Nuclear Medicine CNS Assessment, Protocols, and Interpretation
Nuclear Medicine CNS Assessment, Protocols, and Interpretation
Introduction
Computed tomography (CT) and magnetic resonance imaging (MRI) are the 2 primary diagnostic imaging modalities used to evaluate various pathologies affecting the central nervous system (CNS). Nuclear medicine examinations provide a functional and metabolic assessment of the CNS, complementing the anatomic evaluation typically performed using other modalities such as CT, MRI, and angiography.
The CNS comprises the brain and spinal cord. Nuclear medicine examinations use various radiotracers to assess the brain's metabolic activity through fludeoxyglucose F 18 (F 18 FDG) positron emission tomography (PET) or CT scans, brain perfusion, detect the presence or absence of dopaminergic neurons via dopaminergic transporter scans (DaTscans), and determine cerebrospinal fluid flow using Indium-111 or Technetium 99m (Tc 99m).
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Metabolism and Cellular Transportation
The brain primarily relies on glucose as its main source of energy. A commonly used radiotracer, 18-fluorine deoxyglucose (18F FDG), is transported across the blood-brain barrier and into neuronal cells. Once inside the neuron, F 18 FDG undergoes phosphorylation, converting it into FDG-18-6-phosphate. This phosphorylation process hinders cell exit and enables imaging and localization of areas with potentially abnormal glucose utilization.[1][2]
Areas with high F 18 FDG uptake correspond to increased cellular metabolism, while areas with low uptake correspond to decreased cellular metabolism. Therefore, F 18 FDG PET imaging evaluates both increased and reduced metabolism. This technique is also used to identify seizure foci, evaluate cortical atrophy in patients with neurodegenerative disease, and distinguish tumor necrosis from cancer recurrence.
Parkinson disease results from the loss of presynaptic dopaminergic neurons in the substantia nigra, leading to clinical manifestations such as bradykinesia, resting tremors, and other extrapyramidal symptoms.[3] Although Parkinson disease is typically diagnosed clinically, DaTscan aids in distinguishing between an essential tremor and the resting tremor seen in Parkinson disease. Ioflupane-123, a component of DaTscan imaging, contains iodine-123 and binds to presynaptic dopaminergic neurons in the striatum, facilitating diagnostic differentiation.[4]
Blood-Brain Barrier
The blood-brain barrier is a semi-permeable membrane that prevents the free diffusion of most compounds from the cerebral vasculature into the brain parenchyma.[5] However, lipophilic molecules are not prevented from traversing this barrier. Two such lipophilic molecules used in nuclear medicine CNS assessments are hexamethyl propylene amine (HMPAO) and ethyl cysteinate dimer (ECD). They provide a functional assessment of the brain because neurons take them up in proportion to regional blood flow. Technetium 99 (Tc 99) is labeled to HMPAO and ECD, and these radiotracers are valuable in evaluating cerebral blood flow in conditions such as vascular lesions, carotid stenosis-related vascular insufficiency, transient ischemic attacks, and brain death assessment. In addition, diethylene triamine penta-acetic acid (DTPA) is a hydrophilic compound labeled with Tc 99, which is specifically used to assess cerebral blood flow in cases of brain death.[6]
The American Academy of Neurology defines brain death or death by neurological criteria as the "irreversible cessation of all functions of the entire brain, including the brainstem."[7] The clinical diagnosis of brain death involves multiple components, and nuclear medicine assessment for brain death is conducted to support a clinical diagnosis in specific situations. This approach is particularly important when findings from the physical examination are difficult to obtain due to facial or ocular trauma that prevents cranial nerve testing. In brain death, there is global cerebral swelling and increased intracranial pressure, leading to shunting of blood away from the intracranial arteries.[8]
Cerebrospinal Fluid
Cerebrospinal fluid (CSF) is a clear, watery fluid produced by the choroid plexus that surrounds and protects the brain and spinal cord. CSF circulates through the CNS and is reabsorbed along the cerebral convexities.[9][10] Indium-111 DTPA, a radiotracer with a 67-hour half-life, is used to assess for normal pressure hydrocephalus and CSF leaks. Tc 99m DTPA has a much shorter half-life of 6 hours and is used to evaluate for obstruction of ventriculoperitoneal or ventriculoatrial shunts.
Indications
Nuclear medicine examinations are crucial in assessing various neurological conditions and pathologies by providing functional and metabolic insights. Below are the indications for specific evaluations using nuclear medicine techniques:
- Evaluate for a seizure focus
- Evaluate for brain death
- Evaluate for ischemia, infarction, or vascular insufficiency
- Differentiate brain tumor progression versus radiation necrosis
- Evaluate for dementia and differentiation of the different dementia subtypes
- Evaluate for normal pressure hydrocephalus
- Evaluate for a CSF leak
- Determine ventricular shunt patency
- Evaluation of parkinsonian syndromes
Contraindications
Recent anticoagulation is a relative contraindication to administering radiotracers into the thecal sac. An allergy to sulfa-containing medications is a contraindication to acetazolamide administration.[11] Pregnancy and breastfeeding are relative contraindications to any nuclear medicine examination, with pregnancy typically being a contraindication for CNS nuclear imaging studies in most cases.
Equipment
Equipment Needed for Nuclear Medicine Examinations
PET/CT scanners are essential for conducting F 18 FDG scans. Gamma cameras are required for CSF examinations and the Tc 99 DTPA brain death examination. Single-photon emission computed tomography (SPECT)/CT scanners are necessary for brain perfusion studies and DaTscans.
A glucometer is necessary for F 18 FDG examinations to monitor and ensure the patient’s blood glucose levels remain within an acceptable range. Elevated serum glucose levels could interfere with the accurate localization of F-F-18-FDG.[12] A fluoroscopy machine is recommended to facilitate uncomplicated access to the thecal sac during assessments for a CSF leak or normal pressure hydrocephalus. Additionally, cotton balls or pledgets, along with a well counter, are used when there is clinical suspicion of a skull base leak.[13]
Personnel
Effective nuclear medicine imaging requires coordinated efforts from specialized personnel who have distinct roles in ensuring accurate diagnosis, patient safety, and efficacy of nuclear medicine examinations.
