Introduction
Nasal septoplasty is one of the most commonly performed procedures within otorhinolaryngology (ENT) and plastic surgery. The primary indication for this functional (as opposed to purely aesthetic) surgery is usually septal deviation resulting in significant and symptomatic nasal airway obstruction. Many surgical techniques and approaches have been described; these include endonasal, endoscopic and open procedures. Septoplasty can also be performed alongside or in addition to rhinoplasty, turbinoplasty, or as part of functional endoscopic sinus surgery to improve surgical exposure and access. Operative recovery usually lasts a few weeks, and serious complications are rare. Appropriate patient selection is crucial to maximizing patient outcomes.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Critical to performing a successful septoplasty is a thorough understanding of the anatomy of the nose, specifically the nasal septum. The septum is the main foundational structure of the external nose, providing support to the nasal dorsum, the columella, and the nasal tip. It also separates the nasal cavity, creating two distinct nasal passages that enable laminar airflow and warm and humidify the inspired air. Deviation of the septum can reduce the cross-sectional area of the nasal valve, which subsequently leads to airway obstruction. This can create symptoms of nasal blockage and, in some circumstances, worsen the symptoms of obstructive sleep apnea. Bony spurs resulting from septal deviation may result in epistaxis, headaches ("Sluder syndrome" or rhinogenic headache), and facial pain.
Septal Structures
The septum has three main components: membranous, cartilaginous, and bony (see Image. The Organ of Smell, Bones and Cartilage of the Septum of the Nose). The membranous septum is made up of fibrous tissue and comprises the most anterior portion of the septum, between the caudal end of the quadrangular cartilage (the cartilaginous septum) and the medial crura of the lower lateral cartilages. As the name suggests, the quadrangular cartilage is quadrangular in shape and sits posterior to this membranous septum. It attaches to the maxillary crest inferiorly, the upper lateral cartilages superiorly, and the bony septum posteriorly; there is typically a 1 to 2 cm long, thin cartilaginous "tail" that rests on one side of the bony septum. The bony septum is made up of the vomer, which sits inferior-posterior to the cartilage, and the perpendicular plate of the ethmoid (PPE), which sits superiorly and posteriorly. The ethmoid bone is contiguous with the skull base and sphenoid bone and contains the middle and superior turbinates as well. The nasal bones sit on the medial dorsal aspect of the upper nose, superior to the PPE and joined to the superior aspect of the upper lateral cartilages.
Another critical anatomical concept is the nasal valve, or "internal" nasal valve, which is the narrowest portion of the nasal passage - a triangular area just under the middle portion of the nasal vault. The boundaries of the nasal valve are the dorsal septum, the caudal border of the upper lateral cartilage, and the head of the inferior turbinate.[1] The classically described nasal valve angle is approximately 15 degrees, but it is often closer to 30 degrees; in about 50% of people, there will also be a collection of submucosal glandular and vascular tissue known as a septal swell body or "septal turbinate," which appears to be a wider portion of the dorsal septum at the level of the internal nasal valve.[2][3][4] When viewed unilaterally, the swell body may appear to be a septal deviation; however, it is typically symmetrical bilaterally.
The two surgically critical points of articulation of the septum are its junction with the anterior nasal spine of the maxilla and the "keystone area." The keystone area is located at the confluence between the nasal bones, quadrangular cartilage, upper lateral cartilage, and PPE; it is a crucial area for stability and structure and must be handled carefully during septoplasty in order to avoid disrupting support for the nasal dorsum.[5] The keystone area lies directly deep to the cephalometric point known as the rhinion. If disrupted, it can be technically challenging to repair the keystone area, and a saddle nose deformity may result.
The cartilaginous and bony components of the septum are covered with mucoperichondrium and mucoperiosteum, respectively, which provide innervation and a rich vascular supply. This network of blood vessels enables the warming and humidification of air through the nasal cavity. The surface mucosa is mainly made up of pseudostratified respiratory epithelium. The olfactory epithelium, with its basal, supporting, and olfactory cells, is located more superiorly near the olfactory cleft region of the nasal cavity.
