Sonography Vascular Peripheral Vein Assessment, Protocols, and Interpretation
Sonography Vascular Peripheral Vein Assessment, Protocols, and Interpretation
Introduction
Peripheral venous ultrasound (US) is commonly used to diagnose and evaluate venous disease, including acute thromboembolism, postthrombotic syndrome, and chronic venous insufficiency (see Image. Chronic Venous Insufficiency). Venous duplex ultrasound is quick to perform and easily accessible. It is noninvasive and offers no radiation risk to the patient. When evaluating acute deep venous thrombosis (DVT), ultrasonography is 97% sensitive for proximal DVT and 57% sensitive for calf DVT.[1] Furthermore, duplex ultrasound can delineate venous anatomy, valvular abnormalities and reflux, the extent and pattern of disease, and in doing so, help plan treatment. [2] The US is also used to monitor disease progression over time. [3]
Venous thromboembolism refers to the formation of blood clots in the venous structures. The annual incidence of DVT is 1 in 1000, and DVT is responsible for > 250,000 hospitalizations a year in the United States.[4] The more proximal the blood clot, the more clinically crucial adequate management is. [5] In particular, iliofemoral DVT has a high incidence of recurrent hospitalizations and postthrombotic venous ulceration. [6] Risk factors broadly extend from Virchow's triad of stasis of blood flow, endothelial injury, and hypercoagulability.
Postthrombotic syndrome (PTS) refers to the late complications of venous thromboembolism. The cumulative incidence after symptomatic DVT is 15% to 50%, with most cases developing in 1 to 2 years.[7] Both venous obstruction and the resulting inflammation can lead to valvular damage, reflux, and venous hypertension. [8]
Signs and symptoms of PTS often overlap with chronic venous insufficiency (CVI), a term denoting impaired venous return usually reserved for the superficial system. CVI arises from a combination of genetic predisposition and risk factors such as age, female gender, obesity, and prolonged standing. [9] It appears in younger individuals; approximately 25% to 33% of women and 10% to 20% of men have varicose veins, one of the earliest manifestations of CVI. [10] Up to 5% of the population has a more significant burden with trophic skin changes, including hyperpigmentation, lipodermatosis, and ulceration.[11]
Common clinical symptoms shared with PTS and CVI include pain, cramps, itching, swelling, skin changes, and venous ectasia. The Villalta score is specific for PTS and informs diagnosis, severity, and management stratification. [12] Venous insufficiency is similarly graded according to the CEAP scale. [13] These scoring systems are primarily based upon a thorough history and physical examination. The ultrasound exam then helps confirm the diagnosis and helps map the extent of disease, progression over time, and response to treatment.[14]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Peripheral venous drainage consists of a deep system below the investing muscular fascia and a superficial system above the fascia. Communicating or perforator veins bridge the two systems, directing blood to the deep venous systems. [15] The deep system drains approximately 85% of the total venous volume. Venous anatomy is quite variable, more so than its arterial counterpart, so knowing typical landmarks and venous variants is necessary for an accurate assessment. [15] Compared to arteries, veins are less muscular and more pliable, often more superficial, and have one-way valves preventing the backflow of blood. [16] Venous blood flow is directed towards the heart by these one-way valves, skeletal-muscular contractions, the cardiac pump, and negative intrathoracic pressure during the inspiratory phase of respiration. In turn, venous reflux is often secondary to valvular incompetence, inflammation of the vessel wall, and hemodynamic factors such as pressure gradients.[17]
The lower extremity superficial venous system's main veins include the great and lesser saphenous veins. The great saphenous vein (GSV) arises from the dorsal venous pedal arch, courses anterior to the medial malleolus and medially in the lower leg, anteromedially in the thigh, and joins the common femoral vein (a deep vein) at the femoral triangle. [18] The small saphenous vein (SSV) arises laterally from the dorsal venous pedal arch, courses posterior to the lateral malleolus, and then along the posterior leg between the gastrocnemius muscular heads. [19] The SSV usually joins the popliteal vein (a deep vein) at the knee level but may join with the GSV or extend to the thigh as the vein of Giacomini. Additionally, there are often multiple communicating veins between the GSV and the SSV. [20]
The lower extremity deep venous system consists of the anterior tibial, posterior tibial, and peroneal veins in the calf. [21] The calf veins are usually duplicated. The peroneal vein drains into the posterior tibial vein, which then joins the anterior tibial vein to become the popliteal vein posterior to the knee. The popliteal vein enters the adductor canal in the anterior thigh, becoming the femoral vein. [22] Some refer to the femoral vein portion that is upstream (or distal) to inflow from the profunda femoral vein as the superficial femoral vein; however, this leads to confusion as the 'superficial' femoral vein is a deep vein. The inguinal ligament demarcates the common femoral and external iliac vein junction. [23]
There are approximately 150 perforator veins with one-way valves that direct blood from the superficial to the deep venous systems. [24]
The upper limb veins have variable anatomy in regards to both number and position. The brachial vein is the predominant deep venous drainage structure. The brachial vein can be single or duplicated. [25] It courses anteriorly in the arm, often accompanying the brachial artery and nerves. It originates from the union of the radial and ulnar veins at the cubital fossa and becomes the axillary vein at the teres major's border. The axillary vein becomes the subclavian vein at the lateral border of the first rib, which, in turn, becomes the brachiocephalic vein after joining with the internal jugular vein posterior to the sternoclavicular joint. [26]
The upper extremity superficial system's main veins include the cephalic and basilic veins. The cephalic vein has an anterolateral course in the forearm before giving off the medial cubital vein, draining into the axillary vein. [27] The basilic vein has an anteromedial course in the forearm and continues medially to the upper arm's biceps brachii. The basilic vein drains into the brachial vein just before the brachial vein becomes the axillary vein at the teres major's border.