- A radiologist or nuclear medicine physician determines the appropriate imaging protocol tailored to each patient's clinical needs.
- A nuclear medicine technologist interacts directly with the patient and performs the imaging procedure.
- A nuclear medicine pharmacist is critical in preparing radiotracers and ensuring proper dosing according to established protocols.
Preparation
Interictal Imaging, Dementia Assessment, and Tumor Recurrence or Necrosis Evaluation
When conducting studies to assess tumor recurrence versus radiation necrosis, dementia, or seizure focus, patients are advised to fast for a minimum of 4 hours before the brain PET/CT scan, ensuring that their blood glucose levels are below 200 mg/dL. Diabetic patients should receive long-acting insulin the night before the examination and regular-acting insulin in the morning, with scanning scheduled for the afternoon to optimize imaging conditions.[14]
Brain Perfusion Imaging
When evaluating brain perfusion using Tc 99 HMPAO or Tc 99 ECD, it is crucial to ensure optimal conditions. Patients should be positioned in a quiet, dark room with their eyes open for several minutes before intravenous radiotracer administration. This helps prevent decreased perfusion to the visual cortex, which could affect the examination. In cases where brain perfusion is assessed using acetazolamide, the medication should be administered 20 minutes before the radiotracer injection for optimal results.[6]
For patients undergoing assessment for brain death using Tc 99 DTPA, it is essential to know the coma etiology. Sedatives should be discontinued and a period equivalent to at least 5 half-lives of any sedating medication should pass before initiating the brain death assessment procedure. This ensures accurate and reliable results in determining brain death.[15]
Cerebrospinal Fluid Leaks
CSF leaks are evaluated by intrathecal administration of Indium-111 DTPA. Acetazolamide can slow the flow of CSF and should be discontinued several days before the examination. Cotton pledgets are placed in the external auditory canals and nostrils if the clinical question is a skull base leak. The patient should be brought to the fluoroscopy suite for intrathecal administration of the radiotracer, and the site for lumbar puncture should be sterilized.[13]
Similar to the CSF leak study, Indium-111 DTPA is administered intrathecally when evaluating for normal pressure hydrocephalus.[16]
In cases of suspected obstructed ventriculoperitoneal or ventriculoatrial shunts, the access site is prepared in a sterile manner, and the shunt tubing is accessed and injected with Tc 99m DTPA.[17][18][11]
DaTscan Test
As the radiotracer ioflupane contains radioactive iodine, a precautionary measure is taken by administering potassium iodide or Lugol solution (a solution containing iodide) 1 hour before the radiotracer injection. This helps prevent the thyroid gland from absorbing the radiotracer.[4]
Technique or Treatment
Interictal Imaging, Dementia Assessment, Tumor Recurrence or Necrosis Evaluation
Patients undergo imaging on the PET/CT scanner approximately one hour after receiving intravenous radiotracer. The field of view for these examinations encompasses the entire skull to ensure comprehensive visualization and assessment.[14]
Brain Perfusion Imaging
During the assessment of brain perfusion using Tc 99 HMPAO or ECD radiotracers, patients undergo imaging in a SPECT/CT scanner with the field of view encompassing the entire skull. This comprehensive imaging approach allows for a detailed evaluation of brain perfusion patterns and abnormalities.[6]
For the assessment of brain death using Tc 99 DTPA, frontal and lateral planar images of the head and neck are acquired during the arterial phase after intravenous radiotracer administration. To prevent interference from scalp vasculature, a tourniquet fastened above the ears can be used to restrict blood flow. The examination field of view should include the entire skull and the common carotid arteries for comprehensive evaluation.[6]
Cerebrospinal Fluid Imaging
When assessing for a CSF leak, patients undergo gamma camera imaging at 6 hours post-injection to confirm radiotracer presence in the thecal sac. Subsequent imaging at 24 hours includes orthogonal planar views of the spine and skull. Additional SPECT/CT imaging may be performed as needed. In suspected skull base leak cases, cotton pledgets are inserted into the patient's ears and nose before scanning for leaked radiotracer after the examination.[13]
For evaluations of normal pressure hydrocephalus, planar images are taken at 2-, 4-, 24-, and 48-hour intervals. The field of view of the initial planar images focuses on the injection site to ensure the radiotracer localization is in the thecal sac, whereas later images are of the skull.[19]
The radiotracer is injected directly into the shunt tubing when evaluating for ventriculoatrial or ventriculoperitoneal shunt obstruction. Imaging involves capturing the entire shunt at 10-second intervals every 20 seconds. A final planar image of the distal shunt is obtained at the end of the study.[17][18]
DaTscan Test
Patients undergo scanning in a SPECT/CT machine for approximately 3 to 6 hours following intravenous administration of ioflupane for DaTscan imaging. The study encompasses SPECT/CT imaging with the field of view covering the entire skull.[4]
Abbreviated Standard Operating Procedures For Each Study
Brain death imaging: Due to the critical condition of patients undergoing this study, brain death imaging typically does not require specific patient preparation. Commonly used radiotracers include Tc 99m DTPA, administered at approximately 25 millicuries. Alternatively, if Tc 99m HMPAO is utilized, the activity administered typically falls within a dose range of around 20 millicuries.
Similar to other studies, pediatric dosing considerations for this study should be based on guidelines such as those provided by the European Association of Nuclear Medicine website dosage calculator.[20] Low-energy collimators are commonly used with energy windows set to a 20% window centered at 140 keV. In some cases, using Tc 99m DTPA, a tourniquet may be necessary for optimal results.
