Sonography Physical Principles And Instrumentation
Sonography Physical Principles And Instrumentation
Definition/Introduction
The development of sonography or medical ultrasound was built on the understanding and research of sound, which can be dated as far back as the 6th century. Specifically, sonography can be tied to as far back as the 1700s, when an Italian physicist, Lazaro Spallanzani, researched bats' navigation.[1] In the early 20th century, French physicist Paul Langevin studied more applicable ultrasound uses. He eventually laid the groundwork for SONOR (Sound Navigation and Ranging) as he was commissioned to investigate the sunken Titanic. One of the first medical applications of ultrasound was by Ian Donald in the 1950s. As Ian Donald extrapolated ultrasound's ability to detect flaws and cracks in steel welds, he began researching and applying it to obstetrics and gynecology. He published a fetus at 14 weeks gestational age in the prestigious academic journal, The Lancet.
Basic Ultrasound Physics
As the name implies, ultrasound uses sound waves at a higher frequency than audible for the human ear. For example, the average human ear hears frequencies from the 20 hertz to 20 kHz (kilohertz) range, whereas the typical clinical ultrasound uses frequencies from 1 to 20 MHz (megahertz). A simple understanding of sinusoidal waves is useful for understanding ultrasound better. Wavelength (λ) is inversely proportional to frequency (f) and proportionate to the velocity (v), as depicted in the following equation.
- λ=v/f
Modern-day ultrasounds utilize piezoelectricity technology to convert electricity to sound waves and convert sound waves back into electricity through lead zirconate titanate (PZT), human-made crystals (also called ceramic). The vibration occurs as these crystals are electrically stimulated, which produces longitudinal sound waves in the previously described frequency, thus producing ultrasound waves.[2] PZT crystals are arranged in unique schematics within an ultrasound transducer (also known as a probe) to be clinically applied in various situations. Further details on the variation of these transducers be discussed later.
Like a rock being dropped into a still pond with the waves bouncing off the shore, the sound waves emitted by the transducer resonate and refract back to the transducer. When the ultrasound reverberates off tissue and returns, it induces vibration into the PZT crystals, which are converted back into an electric signal. The processing computer within the ultrasound machine then generates the signals into 2D (2 dimensional) and, in more recent times, 3D (3 dimensional) images. The frequency (and, inversely, wavelength) of the sound waves produced can be modified to produce greater penetration through soft tissue by modifying the power or gain. The lower the frequency, the deeper or greater the sound wave penetrance. Unfortunately, the more penetrance is obtained, the poorer the image quality. Lastly, some common terminology unique to the ultrasound field be discussed. Hyperechoic is defined by an object that is brighter than the surrounding tissue. Hypoechoic or anechoic is the opposite, by which an object is darker. Both of which are determined by the density of the object.
Instrumentation
The following section focus on a variety of transducers. Each transducer is shaped uniquely and has a specific and intentional arrangement of PZT crystals to produce a distinct image. Each ceramic arrangement has a specific use for various clinical scenarios. The phased array transducer is one of the physically smaller probes with a usefully small footprint. Specifically, the phased array probe’s footprint can view between ribs to better view the heart or lungs. Due to the probe's size, the periphery and superficial image have poor focus and detail compared to other transducers. In addition to the footprint, the phased array transducers utilize every crystal within the probe to produce the image.[3] See Image. Ultrasound Phased Array Probe.
The linear transducer does not directly utilize every crystal, but each element is electrically stimulated or vibrated and subsequently excites adjacent crystals. Each crystal within the linear transducer is not given direct electrical stimulation. The advantage of the linear probe is a larger field of view and a higher superficial focus. Common clinical applications of this probe are within the soft tissue and more superficial structures. See Image. Ultrasound Linear Probe.
Endocavity probes are one of the most unique in appearance. The wand-like shape and long neck allow sonographers to extend into orifices such as the oropharynx, rectum, or vagina (also called a transvaginal probe). Common clinical applications include surveillance of early pregnancies. Transabdominal imaging may not provide optimal imaging in smaller fetuses, but it can be diagnosed and used as a therapeutic tool in draining parapharyngeal abscesses. See Image. Endocavity Ultrasound Probe.