Indications
For evaluation of acute thromboembolic disease: suspicion for DVT or PE (such as likely pretest probability of DVT via pulmonary embolism rule-out criteria or Wells score), positive D-dimer.
For evaluation of chronic venous insufficiency: symptomatic varicose veins, asymptomatic varicose veins in patients considering treatment, symptoms of venous hypertension with outward manifestations, recurrence post-treatment.
Equipment
A 5.0 to 7.5 MHz or higher linear transducer is preferred. A curved 3.5 to 5.0 MHz transducer may benefit in challenging cases as it would provide a deeper and wider field view.
Personnel
Ultrasound technicians perform the majority of peripheral ultrasound examinations. Any appropriately trained physician is qualified, and point-of-care ultrasound provided by competent providers can help triage care.
Technique or Treatment
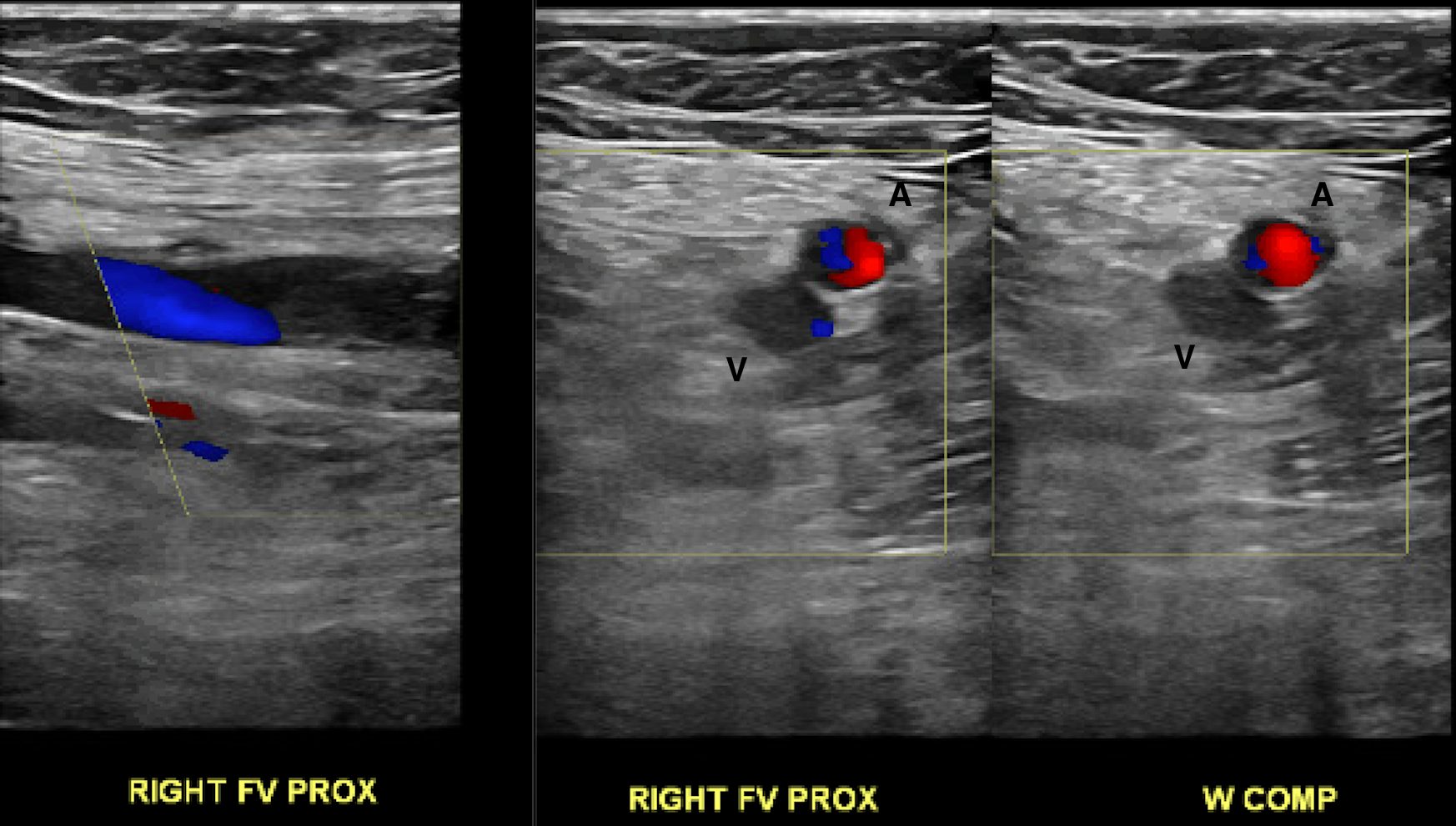
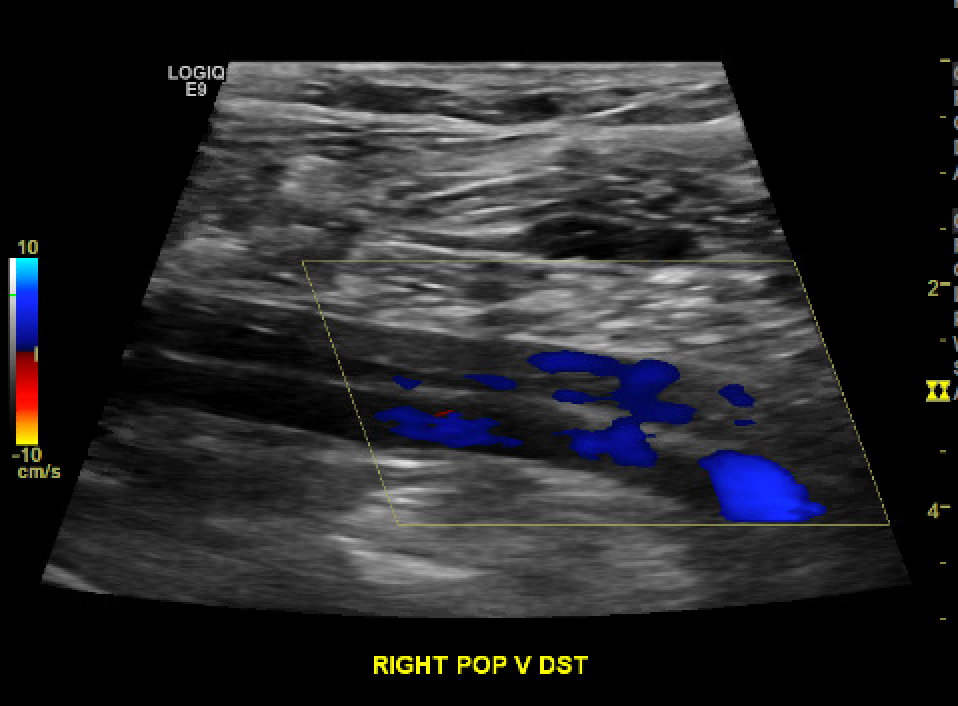
When evaluating deep venous thrombosis, duplex ultrasound with segmental compression every 2 cm is the preferred modality. The patient is positioned supine with the patient's head slightly elevated to promote venous pooling. The patient's leg is externally rotated, and the knee is slightly bent (frog-leg positioning), optimizing the conspicuity of the femoral and popliteal veins. The exam should extend from the common femoral vein at the inguinal ligament to the distal calf veins (anterior tibial, posterior tibial, and peroneal veins). [1][28][1] It is important to note that over 10% of