Cisternography: Cisternography is a valuable diagnostic tool for evaluating normal pressure hydrocephalus and identifying CSF leaks.[21] Informed consent is obtained specifically for the lumbar puncture procedure during this examination. Indium-111 DTPA is administered intrathecally at a dose of 500 microcuries. A 22-gauge or smaller needle is preferred, and patients are positioned horizontally for 2 hours following injection. Imaging uses a medium-energy collimator with energy windows set at 20% for 171 and 245 keV. Delayed images are typically captured at 4 to 6 hours and again at 23 hours after injection, although additional delays can also be obtained.
- For CSF leak studies, appropriately sized pledgets (approximately 1 cm2) with an absorptive capacity of 0.5 mL of water are utilized.
- At the time of pledget removal, approximately 5 mL of venous blood is drawn into a heparinized tube. Pledget and plasma samples are then measured in a well counter, and the results are expressed as a ratio of pledget radioactivity (a ratio >1.5 of CSF or plasma is considered positive for a leak).
Ventricular shunt study: Ventricular shunt patency can be effectively assessed using either Tc 99m DTPA or Indium-111 DTPA radiotracers.[22] A typical dose of 1 millicurie is administered into the shunt reservoir for this purpose. The utilization of Tc 99m DTPA necessitates using a low-energy collimator, while Indium-111 DTPA requires a medium-energy collimator. For Indium-111 DTPA, energy windows are set at 20% for 171 and 245 keV, whereas Tc 99m DTPA imaging requires energy windows set at 20% for 140 keV.
DaTscan: DaTscans utilize iodine-123 ioflupane at a dosage typically ranging from 3 to 5 millicuries. Before imaging, a medication reconciliation is necessary to identify and cease potential interfering drugs.[23] In addition, to prevent thyroid gland uptake due to the iodine-based radiotracer, SSKI (saturated solution of potassium iodide) is administered at a dose of 100 mg (2 drops) approximately 1 hour before imaging.
Tc 99m HMPAO and Tc 99m ECD imaging: Tc 99m HMPAO studies are used for evaluating various clinical indications, including:
- Detection and localization of recurrent brain tumors
- Evaluation of Alzheimer disease
- Localization of seizure foci
- Assessment of brain death
- Evaluation of AIDS dementia
- Acetazolamide studies for mapping brain perfusion distribution in preparation for potential revascularization procedures
Approximately 20 millicuries of Tc 99m HMPAO (or Tc 99m ECD) is administered intravenously after the patient has been in a quiet room for 30 minutes. When used for seizure evaluation, imaging is conducted during both the ictal and interictal states, allowing for comparison of potential sites of increased uptake observed on ictal imaging but not on interictal imaging. Energy windows are set at 20% centered at 140 keV for optimal imaging results.
F 18 FDG brain imaging: F 18 FDG imaging of the brain can be utilized for various indications, including the evaluation of new or recurrent malignancy, assessment of dementia, and identification of seizure foci.[24] Patients undergoing F 18 FDG brain imaging typically fast for 4 to 8 hours before the exam, and strenuous exercise is discouraged 48 hours before the scan. Following the administration of the radiotracer, patients are placed in a quiet, dark environment and advised to refrain from talking to minimize brain activity.
For brain imaging using F 18 FDG, a smaller dosage of approximately 5 millicuries is typically administered.
Complications
Potential complications during intrathecal administration of radiotracer include epidural or subdural hemorrhage, meningitis, or iatrogenic CSF leak. Evaluating ventriculoperitoneal shunt patency carries the risk of introducing contaminants or bacteria into the shunt system. Patients receive potassium iodide or Lugol solution, both containing potassium, before undergoing nuclear medicine exams involving ioflupane, which contains iodine-123. This pre-administration helps prevent the thyroid from absorbing radioactive iodine.[4]
In addition to potential complications such as extravasation during intravenous injections of radiotracer, most nuclear medicine examinations are generally low-risk and do not lead to significant complications.
Clinical Significance
F 18 FDG PET/CT Scans
F 18 FDG PET/CT scans of the brain play a crucial role in supporting the clinical diagnosis of dementia, especially when the clinical presentation is ambiguous or complex. Due to the overlapping brain areas affected by various subtypes of dementia, a high pre-test probability is essential for F 18 FDG examination.
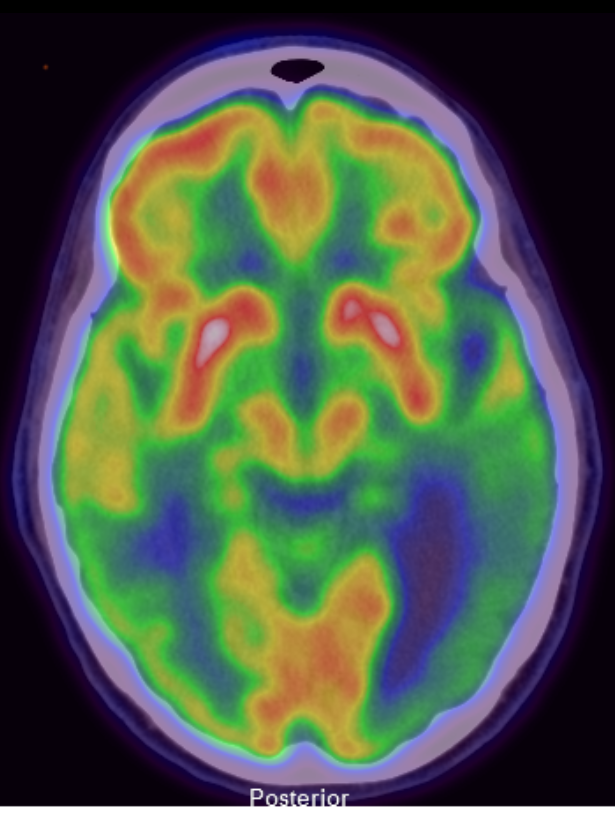
A normal brain 18F-FDG PET/CT scan typically reveals homogeneous cortical uptake throughout the brain. In patients with Alzheimer disease, a characteristic pattern of decreased radiotracer uptake is observed in the posterior cingulate cortex and bilateral posterior parietotemporal regions during the early stages of the disease. As Alzheimer disease progresses, this decreased uptake extends to involve the bilateral frontal lobes (see Image 2. F 18 FDG PET/CT Brain Scan Indicating Alzheimer Disease).[25]