Blood Supply
The blood supply to the nasal septum travels via a collection of arteries derived from the internal and external carotid arteries. The internal carotid artery gives rise to the anterior and posterior ethmoidal arteries (via the ophthalmic artery), which supply the superior aspect of the septum. The external carotid gives rise to the facial and maxillary arteries, the terminal branches of which provide the remaining vascular supply; the facial artery branches form the superior labial artery, which supplies the anterior aspect of the nose. The maxillary artery branches form the greater palatine and sphenopalatine arteries, which supply the inferior and posterior septum. These five arteries (anterior and posterior ethmoid, superior labial, greater palatine, and sphenopalatine) anastomose anteroinferiorly at Little's area on the septum to form Kiesselbach's plexus, which is the most common site of epistaxis.
Innervation
The ophthalmic (CN V1) and maxillary (CN V2) branches of the trigeminal (CN V) nerve innervate the internal and external nose. Specifically, the nasociliary branch of CN V1 gives rise to the anterior and posterior ethmoid nerves, which supply the anterior-superior and posterior-superior aspects of the septum, respectively. The nasopalatine branch of CN V2 supplies the posteroinferior aspect of the septum. The superior alveolar nerve, also a branch of the maxillary nerve, supplies the anterior septum. The olfactory nerve (CN I) is responsible for providing sensory information from the olfactory epithelium to the olfactory bulbs (see Image. The Organ of Smell, Nerves of Septum of the Nose).[6][7]
Indications
The main indication for performing a septoplasty is a nasal septal deformity. This is usually a deviation of the cartilaginous and/or bony parts of the septum into one or both of the nasal passages, reducing the cross-sectional area, hindering airflow, and causing a sensation of nasal blockage. Patients may particularly complain of symptoms of obstruction on exertion or while exercising. The most common cause of the deviation is trauma. Patients must be symptomatic with nasal blockage to warrant functional surgery.
Several scoring systems are available to grade nasal obstructive symptoms. The Nasal Obstruction Symptom Evaluation (NOSE) scale is a validated instrument used to evaluate the degree of obstruction. Those with low scores are unlikely to benefit from surgery.[8][9] It is important to take a thorough history to establish whether concomitant factors may contribute to or cause obstruction, such as trauma, rhinosinusitis, allergies, vasculitis, illicit drug use, chronic use of decongestants, autoimmune disease, or malignancy. In these cases, adequate medical therapy (e.g., intranasal corticosteroids for chronic allergic rhinitis) should be offered prior to considering surgery.[8]
Other indications for septoplasty include recurrent epistaxis, obstructive sleep apnea, sinusitis, and facial pain and/or headaches due to septal spurs that contact a turbinate (Sluder's syndrome).[6] Septoplasty may also be necessary in conjunction with endoscopic sinus, skull base, or orbital surgery in order to provide better access to target structures.[10][11]
Contraindications
There are several contraindications to performing surgery. These include concurrent diseases such as rhinosinusitis or vasculitis or cases in which adequate medical therapy has not been attempted. While nasal sprays will not straighten a deviated septum, decreasing chronic inflammation may relieve symptomatic nasal obstruction sufficiently to obviate the need for operative intervention. Additionally, third-party payers are unlikely to approve an expensive surgical procedure if conservative therapy has not been exhausted first.
Active recreational drug use during the peri-operative period, particularly intranasal cocaine, is highly inadvisable. The vasoconstrictive and mucosal damaging effects of cocaine can prompt complications such as septal perforation, delayed healing, and, ultimately, dorsal collapse with a resulting saddle nose deformity.[12] It is best practice to ensure patients have been abstinent for at least 6 to 12 months before operating, and toxicology screening may be necessary to ensure compliance. Similar precautions should be taken with patients who display signs of rhinitis medicamentosa. Vasoconstrictive decongestant nasal sprays should also be avoided for a significant period pre-operatively, although they can help limit bleeding intra- and post-operatively. While smoking is generally deleterious to patients' health, current evidence does not seem to indicate that it necessarily has a negative impact on the outcomes of nasoseptal surgery.[13]
Patients with unrealistic expectations for aesthetic or functional septoplasty outcomes should not be offered surgery without extensive pre-operative counseling. This point is even more applicable to those undergoing concurrent rhinoplasty. The surgeon and patient need to share achievable expectations, as this will improve post-operative patient and surgeon satisfaction.[14] Similarly, those with septal deviation and deformity, but limited functional symptoms, may experience minimal benefit from surgery.
Patient co-morbidities, functional status (ASA grade), and age all need to be considered to determine whether a general anesthetic would be safe and whether patients would be able to tolerate the post-operative recovery process.