Lastly, the curvilinear or curved-array transducer has a convex shape, whereas the 2 previously described transducers have a more linear shape. The curvilinear probe typically has the largest footprint of the 3. This probe is utilized in the endocervical, intrauterine, or abdominal cavity. The image produced from the probe is “C” shaped, which is reflective of the probe’s shape. Superficial focus is poor, but the advantage is a wider field of view overall.[4] See Image. Ultrasound Curvilinear Probe. With advances in ultrasonography, new research has brought products into the market that utilize a silicon chip in place of piezo crystals that have enhanced ultrasound machines' ability to access multiple images, guiding care beyond PZT imaging.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
To obtain ideal images when scanning human tissue, a sonographer must understand how to optimize its resolution. The following section focuses on the properties of ultrasound to achieve such images and the common artifacts produced from imperfections in both the tissue and the ultrasound that may cause major issues in the final images. A sound wave penetrates deeper into a particular cavity; more interference is met. As such, the reverberant sound waves weaken as they reflect on the transducer. To optimize image quality, one must alter the gain. Altering the gain does not affect the sound wave impulse sent from the transducer but amplifies what is echoed back and received by the PZT crystals. If the produced image is still suboptimal despite optimizing the gain, the intensity or power of the sound wave emitting can be changed. If the image produced is too dim, then the strength of the sound waves may be changed by altering the power. The greater the power of the sound waves, the deeper the waves typically penetrate. Power is measured in decibels (dB). As described above, by increasing the frequency of PZT crystals, a louder sound wave with greater power penetrates deeper but at the cost of more artifacts in the resulting image.
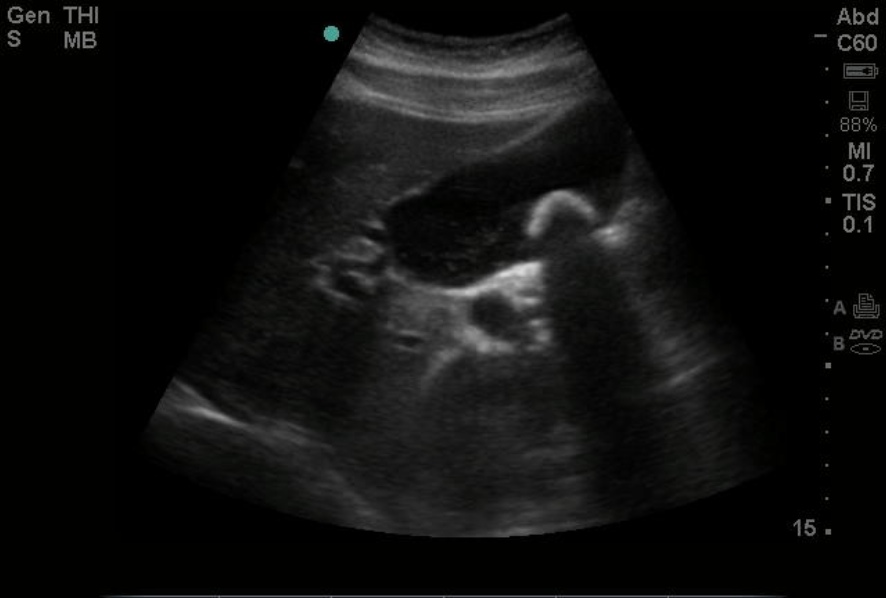
Despite proper optimization, several errors or artifacts can occur in the production of images. In a perfect world, there are some assumptions used to make these images. These assumptions are that sound waves travel in a straight line and reflect in that same line, sound travels at 1,540 m/s, and that the only source of sound wave production is the transducer; any deviation from these assumptions produces artifacts. When a sound wave encounters a medium such as air, which sound waves poorly pass through compared to water-based tissue, the artifact known as a shadow is produced. This appears as a hypoechoic or anechoic area seen distal to the area of gas or air, which obscures any views within the shadow. Besides air, a common cause of shadowing is gallstones, for which the shadow can be diagnostic in identifying gallstones within a gallbladder. See Image. Gallstone on Point-of-Care Ultrasound.
Comet tails typically have the appearance opposite of a shadow. A comet tail has a hyperechoic, or white, presentation. Common causes of this artifact are 2 closely approximated objects, like a mechanical heart valve. With 2 objects close together, there is reverberation, which produces the hyperechoic streak. The last artifact to be discussed is a mirror image. The artificial mirror image is always produced in parallel with the sound beam emitted from an ultrasound transducer. Gas or air is a common culprit in producing various artifacts, and when a smooth and flat pocket of gas interacts with sound waves, it reflects in the same manner as a common household mirror. Sonographers can utilize the mirror artifact and reflective surfaces to obtain views to their advantage.[5][6][7]
Clinical Significance
The clinical significance of sonography spans across multiple medical specialties and fields. Equally important is the training and education surrounding ultrasound, as it is a heavily user-dependent skill and tool. A poor sonographer with the highest quality ultrasound can produce inadequate images. Competent training and experience are crucial. There are 3 regulatory organizations for sonographic certification; Cardiovascular Credentialing International, the American Registry of Radiologic Technologists, and the American Registry for Diagnostic Medical Sonography. Formal training takes 12-18 months and is combined with written and practical knowledge.[8] Outside of ultrasound technician training, medical residents and fellows study ultrasound. Specifically, cardiology fellows focus on cardiac application, nephrology on renal images, and formal fellowships for using ultrasound for emergency medicine residents and attendings. Lastly, radiology residency has formal training in obtaining and interpreting sonographic images.[9]
As described above, the training to properly utilize medical ultrasound has many different pathways. Collectively, technicians, nursing staff, and clinicians benefit from the information obtained from sonography. The coordination subsequently increases patient care and eventual outcomes. In summary, from the foundation of Lazaro Spallanzani’s research of sonography in bats to Ian Donald extrapolating its use from iron welds to the field of obstetrics, ultrasound is now an immediately available and safe diagnostic and therapeutic tool. The utility and applicability of ultrasound span across outpatient obstetrical offices to obtaining critical information within intensive care units. As technology develops, ultrasound machines are becoming smaller and more portable. The future of ultrasound utility continues to expand and likely span almost all medical specialties.