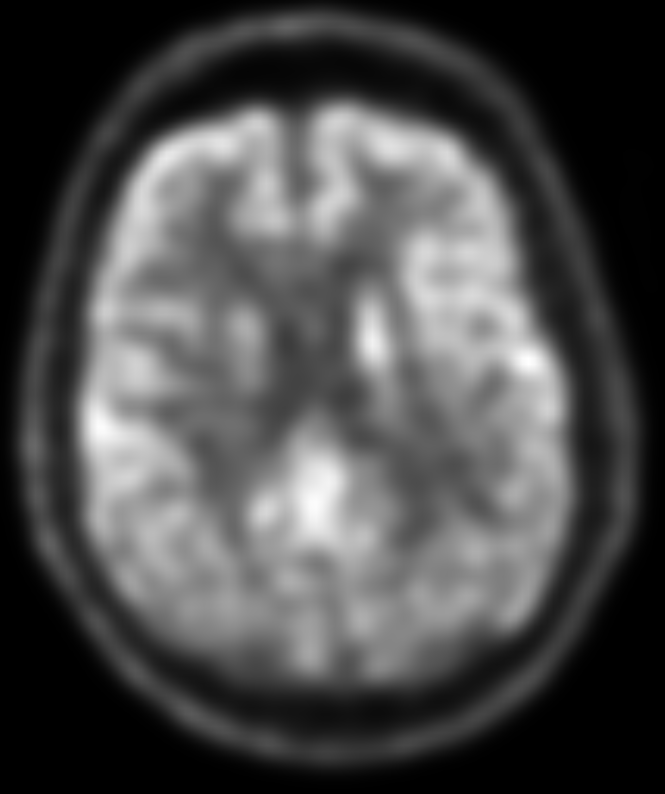
Patients with dementia with Lewy bodies also display reduced radiotracer uptake in the bilateral posterior parietotemporal regions. Additionally, there is decreased metabolism (hypometabolism) noted in the occipital cortex and caudate nuclei in these patients. A notable feature distinguishing Lewy body dementia from Alzheimer disease is the sparing of the posterior cingulate cortex, referred to as the "cingulate island sign" in Lewy body dementia cases. This distinct metabolic pattern aids in the differentiation between Lewy body dementia and Alzheimer disease (see Image 3. F 18 FDG PET/CT Brain Scan Revealing Dementia with Lewy Bodies).[26][27]
Patients diagnosed with frontotemporal dementia typically exhibit decreased radiotracer uptake in the frontal and temporal lobes, along with involvement of the anterior cingulate gyrus (see Image 5. Frontotemporal Dementia).[28] Vascular dementia, also known as multi-infarct dementia, results from multiple areas of infarction in the brain. These patients often experience a step-wise decline in cognitive function, with clinical deficits correlating to the affected areas of the brain.[29] In cases where interpretation may be challenging due to overlapping patterns or potential confounders, various systematic approaches for evaluating F 18 FDG activity have been proposed and can be beneficial for potentially confounding cases.[30]
Tumor necrosis and tumor recurrence can be distinguished on F 18 FDG PET/CT scans by assessing radiotracer uptake. Necrotic areas typically exhibit hypometabolism, while recurrent tumors demonstrate hypermetabolism.[31]
Brain Death Assessment
Tc 99 DTPA can be used for brain death assessment as an angiographic examination. In a normal Tc 99 DTPA brain examination, radiotracer distribution is observed throughout extracranial and intracranial arteries. However, in a Tc 99 DTPA brain examination of a patient determined to be brain dead, the radiotracer is visible in the common and external carotid arteries but not in the intracranial carotid arteries.
Alternatively, brain death assessment can also be performed using Tc 99 HMPAO or Tc 99 ECD with SPECT imaging (see Image 4. Brain Death Study With HMPAO). The absence of Tc 99 uptake in the brain parenchyma during this examination is highly indicative of brain death in the appropriate clinical context (see Image 6. Brain Death Assessment Using Tc99m-Pertechnetate SPECT Imaging).[6]
Brain Perfusion Imaging
Although CT or MRI perfusion evaluation is the standard for assessing brain perfusion, certain patients face limitations such as severe iodinated contrast allergies or incompatible implanted medical devices, precluding them from undergoing contrast-enhanced CT or MRI perfusion examinations. In such cases, brain perfusion can be evaluated using SPECT/CT with Tc 99m HMPAO or ECD. A normal brain perfusion SPECT/CT scan typically reveals uniform and homogeneous radiotracer uptake in the brain cortex, with relatively less uptake observed in the white matter, maintaining a homogeneous pattern. Conversely, areas of ischemia or infarction demonstrate reduced or absent radiotracer uptake, respectively.[6]
Acetazolamide, a carbonic anhydrase inhibitor, may be administered following radiotracer administration in cases where there are concerns about compromised vascular supply due to conditions such as carotid stenosis, vascular pathology, or prior transient ischemic attacks. The administration of acetazolamide results in cerebral vasodilation by elevating intravascular CO2 levels, thereby increasing cerebral blood flow in normal brain parenchyma by up to 30%. However, regions of the brain with preexisting decreased perfusion are unable to further dilate post-acetazolamide administration. As a result, these abnormal areas will not exhibit increased radiotracer uptake following acetazolamide administration.[32]
Seizure Focus
Nuclear medicine examinations become invaluable when conventional first-line diagnostic tools such as MRI and/or EEG fail to provide conclusive or diagnostic results for seizure foci. The assessment for seizure focus using radiotracers presents 2 primary approaches—ictal and interictal imaging. Ictal imaging involves continuous EEG monitoring of hospitalized patients, and Tc 99 HMPAO is injected when they begin to exhibit seizure activity. Tc 99 HMPAO is preferred because the radiotracer persists in the seizure focus for several hours after administration, thereby allowing imaging after seizure activity subsides.[33]
The brain parenchyma involved in the seizure exhibits heightened activity compared to non-involved areas, leading to the accumulation of radiotracer in the seizure focus. Consequently, imaging will depict a cortical region with relatively increased radiotracer uptake. Notably, F 18 FDG brain PET/CT is not a feasible or recommended choice for ictal imaging. This is because the examination requires the patient to remain still in a quiet environment for an hour after radiotracer administration, which is challenging during a seizure episode.