Equipment
A standard septoplasty set usually includes the following. Note that surgeons may only use a fraction of these instruments during the operation, depending on their preferred techniques.
- Headlight
- Hartmann/Vienna nasal speculum
- Cottle nasal speculum
- Killian nasal speculum (3 sizes)
- Killian retractor
- Hills elevator
- #15 blade on a #7 Bard-Parker scalpel handle
- Cottle curved scissors
- Gorney nasal shears
- Jansen-Middleton forceps
- Blakesley nasal forceps
- Takahashi nasal forceps
- Blakesley-Wilde nasal forceps
- Lubet-Barbon nasal dressing forceps
- Ferguson or Frazier suction tip
- Bipolar diathermy forceps
- Adson-Brown forceps
- Cushing-Brown bayonet forceps
- Jansen nasal dressing forceps
- Cottle lower lateral forceps
- Cotton applicator
- McKenty raspatory
- Freer chisel
- Freer elevator
- Freer septal knife
- Pierce elevator
- Cottle elevator
- Woodson elevator
- Cottle chisel
- Cottle metal mallet
- Backhaus towel forceps
- Needle holder
- Medicine cup
- Hopkins rod, light source, and stack (if endoscopic)
- Silastic splints
- 5-0 and 4-0 chromic gut suture
- Mupirocin ointment
Personnel
ENT surgeons usually carry out septoplasty procedures. Among otolaryngologists, simple septoplasties are performed by most ENT surgeons. Revision surgery or more complex procedures, with or without rhinoplasty, may be performed by rhinology or facial plastic surgery specialists. Some plastic surgeons also perform septoplasties. Additional personnel required to complete the operation includes an anesthetist, a scrub nurse, a surgical technologist, theatre and recovery nurses, and coordinators.
Preparation
History
A complete history detailing the nature and extent of nasal symptoms should be obtained. Symptoms of other sinonasal or systemic pathology, including allergies, should be evaluated. Scoring systems such as the NOSE scale can be used to grade nasal obstructive symptoms.[9] A detailed drug history with a particular focus on intranasal decongestants and corticosteroids, in addition to any recreational drug use, should be obtained. If the patient smokes, cessation should be considered, although smoking has not been shown definitely to worsen outcomes in nasoseptal surgery.[13] It is useful to elucidate whether the patient has had previous nasal or sinus surgery, issues with prior anesthetics, or bleeding conditions.
Physical Examination
Patients should be thoroughly examined in the outpatient clinic. A full head and neck examination should be carried out, followed by an anterior rhinoscopy with a nasal speculum. Flexible nasendoscopy can be performed to look for signs of sinonasal disease or masses in the posterior nasal space, particularly when the results of anterior rhinoscopy do not correspond to the patient history (e.g., severe nasal obstruction symptoms in the presence of normal-appearing turbinates and a midline anterior septum). When evaluating the nasal septum, the surgeon should look at the quality of the mucosa (assessing for signs of inflammation) and the size and nature of the turbinates, particularly the inferior turbinates. If there is poor access due to overly large turbinates, a turbinoplasty may also be indicated.
The septum should be palpated to evaluate the size, location, and nature of the deviation, noting specifically whether this seems cartilaginous or bony and whether there are any septal perforations, dislocations, or bony spurs. If a prior septoplasty has been performed, septal palpation with a cerumen curette or cotton-tipped applicator to determine how much cartilage remains within the septum is also important. An external examination should be made, noting any additional deformity, the dynamic collapse of the alae with inspiration, and the degree of nasal tip support. Lastly, Cottle’s maneuver should be performed to assess internal valve stenosis.[8] A thorough examination will assist in determining whether surgery is appropriate, what the level of difficulty would be, and what approach and technique would be most suited for the patient.
Technique or Treatment
Endonasal
Preparation
- The patient is positioned supine with the head tilted slightly towards the surgeon.
- Prominent nasal hairs are trimmed.
- Some surgeons prefer to decongest the nose with oxymetazoline or Moffatt's solution.
- Local anesthetic is infiltrated bilaterally in the sub-mucoperichondrial plane, using 1% lidocaine with epinephrine (1:100,000) until the mucosa is well blanched. This assists in the hydro-dissection of the planes in addition to analgesia and hemostasis.
Raising Mucoperichondrial Flaps
- A nasal speculum is used to expose the caudal edge of the cartilaginous septum. An incision is made using a #15 blade along this caudal edge to expose the cartilage. Typically, a hemi-transfixion (vertical incision through one side of the membranous septum) or Killian's incision (vertical incision more posteriorly, over the quadrangular cartilage) is used. If the septoplasty is performed in conjunction with an open rhinoplasty, the septum may be approached from above after the upper lateral cartilage is disarticulated from the dorsal quadrangular cartilage.
- Dissecting scissors and Freer or Cottle elevators are then used to develop a sub-mucoperichondrial plane and dissect posteriorly to reveal the quadrangular cartilage, PPE, and vomer. Exposure of the bare cartilage is very important to ensure a well-vascularized and robust mucoperichondrial flap is elevated; the quadrangular cartilage usually appears a pearly white/blue color. Care must be taken not to perforate the mucosa, especially if dissection over bony spurs or deviations is necessary. A second flap on the contralateral side may then be raised through the same incision.[8] If a mucosal perforation has occurred on one side, extreme care must be taken not to perforate the contralateral side; bilateral, opposing perforations can lead to a septal perforation post-operatively. As the surgeon reaches farther posteriorly, longer nasal specula will be required to obtain good visualization unless an endoscope is employed.
Septal Deviation Correction
- Evaluation of the location, direction, and nature (cartilaginous/bony) of the deformity is made.
- Various instruments can be used to incise the septum, and sometimes these are used in combination. A blade, a Freer elevator, Jansen-Middleton forceps, or Takahashi forceps are used to remove the deviated piece of cartilage. To maintain nasal dorsum and tip stability, an "L-strut" shape of quadrangular cartilage is preserved (dorsal and caudal edges); the posterior aspect of the cartilage is, therefore, often the area that is removed. In the event that the deviation involves the L-strut, an extracorporeal septoplasty or an open approach with grafting may be required (see below). The dorsal and caudal limbs of the L-strut, as well as their attachments to bone, should remain 10 to 15 mm in width to guarantee sufficient support for the external nose. A twisting movement is often employed to remove sections of cartilage or bone; care must be taken not to twist too forcefully outside of the anterior-posterior axis as this can cause a fracture of the cribriform plate and potential leak of cerebrospinal fluid.[6][10] Care should also be taken to avoid disruption of the keystone area during the removal of deviated portions of the septum.
- Removed cartilage should be saved in normal saline or a surgical gauze sponge dampened with normal saline. Pieces of native cartilage can be reshaped and used to reinforce the L-strut if necessary or to contribute to grafts used in rhinoplasty.
Closure
- The mucoperichondrial flaps are laid back into position against the septum. Interrupted stitches using absorbable suture material (e.g., chromic gut) are used to close the incision.
- Running mattress, or "quilting," sutures are often made through and through the septum to close any dead space and reapproximate the flaps to avoid hematoma accumulation post-operatively. If the mucoperichondrial flaps remain completely intact at the end of the operation, some surgeons will place a small incision inferiorly in one flap to facilitate the egress of any fluid that may collect within the septum. Some surgeons prefer to use a septal stapler rather than placing a quilting suture by hand.
- Silastic splints are sometimes required. If necessary, these are cut to size, placed adjacent to the septum, and sutured transseptally for easy removal in the outpatient clinic. The splints aim to prevent adhesions to the turbinates if these are hypertrophied or if turbinoplasties have also been performed.
- An antibiotic cream can be applied intranasally.
Post-operative Care
- Patients are usually able to be discharged on the same day.
- Topical antibiotic cream and oral analgesia are prescribed.
- Oxymetazoline and saline sprays may also be useful for providing a clean intranasal environment during the healing process.
- The patient will need to be seen in 1 to 2 weeks for post-operative assessment and removal of splints if used.
- Antibiotics are not typically required. If gauze packing is placed to stabilize comminuted bone fragments, antibiotics to prevent staphylococcal toxic shock may be warranted.
Endoscopic
The various steps and techniques used are similar to the traditional endonasal method. Rather than a nasal speculum and headlight, however, a 0-degree Hopkins rod endoscope is used to visualize the nasal structures and septum. The endoscope can be placed between mucosal flaps to ensure adequate resection is achieved.[6] This method may more commonly be used in conjunction with functional endoscopic sinus surgery (FESS), wherein endoscopic techniques will already be in use, and the septoplasty required may be needed only to provide access or visualization to a particular area within the nasal cavity. Frequently, the endoscopic method is employed via a Killian incision, which may be made just anterior to a bony spur to facilitate access to an isolated septal deformity and avoid elevation of complete mucoperichondrial flaps all the way back from a hemi-transfixion incision.
Endoscopic techniques enable enhanced visualization and a magnified view of the anatomy. This is particularly helpful when teaching more junior surgeons. Endonasal procedures are notoriously difficult to observe, which makes training challenging. In revision cases where scarring or adhesions are present, endoscopic visualization can also help identify the correct dissection planes.[6]
However, endoscopic septoplasties may be technically more challenging than endonasal methods. It may take even senior surgeons trained in endonasal and FESS surgery approximately 60 procedures before achieving satisfactory operative times and an acceptable rate of complications.[15] Anterior defects may be particularly challenging to correct endoscopically, as the surgeon cannot easily stabilize the endoscope and may have to use a "free-hand" technique.[6]
Extracorporeal
Complex septal deformities in all three areas (quadrangular cartilage, PPE, and vomer) or previous comminuted fractures may make applying the aforementioned techniques very challenging or impossible.[16] In this case, extracorporeal methods may be needed, wherein the whole septal cartilage is removed, the deviation corrected, and the septum replaced. This may be performed as an open procedure through a rhinoplasty approach, as part of a septorhinoplasty, or alternatively, it can be performed as a closed procedure via an extended hemi-transfixion incision.[10]
Once the septum has been removed, several techniques may be used to straighten it. These include making partial-thickness scoring incisions on the concave side of the deviation, incising fracture lines and re-suturing component parts together, affixing grafts to overlap and splint the deviated cartilage, or drilling/filing sections of the septum. An alternative technique particularly useful when minimal cartilage remains within the septum involves suturing excised septal cartilage fragments to a polydioxanone scaffolding plate.[17] The cartilage may be rotated and replaced in a different orientation if this provides superior structural support. It must then be reimplanted and sutured to the lateral cartilages and anterior nasal spine.[16]
In cases with minimal cartilaginous septum remaining (due to previous surgery or necrosis), a neoseptum may be made from fragments of existing septal cartilage or autologous material, e.g., costal cartilage. Homologous grafts (homologous costal cartilage harvested from cadaveric donors) can also be used.[18]
Complications
Several complications may occur following septoplasty; these must be fully described and explained to the patient during the informed consent discussion. The most common is excessive bleeding; some oozing is expected, but more extensive bleeding is manageable with nasal packing and may require cautery in rare circumstances. Septal hematomas can occur when bleeding occurs beneath the mucoperichondrium, and these require drainage or aspiration to prevent infection development of septal perforation and/or saddle nose deformity. Perforations can also occur due to bilateral, opposing mucosal lacerations intra-operatively; if a perforation approaches the keystone area, a saddle nose deformity may develop, which will likely necessitate revision surgery.
Infection, nasal obstruction, and prolonged healing can occur in a proportion of patients. Infections are rare and can generally be cleared with oral antibiotics; the vast majority of patients will make a full recovery within a few weeks. Hyposmia has been described in some patients (more frequently with concurrent turbinoplasty procedures); this usually resolves within six months. Intranasal adhesions can occur, but the use of silastic splints minimizes the risk of this complication. Lastly, patients may experience numbness or sensitivity of the upper teeth or lip due to intraoperative manipulation of the nasopalatine nerve; this problem is usually short-lived, and normal sensation returns within a few months.[19]
The most common long-term complaint after septoplasty with or without turbinoplasty is that the improvement in nasal breathing is insufficient; this may result from a number of reasons, including repeated trauma, weight gain, cartilage migration over time, and technical error, among others.[20][21] Counseling patients about this risk pre-operatively is critical.
Clinical Significance
Septoplasty is one of the most frequently performed ENT procedures. Systematic reviews have revealed that long-term outcomes are favorable for most patients with septal deviation-related obstructive symptoms. Despite this, a significant proportion of patients experience recurrence of obstructive symptoms post-surgery, and satisfaction rates can be highly variable (50% to 100% satisfaction).[20]
Postoperative outcomes are commonly assessed by patient satisfaction, quality of life, and symptom improvement questionnaires. It is challenging to evaluate symptom improvement, given that it is often very subjective and variable throughout the day, and objective measurements, such as acoustic rhinometry and rhinomanometry, do not always align with patient perception.[20] Some studies have shown a decline in long-term outcomes for patients. One study showed that 26% of patients had no nasal obstruction after nine years compared to 51% at nine months post-operatively.[22] Another study reported that 53% of patients were symptom-free at six months post-operatively, but only 18% remained symptom-free at 34 to 70 months.[23] This may demonstrate that the effects of surgery are short-lived for some patients or that patients who present initially after a nasal trauma will remain at risk for additional nasal trauma after surgery. Persistence or recurrence of nasal allergic symptoms may also account for the high rate of recidivistic obstruction. In most cases, septoplasty is combined with inferior turbinoplasty, which may also confound results.
Evaluation of candidates for surgery is made on a clinical basis, and it is not entirely clear in the literature what prognostic factors determine successful postoperative outcomes. A combination of objective and subjective measures to identify appropriate patients may help improve outcomes in the future.
In countries such as the UK, which operate on a state-funded healthcare system, it is imperative to demonstrate that such operations significantly improve patient quality of life and symptom burden to justify funding. Patients must be evaluated clinically, and the decision to proceed with surgery must be based upon evidence that suggests a good postoperative outcome is likely.
Enhancing Healthcare Team Outcomes
Managing patients who require septoplasty pre-, intra-, and post-operatively requires interprofessional teamwork. Outpatient assessment in ENT clinics involves a team of ENT surgeons, nurses, and administrative staff, each contributing to the case from their expertise. Intra-operative staff (as detailed above) are critical for carrying out the operation smoothly and successfully, minimizing complications in the early postoperative period and the long term. All interprofessional team members are responsible for monitoring the patient before, during, and after the procedure and must communicate with the appropriate clinical staff if they note any issues or concerns. The entire process requires meticulous documentation in the patient's permanent health record so that all care team members can access the same updated and accurate patient information. This interprofessional approach will yield the optimal results with the fewest adverse events.[Level 5]
Media
(Click Image to Enlarge)
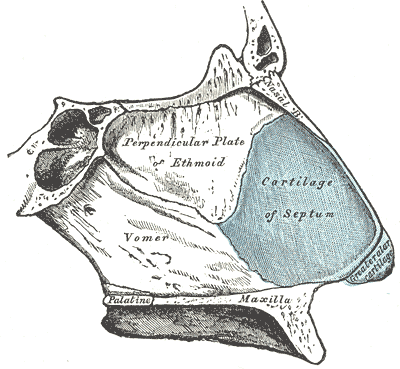
The Organ of Smell, Bones and Cartilage of the Septum of the Nose. Bones and cartilage of the septum of the nose, viewed from the right side: the perpendicular plate of the ethmoid, vomer, maxilla, palatine, septum cartilage, and greater alar cartilage.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)

The Organ of Smell, Nerves of Septum of the Nose. Right side, filaments from the olfactory nerve and bulb.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
References
Wexler DB, Davidson TM. The nasal valve: a review of the anatomy, imaging, and physiology. American journal of rhinology. 2004 May-Jun:18(3):143-50 [PubMed PMID: 15283487]
Wexler D, Braverman I, Amar M. Histology of the nasal septal swell body (septal turbinate). Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2006 Apr:134(4):596-600 [PubMed PMID: 16564379]
Ng BA, Ramsey RG, Corey JP. The distribution of nasal erectile mucosa as visualized by magnetic resonance imaging. Ear, nose, & throat journal. 1999 Mar:78(3):159, 163-6 [PubMed PMID: 10188352]
Miman MC, Deliktaş H, Ozturan O, Toplu Y, Akarçay M. Internal nasal valve: revisited with objective facts. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2006 Jan:134(1):41-7 [PubMed PMID: 16399179]
Simon PE, Lam K, Sidle D, Tan BK. The nasal keystone region: an anatomical study. JAMA facial plastic surgery. 2013 May:15(3):235-7. doi: 10.1001/jamafacial.2013.777. Epub [PubMed PMID: 23539211]
Shah J, Roxbury CR, Sindwani R. Techniques in Septoplasty: Traditional Versus Endoscopic Approaches. Otolaryngologic clinics of North America. 2018 Oct:51(5):909-917. doi: 10.1016/j.otc.2018.05.007. Epub 2018 Jul 17 [PubMed PMID: 30025848]
Sobiesk JL, