacent to each other, having a fragmented appearance. A cystic morphology has also been described with a hyperechoic wall and anechoic internally. Acute calcific tendinitis shows increased doppler flow and thickening of the subacromial-subdeltoid bursa, an additional feature of the acute inflammatory phase. Ultrasound-guided barbotage constitutes a needling and lavage procedure. The needling portion of the procedure attempts to break down the hydroxyapatite crystals deposited within the rotator cuff tendon being treated. The lavage procedure attempts to remove the resultant fragmented crystals. This procedure is also coupled with corticosteroid injection of the subacromial-subdeltoid bursa for further pain relief.
Indications
The treatment options of calcific tendinitis often include oral analgesics. Additional treatment options include corticosteroid injection and ultrasound-guided barabotage. Surgical treatment and extracorporeal shockwave therapy are additional considerations depending on presentation and prior treatment course.
Contraindications
Some considerations with regards to absolute and relative contraindications include:
- A septic joint
- Cellulitis of the skin entry site
- Anaphylaxis/allergy to the selected therapeutic agents intended to be used
Equipment
While equipment can vary institutionally, the following list includes possible equipment and pharmacotherapeutic mixture used in this procedure:
- A sterile drape or towels to create a sterile field.
- Sterilizing solution such as chlorhexidine.
- An ultrasound machine with a high-frequency transducer (5 to 12 MHz).
- 1% lidocaine (typically 10 to 20 mL)
- Saline syringes (typically 2 to 3 saline flushes can be used)
- An 18-gauge needle (provider preference)
- A mixture of 1 mL of betamethasone 6 mg/mL (or other steroids such as triamcinolone) and 2 mL of bupivacaine 0.5% (or other anesthetics such as lidocaine or ropivacaine).
Personnel
Personnel includes:
- The clinician performing the procedure
- A sonographer (or possibly any assistant to the physician)
Preparation
Evaluation of ultrasound-guided barbotage of the rotator cuff tendons should begin with a thorough history and physical examination. In addition, a shoulder physical exam to include evaluation of other etiologies such as a septic joint should be performed. Laboratory analysis to include white blood cell count and inflammatory markers should also be obtained in the patient's initial workup.
Clinicians performing the procedures should have a basic understanding of the sonographic anatomy of the shoulder joint. Sonography has gradually replaced fluoroscopy in treating calcific tendinitis of the shoulder due to its ease of use and the radiation involved in fluoroscopy. Sonography is also able to easily evaluate for subtle changes in joint pathology and can evaluate for tears and degree of calcific deposits within the tendon. The humeral head (particularly the bicipital groove), coracoid process, the distal clavicle, and the acromion are often used as landmarks in localizing the supraspinatus tendon.
Before beginning the procedure, a clinician needs to have a thorough discussion with the patient regarding the following:
- The steps involved in the procedure, including the use of a needle to break up the crystalline deposits
- The potential for pain or significant discomfort during the procedure
- Prior medical conditions or surgeries that could affect the procedure
- The possible complications of the procedure such as skin or joint infection, steroid flare (usually within the first 24 hours), and tendon rupture.
Before beginning the procedure, an initial ultrasound exam should be performed to confirm and document the presence of hydroxyapatite deposition within the intended tendon target. A final time-out should also be performed before beginning the procedure. The skin entry site should then be cleaned, and a sterile drape should be put in place. The ultrasound probe is covered with a sterile sleeve.
- Optimal patient positioning includes:
- Patient in the supine position
- Arm in full extension
- Arm either rotated internally or externally (depending on the location of the HADD)
- Alternative positioning (can include the patient sitting in a partially reclined chair if they are unable to lie flat)
Technique or Treatment
- Ultrasound should be performed to localize the crystals and the skin marked at the planned needle entry site.
- The skin should be sterilized with a topical solution such as chlorhexidine, and a sterile drape should be placed around the procedure site.
- The ultrasound probe should be covered with a sterile cover.
- The skin should be anesthetized with 1% lidocaine. The subcutaneous soft tissues can also be anesthetized at the site of the proposed needle tract.
- Under ultrasound guidance, an 18-gauge needle should be advanced to the location of the coarse calcifications seen on ultrasound. Aspiration of the crystals should be attempted first without lavage.
- After attempts at aspiration, sterile saline should be pulsed through the needle, and then aspiration should be attempted through the same needle (lavage). The saline injection helps in the aspiration of calcium deposits. Care is necessary to confirm the visualization of multiple aspirate samples of calcifications.
- Lavage can be continued (with multiple needles) until a significant amount of calcium is felt to have been fragmented and aspirated.
- Upon completing the barbotage and lavage, a small volume of lidocaine can be injected into the residual calcium crystal deposits. However, the steroid should not be injected into the tendon as it can weaken the tendon and lead to rupture.
- A mixture of 1 mL of betamethasone 6 mg/mL and 2 mL of bupivacaine 0.5% should then be injected into the subacromial/subdeltoid bursa. This helps with pain relief in the event that calcium crystals are released into the bursa.
- Hemostasis should be achieved, and the placement of a sterile dressing is the final step.
Points to Consider
- A two-needle technique can also be employed, with two needles placed at the site of the calcific deposits: one needle is used to inject saline and the other used for aspiration. This would require an assistant to either hold the ultrasound probe or control/place one of the needles.
- A high-frequency (5 to 12 MHz) transducer is often preferred in performing the procedure. This is because high-frequency transducers allow for better resolution of superficial structures, while lower frequency transducers are used for the assessment of deeper structures such as abdominal or pelvic ultrasound.
- Residual calcifications can (and usually do) persist after the lavage. However, the procedure generally results in decreased pain and more rapid resorption of the crystals, although some literature suggests similar outcomes with conservative treatment and barbotage. [9]
- If the needle tip is difficult to visualize when situated at the crystal, needle movement can help localize the needle tip. Visualization of the needle is also improved by having the needle more parallel to the probe. This can be accomplished by "heel-toeing" (depressing one side of the transducer more than the other). Alternatively, some ultrasound machines and transducers allow for beam-steering, which can have a similar effect.
- Visualization of the needle tip can be difficult due to the acoustic features of the crystalline deposits.
- Fragmented calcium often appears as milk-like fluid or punctate solid debris within the lavage syringe.
- Ill-defined calcification, which suggests early and acute disease, is most responsive to this procedure.
Complications
Complications include bruising, hematoma formation, infections, and acute exacerbation of pain. In some institutions, patients are often advised to make arrangements for transport. Driving is possible the day after the procedure based on patient comfort. Patients are advised to take oral analgesics for pain relief in the acute post-procedural setting in the days following the procedure. Other complications also include bursitis that develops post-procedure or, in rare cases, tendon rupture.
Clinical Significance
Various associations that involve hormonal, genetic, and metabolic syndromes have been associated with HADD. When asymptomatic, HADD is often discovered incidentally on radiographs. Clinical symptoms can include acute pain, chronic mild pain, and acute-on-chronic pain. Patients can also present with fever. Acute episodes can present with swelling and warmth, in which case a septic joint should be included in the differential diagnosis. Other symptoms include a limited range of motion. Laboratory analysis can include mildly elevated inflammatory markers in the acute setting. Pain is often exacerbated during movement. Pain is also often confined to the affected joint and can be exacerbated during nighttime. Milwaukee shoulder refers to a destructive arthropathy of the shoulder joint involving the rupture/deposition of hydroxyapatite crystals into the glenohumeral joint. [14] When this process occurs in the hands or feet and the HA deposits in the periarticular soft tissues, it is termed periarticular calcific arthritis.
Enhancing Healthcare Team Outcomes
The role of a sonographer or technologist is important in helping the clinician during the procedure, considering optimal visualization of the needle or needles (in a two-needle technique) is critical to avoiding damage to adjacent structures. Patient comfort is also important in properly performing this sometimes technically challenging procedure as significant patient movement can affect satisfactory targeting of the hydroxyapatite crystals within the rotator cuff tendon. Movement can affect needle trajectory and injury to the adjacent musculature or tendons. The sonographer or technologist plays an important role in positioning the patient and limiting patient movement by helping with patient comfort. In addition, the technologist operates the ultrasound control panel while the clinician maintains the sterile field, handles the ultrasound probe, and performs the procedure.
Management of HADD via ultrasound-guided barbotage is best accomplished using an interprofessional healthcare team that includes clinicians (including mid-level practitioners), specialists, nurses, and sonographers, working collaboratively to bring about optimal patient outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Hayes CW, Conway WF. Calcium hydroxyapatite deposition disease. Radiographics : a review publication of the Radiological Society of North America, Inc. 1990 Nov:10(6):1031-48 [PubMed PMID: 2175444]
Bardin T, Richette P. [Basic calcium phosphate crystal deposition disease]. Presse medicale (Paris, France : 1983). 2011 Sep:40(9 Pt 1):850-5. doi: 10.1016/j.lpm.2011.04.011. Epub 2011 Jul 6 [PubMed PMID: 21737231]
Daftary AR, Karnik AS. Perspectives in ultrasound-guided musculoskeletal interventions. The Indian journal of radiology & imaging. 2015 Jul-Sep:25(3):246-60. doi: 10.4103/0971-3026.161445. Epub [PubMed PMID: 26288519]
Level 3 (low-level) evidenceUpton SJ,Ly JQ,Beall DP,Folio L, Radiology corner. Answer to last month's radiology case and image (case [PubMed PMID: 17153560]
Level 3 (low-level) evidenceBeckmann NM. Calcium Apatite Deposition Disease: Diagnosis and Treatment. Radiology research and practice. 2016:2016():4801474. doi: 10.1155/2016/4801474. Epub 2016 Nov 30 [PubMed PMID: 28042481]
Hongsmatip P, Cheng KY, Kim C, Lawrence DA, Rivera R, Smitaman E. Calcium hydroxyapatite deposition disease: Imaging features and presentations mimicking other pathologies. European journal of radiology. 2019 Nov:120():108653. doi: 10.1016/j.ejrad.2019.108653. Epub 2019 Sep 8 [PubMed PMID: 31550638]
Shawky A, Elnady B, El-Morshidy E, Gad W, Ezzati A. Longus colli tendinitis. A review of literature and case series. SICOT-J. 2017:3():48. doi: 10.1051/sicotj/2017032. Epub 2017 Jun 28 [PubMed PMID: 28664844]
Level 2 (mid-level) evidencePaparo F,Fabbro E,Ferrero G,Piccazzo R,Revelli M,Camellino D,Garlaschi G,Cimmino MA, Imaging studies of crystalline arthritides. Reumatismo. 2012 Jan 19; [PubMed PMID: 22303533]
Klontzas ME, Vassalou EE, Zibis AH, Karantanas AH. Hydroxyapatite deposition disease around the hip: outcomes of CT-guided treatment. Diagnostic and interventional radiology (Ankara, Turkey). 2016 Sep-Oct:22(5):466-70. doi: 10.5152/dir.2016.15616. Epub [PubMed PMID: 27537854]
Volk AG, Vangsness CT Jr. An anatomic study of the supraspinatus muscle and tendon. Clinical orthopaedics and related research. 2001 Mar:(384):280-5 [PubMed PMID: 11249176]
Gatt DL, Charalambous CP. Ultrasound-guided barbotage for calcific tendonitis of the shoulder: a systematic review including 908 patients. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2014 Sep:30(9):1166-72. doi: 10.1016/j.arthro.2014.03.013. Epub 2014 May 10 [PubMed PMID: 24813322]
Level 1 (high-level) evidenceComfort TH,Arafiles RP, Barbotage of the shoulder with image-intensified fluoroscopic control of needle placement for calcific tendinitis. Clinical orthopaedics and related research. 1978 Sep; [PubMed PMID: 709929]
Lanza E, Banfi G, Serafini G, Lacelli F, Orlandi D, Bandirali M, Sardanelli F, Sconfienza LM. Ultrasound-guided percutaneous irrigation in rotator cuff calcific tendinopathy: what is the evidence? A systematic review with proposals for future reporting. European radiology. 2015 Jul:25(7):2176-83. doi: 10.1007/s00330-014-3567-1. Epub 2015 Jan 13 [PubMed PMID: 25583182]
Level 2 (mid-level) evidenceGarcia GM, McCord GC, Kumar R. Hydroxyapatite crystal deposition disease. Seminars in musculoskeletal radiology. 2003 Sep:7(3):187-93 [PubMed PMID: 14593560]