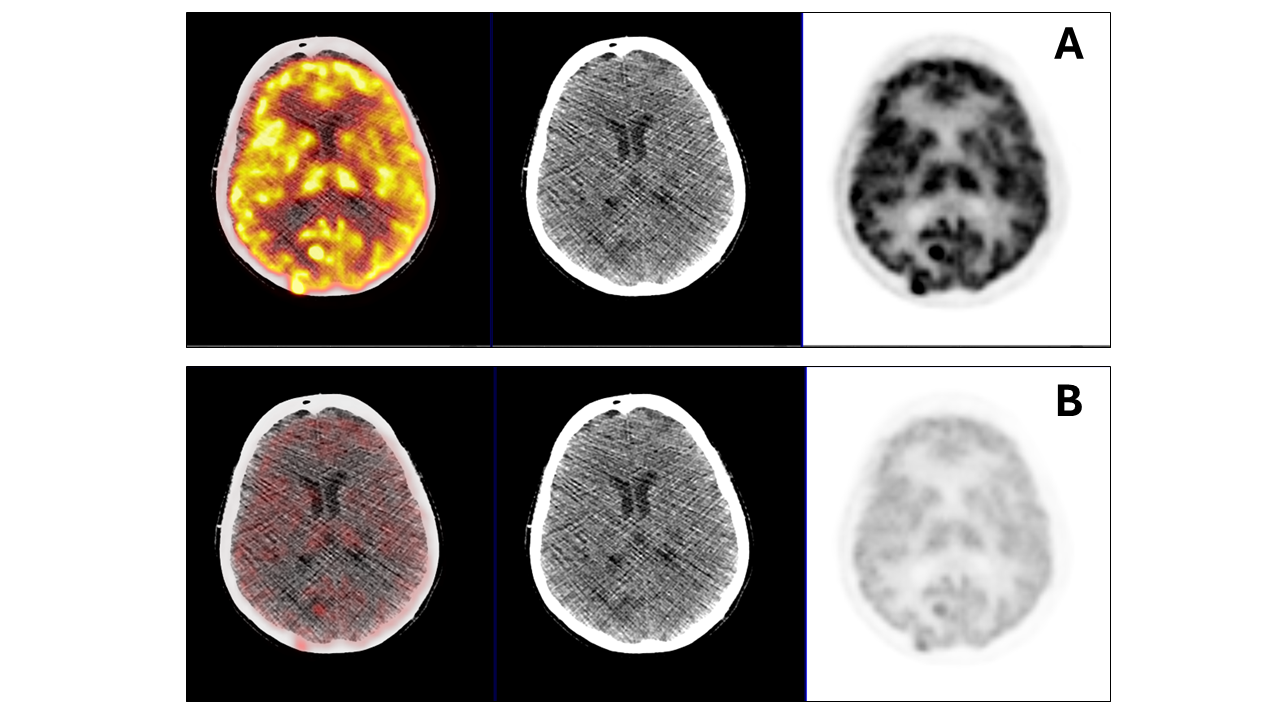
Interictal assessment involves an F 18 FDG PET/CT of the brain when the patient is in between seizures, although it is not as sensitive as an ictal examination. A positive F 18 FDG PET/CT will reveal an area of relatively decreased metabolism. Notably, nuclear medicine assessment for seizure foci beyond the temporal lobe is less precise, and potential extratemporal lobe lesions may not be evident on either ictal or interictal imaging (see Image 1. F 18 FDG PET/CT Brain Scan Revealing Lesions).[34][35]
Cerebrospinal Fluid Analysis
Evaluation for CSF abnormalities, including CSF leaks, ventriculostomy shunt failure, or communicating hydrocephalus, is typically pursued when clinical examinations, CT scans, and/or MRI results are inconclusive or contraindicated. This assessment involves administering radiotracer either directly into the thecal sac or through the ventriculostomy catheter.
A normal cisternogram during the assessment for normal pressure hydrocephalus will demonstrate the radiotracer ascending the spinal canal. After 24 hours, the radiotracer will be absent from the lateral ventricles and perimesencephalic cisterns, and will be present in the bilateral cerebral convexities. In patients with communicating hydrocephalus, the radiotracer may or may not clear from the perimesencephalic cisterns and will persist in the lateral ventricles beyond 24 hours.[36]
When evaluating for a CSF leak, the presence of Indium-111 DTPA accumulating outside of the thecal sac is indicative of a potential CSF leak. After the study, the cotton pledgets placed in the nostrils and ears are removed and analyzed using a well counter. The presence of radioactive material in the pledgets suggests a leak at the skull base.[13]
Suspected Cerebrospinal Fluid Shunt Failure
An unobstructed, properly functioning ventriculoperitoneal or ventriculoatrial shunt system allows immediate passage of the radiotracer into the distal aspect of the shunt, diffusing throughout the peritoneum or right atrium. Conversely, an obstructed ventriculoperitoneal shunt will exhibit significantly delayed or no passage of radiotracer into the distal part of the system. To exclude the possibility of an adhesion or fibrous cap obstructing the distal tip, the radiotracer must diffuse beyond the tip of the distal shunt tube.[37]
DaTscan
A normal DaTscan demonstrates radiotracer uptake in the caudate and putamen regions, forming a shape resembling a comma. In Parkinson disease, where presynaptic dopaminergic neurons are lost, the radiotracer uptake in the putamen diminishes, causing the radiotracer uptake pattern to appear as a sphere in the caudate head area on a DaTscan.[4] As the disease progresses, reduced activity in the caudate can also become evident.