patients may have a duplicated femoral vein, which, if present, should be interrogated (see Image. Chronic Nonocclusive DVT Within a Duplicated Popliteal Vein). While rare, isolated profunda femora clots can also be missed if not part of the routine exam. Thrombosis in the greater saphenous vein (a superficial vein) within 3 cm of the saphenofemoral junction is often treated the same as deep venous thrombosis due to the high likelihood of propagation.[29]
Proper compression is paramount. [30] The probe should be perpendicular to the vein, and pressure should be applied until the artery is compressed slightly. Lack of coaptation of the vessel wall during graded compression is the most sensitive parameter, and complete coaptation effectively rules out venous thrombosis. Color Doppler helps evaluate the degree of flow obstruction. Grayscale can help age and characterize the thrombus. [31]
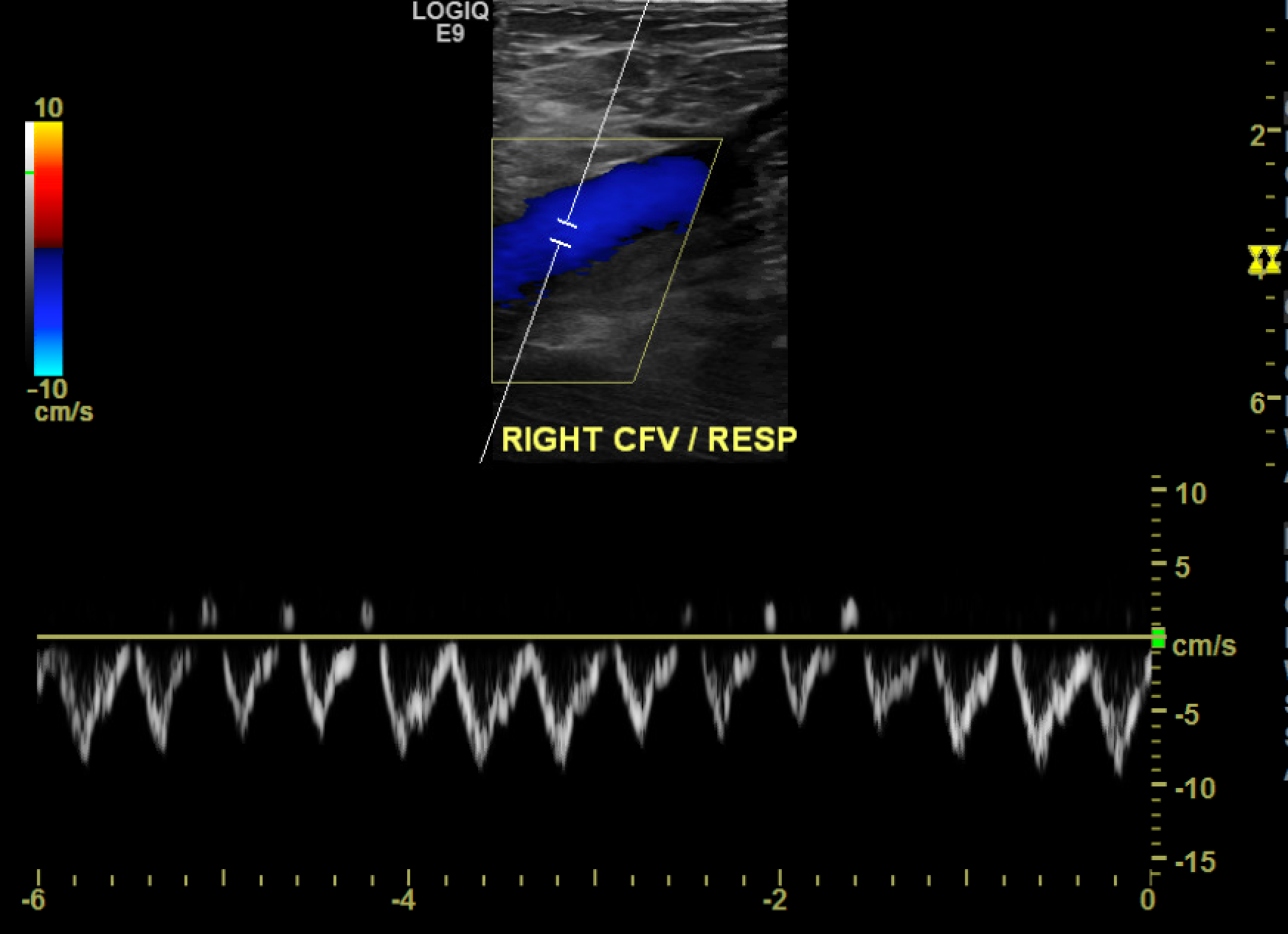
Only color and spectral Doppler are used at the proximal common femoral and popliteal veins since satisfactory compression is technically infeasible in these areas (see Image. Color Doppler Depicting Loss of Phasicity in the Common Femoral Vein). At the common femoral vein, spectral Doppler evaluates for cardiac pulsatility and respiratory phasicity, which indirectly suggest patency between the common femoral vein and the heart as an occlusive disease would prevent the expected propagation of these cyclical changes (see Image. Normal Respiratory Phasicity). Similarly, augmentation of the calf veins by manual compression should manifest as a sharp peak in the popliteal waveform, indicating at least partial patency of the deep venous structures in the calf. [32] This maneuver is particularly helpful as the infra-popliteal veins are often problematic to delineate due to overlying edema or trophic skin changes.[1]
The upper extremity deep venous system can similarly undergo interrogation. The patient is positioned supine, with the patient's head rotated away from the side being evaluated to delineate the internal jugular and subclavian veins better. An infraclavicular approach often identifies the subclavian vein, while the central portion is often best seen from a supraclavicular approach. The arm is externally rotated and repositioned as needed to assess the arm veins. In areas where compression is not feasible due to overlying bony prominences such as the subclavian vein, the patient can be asked to take a sniff of air or Valsalva. The vein is watched in real-time during the maneuver to ensure a proper venous response (venous collapse during inspiration or sniffing and enlargement during Valsalva). It is helpful to distinguish between acute venous thrombosis and chronic postthrombotic change. Acute thrombus manifests as hypoechoic or anechoic material within the vessel lumen. [33] The vein tends to be distended by thrombus and is often larger than the paired artery. Also, there may be a central free-floating component. See Image. Acute, Occlusive Deep Venous Thrombosis Hypoechoic Thrombus Distends the Vein.
After an acute episode, the vein may completely heal and appear normal or scar down. Over time, the thrombus becomes more echogenic and adherent to the wall. [34] Wall thickening and formation of synechiae/fibrous cords may result in a degree of chronic obstruction. Valvular thickening may result in valvular abnormalities such as restricted cusp motion. These chronic changes often result in a diminutive appearance of the vessel with compensatory collateral formation. [35]
Several technical and interpretative errors require monitoring: lack of adequate compression, mislabeling of the venous structures by the sonographer, and incorrect ultrasound gain and color settings. While color flow is often absent in acute thrombus, flow can be seen in a partially occlusive or recanalized thrombus. Further, slow flow can appear as mobile low-level echoes that could be mistaken for thrombosis.[36]
When evaluating venous insufficiency, an attempt is made to recreate the conditions contributing to venous insufficiency. The patient, if able to, is asked to stand with weight over the leg not being evaluated. The patient is asked to relax the musculature in the leg of interest. The knees are in slight flexion. The greater saphenous vein is best evaluated by first finding the saphenofemoral junction. [37] The small saphenous vein is best evaluated by finding its origin at the calf and followed the vein until termination. The veins are examined with an axial grayscale technique. Vein diameters are measured, and major tributaries are followed to their termination. Varicose tributaries can be traced to visible varicose veins, confirming their etiology. The deep venous system is interrogated with the patient supine, similar to the positioning for evaluation of deep venous thrombosis.
Average measurements are up to 5 mm for the greater saphenous and up to 3 mm for the smaller saphenous veins. [38] These are often significantly dilated and can measure up to 20 mm with long-standing insufficiency. Maximal dimensions are seen just caudal to an incompetent valve. Likewise, any perforators that measure greater than 3.5 mm are likely incompetent.[39]
The saphenofemoral junction is assessed for reflux in longitudinal and short-axis views. The first competent valve can be identified by asking the patient to perform a Valsalva maneuver. The increased intraabdominal pressure will cause reflux past any damaged or incompetent valves. Each vein is then evaluated with color Doppler while sequential compression and relaxation are applied to a more peripheral segment. This can be performed manually or with a sequentially inflated and deflated blood pressure cuff. For example, one could position an automatic blood pressure cuff distal to the vein segment being interrogated, inflate the cuff to 80 mmHg, wait 4 seconds, and then deflate the cuff. The duration of reversed color flow after relaxation is measured. Pulsed Doppler helps quantify the reflux time and volume. Any reflux or reversed flow lasting longer than 0.5 seconds in the superficial system or 1.0 seconds in the deep system is considered clinically significant.[40][41]
When monitoring for progression, it is essential to keep any confounding factors to a minimum and perform the exam with similar positioning, reflux-eliciting maneuver, and time of day.[42]
Clinical Significance
Acute DVT and chronic venous disease are common conditions with a significant burden on patient morbidity, mortality, and quality of life. The Venous Insufficiency Epidemiological and Economical Study (VEINES) showed a correlation between varicosities, venous disease, and decreased life scores.[43] Duplex ultrasonography of the peripheral venous system allows accurate, rapid assessment of venous disease. It can delineate the extent of the disease, guiding management. The exam is non-invasive and offers no radiation dose to the patient. Moreover, it is easy to learn and simple to perform, costly, and widely available.
Enhancing Healthcare Team Outcomes
Diagnosing and treating venous disease requires cooperation and communication between multiple healthcare providers. There is often overlap between who is medically managing and treating the patient. Peripheral venous disease is at an interface involving interventional radiologists, interventional cardiologists, vascular surgeons, hematologists/oncologists, and primary care physicians. This interplay can lead to confusing and sometimes counterproductive recommendations. A collaborative effort such as a specialized vein clinic with clearly defined roles is one of the current solutions to ensure the best possible outcomes.[44] [Level 3]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)

Chronic Venous Insufficiency. The I stands for inflation of the blood pressure cuff; D for deflation. The duration of flow reversal after deflation is measured. Valvular insufficiency is considered for times greater than 0.5 seconds for superficial and greater than 1.0 seconds for deep veins.
Contributed by E Negussie, MD
References
Needleman L, Cronan JJ, Lilly MP, Merli GJ, Adhikari S, Hertzberg BS, DeJong MR, Streiff MB, Meissner MH. Ultrasound for Lower Extremity Deep Venous Thrombosis: Multidisciplinary Recommendations From the Society of Radiologists in Ultrasound Consensus Conference. Circulation. 2018 Apr 3:137(14):1505-1515. doi: 10.1161/CIRCULATIONAHA.117.030687. Epub [PubMed PMID: 29610129]
Level 3 (low-level) evidenceMalgor RD, Labropoulos N. Diagnosis of venous disease with duplex ultrasound. Phlebology. 2013 Mar:28 Suppl 1():158-61. doi: 10.1177/0268355513476653. Epub [PubMed PMID: 23482553]
Meissner MH. Duplex follow-up of patients with DVT: does it have clinical significance? Seminars in vascular surgery. 2001 Sep:14(3):215-21 [PubMed PMID: 11561283]
Sharafuddin MJ,Sun S,Hoballah JJ,Youness FM,Sharp WJ,Roh BS, Endovascular management of venous thrombotic and occlusive diseases of the lower extremities. Journal of vascular and interventional radiology : JVIR. 2003 Apr; [PubMed PMID: 12682198]
Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation. 1996 Jun 15:93(12):2212-45 [PubMed PMID: 8925592]
Liu D, Peterson E, Dooner J, Baerlocher M, Zypchen L, Gagnon J, Delorme M, Sing CK, Wong J, Guzman R, Greenfield G, Moodley O, Yenson P, Interdisciplinary Expert Panel on Iliofemoral Deep Vein Thrombosis (InterEPID). Diagnosis and management of iliofemoral deep vein thrombosis: clinical practice guideline. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2015 Nov 17:187(17):1288-1296. doi: 10.1503/cmaj.141614. Epub 2015 Sep 28 [PubMed PMID: 26416989]
Level 1 (high-level) evidenceKahn SR, Ginsberg JS. Relationship between deep venous thrombosis and the postthrombotic syndrome. Archives of internal medicine. 2004 Jan 12:164(1):17-26 [PubMed PMID: 14718318]
Spiridon M,Corduneanu D, Chronic Venous Insufficiency: a Frequently Underdiagnosed and Undertreated Pathology. Maedica. 2017 Jan [PubMed PMID: 28878840]
Jawien A. The influence of environmental factors in chronic venous insufficiency. Angiology. 2003 Jul-Aug:54 Suppl 1():S19-31 [PubMed PMID: 12934754]
Level 2 (mid-level) evidenceNicolaides AN, Cardiovascular Disease Educational and Research Trust, European Society of Vascular Surgery, ,The International Angiology Scientific Activity Congress Organization, International Union of Angiology, Union Internationale de Phlebologie at the Abbaye des Vaux de Cernay. Investigation of chronic venous insufficiency: A consensus statement (France, March 5-9, 1997). Circulation. 2000 Nov 14:102(20):E126-63 [PubMed PMID: 11076834]
Level 3 (low-level) evidenceOklu R, Habito R, Mayr M, Deipolyi AR, Albadawi H, Hesketh R, Walker TG, Linskey KR, Long CA, Wicky S, Stoughton J, Watkins MT. Pathogenesis of varicose veins. Journal of vascular and interventional radiology : JVIR. 2012 Jan:23(1):33-9; quiz 40. doi: 10.1016/j.jvir.2011.09.010. Epub 2011 Oct 26 [PubMed PMID: 22030459]
Galanaud JP,Holcroft CA,Rodger MA,Kovacs MJ,Betancourt MT,Wells PS,Anderson DR,Chagnon I,Le Gal G,Solymoss S,Crowther MA,Perrier A,White RH,Vickars LM,Ramsay T,Kahn SR, Comparison of the Villalta post-thrombotic syndrome score in the ipsilateral vs. contralateral leg after a first unprovoked deep vein thrombosis. Journal of thrombosis and haemostasis : JTH. 2012 Jun [PubMed PMID: 22646832]
Zegarra TI, Tadi P. CEAP Classification Of Venous Disorders. StatPearls. 2024 Jan:(): [PubMed PMID: 32491342]
Soosainathan A, Moore HM, Gohel MS, Davies AH. Scoring systems for the post-thrombotic syndrome. Journal of vascular surgery. 2013 Jan:57(1):254-61. doi: 10.1016/j.jvs.2012.09.011. Epub 2012 Nov 20 [PubMed PMID: 23182156]
Level 1 (high-level) evidenceMeissner MH. Lower extremity venous anatomy. Seminars in interventional radiology. 2005 Sep:22(3):147-56. doi: 10.1055/s-2005-921948. Epub [PubMed PMID: 21326687]
Tucker WD, Arora Y, Mahajan K. Anatomy, Blood Vessels. StatPearls. 2023 Jan:(): [PubMed PMID: 29262226]
Raffetto JD. Pathophysiology of Chronic Venous Disease and Venous Ulcers. The Surgical clinics of North America. 2018 Apr:98(2):337-347. doi: 10.1016/j.suc.2017.11.002. Epub 2018 Jan 5 [PubMed PMID: 29502775]
Shah DM, Chang BB, Leopold PW, Corson JD, Leather RP, Karmody AM. The anatomy of the greater saphenous venous system. Journal of vascular surgery. 1986 Feb:3(2):273-83 [PubMed PMID: 3944931]
Schweighofer G, Mühlberger D, Brenner E. The anatomy of the small saphenous vein: fascial and neural relations, saphenofemoral junction, and valves. Journal of vascular surgery. 2010 Apr:51(4):982-9. doi: 10.1016/j.jvs.2009.08.094. Epub [PubMed PMID: 20022210]
Baliyan V, Tajmir S, Hedgire SS, Ganguli S, Prabhakar AM. Lower extremity venous reflux. Cardiovascular diagnosis and therapy. 2016 Dec:6(6):533-543. doi: 10.21037/cdt.2016.11.14. Epub [PubMed PMID: 28123974]
Hochauf S, Sternitzky R, Schellong SM. [Structure and function of the peripheral venous system]. Herz. 2007 Feb:32(1):3-9 [PubMed PMID: 17323029]
Migirov A, Arbor TC, Vilella RC. Anatomy, Abdomen and Pelvis: Adductor Canal (Subsartorial Canal, Hunter Canal). StatPearls. 2024 Jan:(): [PubMed PMID: 32310506]
Lytle WJ. Inguinal anatomy. Journal of anatomy. 1979 May:128(Pt 3):581-94 [PubMed PMID: 468709]
Tolu I, Durmaz MS. Frequency and Significance of Perforating Venous Insufficiency in Patients with Chronic Venous Insufficiency of Lower Extremity. The Eurasian journal of medicine. 2018 Jun:50(2):99-104. doi: 10.5152/eurasianjmed.2018.18338. Epub 2018 Apr 30 [PubMed PMID: 30002576]
Anaya-Ayala JE, Younes HK, Kaiser CL, Syed O, Ismail N, Naoum JJ, Davies MG, Peden EK. Prevalence of variant brachial-basilic vein anatomy and implications for vascular access planning. Journal of vascular surgery. 2011 Mar:53(3):720-4. doi: 10.1016/j.jvs.2010.09.072. Epub 2010 Dec 8 [PubMed PMID: 21144691]
Level 2 (mid-level) evidenceYang HJ, Gil YC, Jin JD, Cho H, Kim H, Lee HY. Novel findings of the anatomy and variations of the axillary vein and its tributaries. Clinical anatomy (New York, N.Y.). 2012 Oct:25(7):893-902. doi: 10.1002/ca.22086. Epub 2012 May 23 [PubMed PMID: 22623347]
Au FC. The anatomy of the cephalic vein. The American surgeon. 1989 Oct:55(10):638-9 [PubMed PMID: 2802390]
. AIUM Practice Parameter for the Performance of a Peripheral Venous Ultrasound Examination. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2020 May:39(5):E49-E56. doi: 10.1002/jum.15263. Epub 2020 Mar 12 [PubMed PMID: 32162338]
Scovell SD, Ergul EA, Conrad MF. Medical management of acute superficial vein thrombosis of the saphenous vein. Journal of vascular surgery. Venous and lymphatic disorders. 2018 Jan:6(1):109-117. doi: 10.1016/j.jvsv.2017.08.016. Epub 2017 Oct 31 [PubMed PMID: 29097174]
Prandoni P, Lensing AW, Bernardi E, Villalta S, Bagatella P, Girolami A, DERECUS Investigators Group. The diagnostic value of compression ultrasonography in patients with suspected recurrent deep vein thrombosis. Thrombosis and haemostasis. 2002 Sep:88(3):402-6 [PubMed PMID: 12353067]
Baxter GM, McKechnie S, Duffy P. Colour Doppler ultrasound in deep venous thrombosis: a comparison with venography. Clinical radiology. 1990 Jul:42(1):32-6 [PubMed PMID: 2202536]
Kim ES, Sharma AM, Scissons R, Dawson D, Eberhardt RT, Gerhard-Herman M, Hughes JP, Knight S, Marie Kupinski A, Mahe G, Neumyer M, Poe P, Shugart R, Wennberg P, Williams DM, Zierler RE. Interpretation of peripheral arterial and venous Doppler waveforms: A consensus statement from the Society for Vascular Medicine and Society for Vascular Ultrasound. Vascular medicine (London, England). 2020 Oct:25(5):484-506. doi: 10.1177/1358863X20937665. Epub 2020 Jul 15 [PubMed PMID: 32667274]
Level 3 (low-level) evidenceLinkins LA, Stretton R, Probyn L, Kearon C. Interobserver agreement on ultrasound measurements of residual vein diameter, thrombus echogenicity and Doppler venous flow in patients with previous venous thrombosis. Thrombosis research. 2006:117(3):241-7 [PubMed PMID: 16378830]
Rubin JM, Xie H, Kim K, Weitzel WF, Emelianov SY, Aglyamov SR, Wakefield TW, Urquhart AG, O'Donnell M. Sonographic elasticity imaging of acute and chronic deep venous thrombosis in humans. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2006 Sep:25(9):1179-86 [PubMed PMID: 16929019]
Karande GY, Hedgire SS, Sanchez Y, Baliyan V, Mishra V, Ganguli S, Prabhakar AM. Advanced imaging in acute and chronic deep vein thrombosis. Cardiovascular diagnosis and therapy. 2016 Dec:6(6):493-507. doi: 10.21037/cdt.2016.12.06. Epub [PubMed PMID: 28123971]
Rose SC,Nelson TR, Ultrasonographic modalities to assess vascular anatomy and disease. Journal of vascular and interventional radiology : JVIR. 2004 Jan; [PubMed PMID: 14709684]
Souroullas P, Barnes R, Smith G, Nandhra S, Carradice D, Chetter I. The classic saphenofemoral junction and its anatomical variations. Phlebology. 2017 Apr:32(3):172-178. doi: 10.1177/0268355516635960. Epub 2016 Jul 9 [PubMed PMID: 26924361]
Joh JH, Park HC. The cutoff value of saphenous vein diameter to predict reflux. Journal of the Korean Surgical Society. 2013 Oct:85(4):169-74. doi: 10.4174/jkss.2013.85.4.169. Epub 2013 Sep 30 [PubMed PMID: 24106683]
Min RJ, Khilnani NM, Golia P. Duplex ultrasound evaluation of lower extremity venous insufficiency. Journal of vascular and interventional radiology : JVIR. 2003 Oct:14(10):1233-41 [PubMed PMID: 14551269]
Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2014 Jul 22:130(4):333-46. doi: 10.1161/CIRCULATIONAHA.113.006898. Epub [PubMed PMID: 25047584]
Zygmunt JA. Duplex ultrasound for chronic venous insufficiency. The Journal of invasive cardiology. 2014 Nov:26(11):E149-55 [PubMed PMID: 25364006]
Lurie F, Comerota A, Eklof B, Kistner RL, Labropoulos N, Lohr J, Marston W, Meissner M, Moneta G, Neglén P, Neuhardt D, Padberg F Jr, Welsh HJ. Multicenter assessment of venous reflux by duplex ultrasound. Journal of vascular surgery. 2012 Feb:55(2):437-45. doi: 10.1016/j.jvs.2011.06.121. Epub 2011 Dec 16 [PubMed PMID: 22178437]
Level 2 (mid-level) evidenceAbenhaim L, Kurz X. The VEINES study (VEnous Insufficiency Epidemiologic and Economic Study): an international cohort study on chronic venous disorders of the leg. VEINES Group. Angiology. 1997 Jan:48(1):59-66 [PubMed PMID: 8995345]
Level 2 (mid-level) evidenceChristenson JT. The impact of the creation of a venous surgical centre within the department of cardiovascular surgery at a university hospital. Phlebology. 2007:22(2):70-4 [PubMed PMID: 18268853]