Nursing, Allied Health, and Interprofessional Team Interventions
Just as Ian Donald saw the use of ultrasound in the field of obstetrics, many have followed and seen its utility in other medical fields: cardiology, vascular surgery, anesthesiology, pain medicine, radiology, general surgery, nephrology, ophthalmology, urology, emergency medicine, obstetrics, and gynecology.[3][10] Ultrasound is a quick, efficient, and safe diagnostic and therapeutic tool within the medical field. Team efforts can be taken to influence medical care and decision-making.
Media
(Click Image to Enlarge)
References
Ledermann D W. [Reading Spallanzani today]. Revista chilena de infectologia : organo oficial de la Sociedad Chilena de Infectologia. 2020 Feb:37(1):64-68. doi: 10.4067/S0716-10182020000100064. Epub [PubMed PMID: 32730402]
Mantsevich SN, Yushkov KB. Optimization of piezotransducer dimensions for quasicollinear paratellurite AOTF. Ultrasonics. 2021 Apr:112():106335. doi: 10.1016/j.ultras.2020.106335. Epub 2020 Dec 30 [PubMed PMID: 33395592]
Prabhu M, Raju D, Pauli H. Transesophageal echocardiography: instrumentation and system controls. Annals of cardiac anaesthesia. 2012 Apr-Jun:15(2):144-55. doi: 10.4103/0971-9784.95080. Epub [PubMed PMID: 22508208]
Matthias I, Panebianco NL, Maltenfort MG, Dean AJ, Baston C. Effect of Machine Settings on Ultrasound Assessment of B-lines. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2020 Dec 2:40(10):2039-46. doi: 10.1002/jum.15581. Epub 2020 Dec 2 [PubMed PMID: 33289208]
Goffi A, Kruisselbrink R, Volpicelli G. The sound of air: point-of-care lung ultrasound in perioperative medicine. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 2018 Apr:65(4):399-416. doi: 10.1007/s12630-018-1062-x. Epub 2018 Feb 6 [PubMed PMID: 29411300]
Sites BD, Brull R, Chan VW, Spence BC, Gallagher J, Beach ML, Sites VR, Hartman GS. Artifacts and pitfall errors associated with ultrasound-guided regional anesthesia. Part I: understanding the basic principles of ultrasound physics and machine operations. Regional anesthesia and pain medicine. 2007 Sep-Oct:32(5):412-8 [PubMed PMID: 17961841]
Level 3 (low-level) evidenceSchafer ME. Fundamentals of High-Resolution Ultrasound in Breast Implant Screening for Plastic Surgeons. Clinics in plastic surgery. 2021 Jan:48(1):59-69. doi: 10.1016/j.cps.2020.08.001. Epub 2020 Sep 19 [PubMed PMID: 33220905]
Haynes KW. The Importance of Professional Values From Radiologic Technologists' Perspective. Radiologic technology. 2020 Jul:91(6):525-532 [PubMed PMID: 32606230]
Level 3 (low-level) evidenceShokoohi H, Duggan NM, Adhikari S, Selame LA, Amini R, Blaivas M. Point-of-care ultrasound stewardship. Journal of the American College of Emergency Physicians open. 2020 Dec:1(6):1326-1331. doi: 10.1002/emp2.12279. Epub 2020 Oct 11 [PubMed PMID: 33392540]
Guner NG, Yurumez Y, Yucel M, Alacam M, Guner ST, Ercan B. Effects of Point-of-care Ultrasonography on the Diagnostic Process of Patients Admitted to the Emergency Department with Chest Pain: A Randomised Controlled Trial. Journal of the College of Physicians and Surgeons--Pakistan : JCPSP. 2020 Dec:30(12):1262-1268. doi: 10.29271/jcpsp.2020.12.1262. Epub [PubMed PMID: 33397050]
Level 1 (high-level) evidence