Enhancing Healthcare Team Outcomes
The interprofessional healthcare team involved in a nuclear medicine study of the CNS comprises a clinician who orders the study, a nuclear medicine technologist responsible for preparing the patient and administering the exam, a nuclear pharmacist who prepares the radiotracer, and a nuclear medicine physician overseeing the study.
Optimizing patient outcomes requires seamless collaboration among the multidisciplinary healthcare team. The clinician ordering the scan should utilize first-line diagnostic tools to refine the differential diagnosis before proceeding with the nuclear medicine assessment. The nuclear medicine technologist must adhere to department protocols while conducting the examination. The nuclear medicine pharmacist is responsible for ensuring the correct dosage and safe handling of radioactive material in accordance with department protocols. The nuclear medicine physician is critical in developing tailored protocols to address specific clinical inquiries, providing supervision and support to the team, and ultimately interpreting the examination findings and communicating them clearly and succinctly to the ordering provider.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
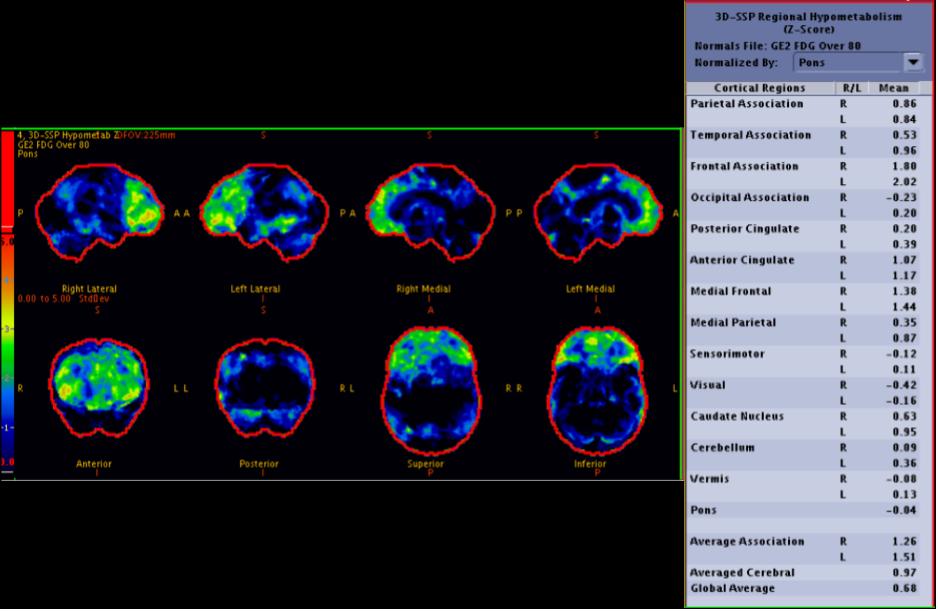
Frontotemporal Dementia. PET/CT images were obtained following intravenous injection of F18 FDG. The image shows significantly reduced F18 FDG uptake in the bilateral frontal lobes, indicated by areas highlighted in green and representing regions with elevated z scores. The z score in this study quantifies the standard deviations by which the patient's mean F18 uptake in a specific brain area differs from that of an age-matched group. Higher z scores are indicative of reduced metabolism in those areas. The notably elevated z scores in the frontal cortices strongly suggest a diagnosis consistent with frontotemporal dementia.
Images contributed by Mathew Chakko, MD
(Click Image to Enlarge)

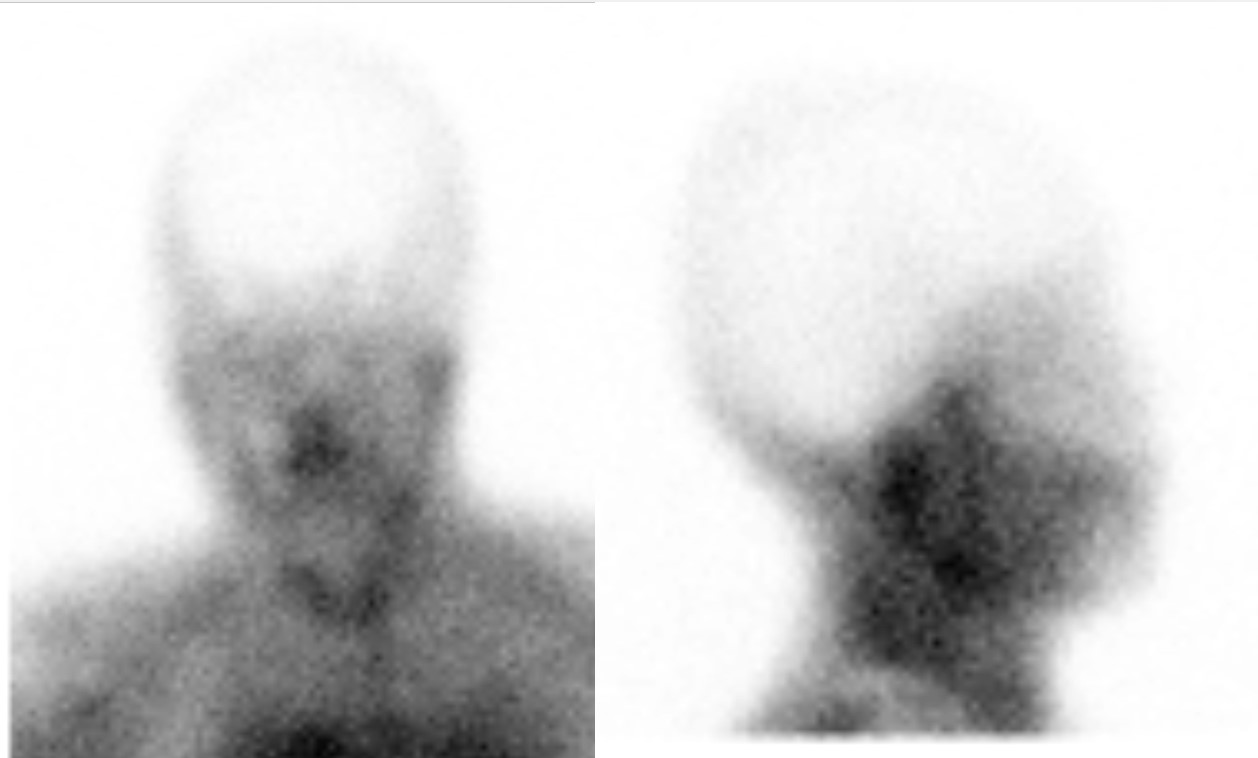
Brain Death Assessment Using Tc99m-Pertechnetate SPECT Imaging. Planar SPECT images obtained 30 minutes after intravenous injection of Tc99m-pertechnetate depict absent radiotracer activity across the bilateral cerebral hemispheres. This absence is attributed to cerebral edema and tamponade of the cerebral arterial vasculature. Due to the lack of flow via the internal carotid arteries, blood redistributes to the external carotid arteries, supplying the face and nose. Consequently, the characteristic "hot nose" sign is observed, marked by increased radiotracer activity in the nasal mucosa (red arrow).
Images contributed by Mathew Chakko, MD
References
Maldonado KA, Alsayouri K. Physiology, Brain. StatPearls. 2024 Jan:(): [PubMed PMID: 31869182]
Ashraf MA, Goyal A. Fludeoxyglucose (18F). StatPearls. 2024 Jan:(): [PubMed PMID: 32491585]
Zafar S, Yaddanapudi SS. Parkinson Disease. StatPearls. 2024 Jan:(): [PubMed PMID: 29261972]
Banks KP, Peacock JG, Clemenshaw MN, Kuo PH. Optimizing the Diagnosis of Parkinsonian Syndromes With (123)I-Ioflupane Brain SPECT. AJR. American journal of roentgenology. 2019 Aug:213(2):243-253. doi: 10.2214/AJR.19.21088. Epub 2019 Apr 17 [PubMed PMID: 30995085]
Gawdi R, Shumway KR, Emmady PD. Physiology, Blood Brain Barrier. StatPearls. 2024 Jan:(): [PubMed PMID: 32491653]
Kaechele AP, Chakko MN. Nuclear Medicine Cerebral Perfusion Scan. StatPearls. 2024 Jan:(): [PubMed PMID: 35881740]
Lewis A, Kirschen MP, Greer D. The 2023 AAN/AAP/CNS/SCCM Pediatric and Adult Brain Death/Death by Neurologic Criteria Consensus Practice Guideline: A Comparison With the 2010 and 2011 Guidelines. Neurology. Clinical practice. 2023 Dec:13(6):e200189. doi: 10.1212/CPJ.0000000000200189. Epub 2023 Oct 11 [PubMed PMID: 37829552]
Level 1 (high-level) evidenceStarr R, Tadi P, Weisbrod LJ, Pfleghaar N. Brain Death. StatPearls. 2024 Jan:(): [PubMed PMID: 30844186]
Telano LN, Baker S. Physiology, Cerebral Spinal Fluid. StatPearls. 2024 Jan:(): [PubMed PMID: 30085549]
Huff T, Tadi P, Weisbrod LJ, Varacallo M. Neuroanatomy, Cerebrospinal Fluid. StatPearls. 2024 Jan:(): [PubMed PMID: 29262203]
Farzam K, Abdullah M. Acetazolamide. StatPearls. 2024 Jan:(): [PubMed PMID: 30335315]
Sprinz C, Zanon M, Altmayer S, Watte G, Irion K, Marchiori E, Hochhegger B. Effects of blood glucose level on 18F fluorodeoxyglucose (18F-FDG) uptake for PET/CT in normal organs: an analysis on 5623 patients. Scientific reports. 2018 Feb 1:8(1):2126. doi: 10.1038/s41598-018-20529-4. Epub 2018 Feb 1 [PubMed PMID: 29391555]
Grantham VV, Blakley B, Winn J. Technical review and considerations for a cerebrospinal fluid leakage study. Journal of nuclear medicine technology. 2006 Mar:34(1):48-51 [PubMed PMID: 16517969]
Noble RM. (18)F-FDG PET/CT Brain Imaging. Journal of nuclear medicine technology. 2021 Sep:49(3):215-216. doi: 10.2967/jnmt.121.263000. Epub [PubMed PMID: 34479955]
Donohoe KJ, Agrawal G, Frey KA, Gerbaudo VH, Mariani G, Nagel JS, Shulkin BL, Stabin MG, Stokes MK. SNM practice guideline for brain death scintigraphy 2.0. Journal of nuclear medicine technology. 2012 Sep:40(3):198-203. doi: 10.2967/jnmt.112.105130. Epub 2012 Jun 28 [PubMed PMID: 22743146]
Level 1 (high-level) evidenceSehgal AK, Sethi RS, Raghavan S, Namgyal PA. Radionuclide cisternography: A prudent investigation in diagnosing spontaneous intracranial hypotension. Indian journal of nuclear medicine : IJNM : the official journal of the Society of Nuclear Medicine, India. 2013 Jan:28(1):42-4. doi: 10.4103/0972-3919.116815. Epub [PubMed PMID: 24019678]
Graham M. Ventricular Shunt Patency. Journal of nuclear medicine technology. 2023 Dec 5:51(4):277-278. doi: 10.2967/jnmt.123.266765. Epub 2023 Dec 5 [PubMed PMID: 37963779]
Graham MM. Practical Aspects of Nuclear Medicine Ventriculoperitoneal Shunt Evaluation. Journal of nuclear medicine technology. 2023 Dec 5:51(4):271-276. doi: 10.2967/jnmt.123.266203. Epub 2023 Dec 5 [PubMed PMID: 37963777]
Thut DP, Kreychman A, Obando JA. ¹¹¹In-DTPA cisternography with SPECT/CT for the evaluation of normal pressure hydrocephalus. Journal of nuclear medicine technology. 2014 Mar:42(1):70-4. doi: 10.2967/jnmt.113.128041. Epub 2014 Jan 24 [PubMed PMID: 24463341]
Lassmann M, Biassoni L, Monsieurs M, Franzius C, EANM Dosimetry and Paediatrics Committees. The new EANM paediatric dosage card: additional notes with respect to F-18. European journal of nuclear medicine and molecular imaging. 2008 Sep:35(9):1666-8. doi: 10.1007/s00259-008-0799-9. Epub 2008 Jun 24 [PubMed PMID: 18574583]
Vemuri NV, Karanam LSP, Manchikanti V, Dandamudi S, Puvvada SK, Vemuri VK. Imaging review of cerebrospinal fluid leaks. The Indian journal of radiology & imaging. 2017 Oct-Dec:27(4):441-446. doi: 10.4103/ijri.IJRI_380_16. Epub [PubMed PMID: 29379240]
Chiewvit S, Nuntaaree S, Kanchaanapiboon P, Chiewvit P. Assessment lumboperitoneal or ventriculoperitoneal shunt patency by radionuclide technique: a review experience cases. World journal of nuclear medicine. 2014 May:13(2):75-84. doi: 10.4103/1450-1147.139135. Epub [PubMed PMID: 25191120]
Level 3 (low-level) evidenceSeifert KD, Wiener JI. The impact of DaTscan on the diagnosis and management of movement disorders: A retrospective study. American journal of neurodegenerative disease. 2013:2(1):29-34 [PubMed PMID: 23515233]
Level 2 (mid-level) evidenceKnowlton RC. The role of FDG-PET, ictal SPECT, and MEG in the epilepsy surgery evaluation. Epilepsy & behavior : E&B. 2006 Feb:8(1):91-101 [PubMed PMID: 16406729]
Ishii K. PET approaches for diagnosis of dementia. AJNR. American journal of neuroradiology. 2014 Nov-Dec:35(11):2030-8. doi: 10.3174/ajnr.A3695. Epub 2013 Aug 14 [PubMed PMID: 23945233]
Kantarci K, Boeve BF, Przybelski SA, Lesnick TG, Chen Q, Fields J, Schwarz CG, Senjem ML, Gunte JL, Jack CR, Min P, Jain M, Miyagawa T, Savica R, Graff-Radford J, Botha H, Jones DT, Knopman DS, Graff-Radford N, Ferman TJ, Petersen RC, Lowe VJ. FDG PET metabolic signatures distinguishing prodromal DLB and prodromal AD. NeuroImage. Clinical. 2021:31():102754. doi: 10.1016/j.nicl.2021.102754. Epub 2021 Jul 4 [PubMed PMID: 34252877]
Haider A, Spurling BC, Sánchez-Manso JC. Lewy Body Dementia. StatPearls. 2024 Jan:(): [PubMed PMID: 29494048]
Khan I, De Jesus O. Frontotemporal Lobe Dementia. StatPearls. 2024 Jan:(): [PubMed PMID: 32644712]
Sanders AE, Schoo C, Kalish VB. Vascular Dementia. StatPearls. 2024 Jan:(): [PubMed PMID: 28613567]
Sawyer DM, Kuo PH. Top-Down Systematic Approach to Interpretation of FDG-PET for Dementia. Clinical nuclear medicine. 2018 Jun:43(6):e212-e214. doi: 10.1097/RLU.0000000000002115. Epub [PubMed PMID: 29659399]
Level 1 (high-level) evidenceSharma A, Kumar R. Metabolic Imaging of Brain Tumor Recurrence. AJR. American journal of roentgenology. 2020 Nov:215(5):1199-1207. doi: 10.2214/AJR.19.22624. Epub 2020 Sep 22 [PubMed PMID: 32960666]
Vagal AS, Leach JL, Fernandez-Ulloa M, Zuccarello M. The acetazolamide challenge: techniques and applications in the evaluation of chronic cerebral ischemia. AJNR. American journal of neuroradiology. 2009 May:30(5):876-84. doi: 10.3174/ajnr.A1538. Epub 2009 Feb 26 [PubMed PMID: 19246526]
Prener M, Drejer V, Ziebell M, Jensen P, Madsen CG, Olsen S, Thomsen G, Pinborg LH, Paulson OB. Ictal and interictal SPECT with (99m) Tc-HMPAO in presurgical epilepsy. II: Methodological considerations on hyper- and hypoperfusion. Epilepsia open. 2023 Dec:8(4):1503-1511. doi: 10.1002/epi4.12833. Epub 2023 Oct 12 [PubMed PMID: 37750050]
Kim S, Mountz JM. SPECT Imaging of Epilepsy: An Overview and Comparison with F-18 FDG PET. International journal of molecular imaging. 2011:2011():813028. doi: 10.1155/2011/813028. Epub 2011 Jul 14 [PubMed PMID: 21785722]
Level 3 (low-level) evidenceAkdemir ÜÖ, Atay Kapucu LÖ. Nuclear Medicine Imaging in Pediatric Neurology. Molecular imaging and radionuclide therapy. 2016 Feb 5:25(1):1-10. doi: 10.4274/mirt.49389. Epub [PubMed PMID: 27299282]
Khan SH, Rather TA, Sinha S. Cerebral Spinal Fluid Cisternography in Normal Pressure Hydrocephalus of the Elderly. Indian journal of nuclear medicine : IJNM : the official journal of the Society of Nuclear Medicine, India. 2017 Jul-Sep:32(3):250-251. doi: 10.4103/ijnm.IJNM_55_17. Epub [PubMed PMID: 28680223]
Vettiyil B, Bessette S, McQuiston S, Greiner F. Nuclear Medicine to Evaluate Complications of Cerebral Shunts: Two Cases and Review of Literature. World journal of nuclear medicine. 2015 Sep-Dec:14(3):212-5. doi: 10.4103/1450-1147.163263. Epub [PubMed PMID: 26420995]
Level 3 (low-level) evidence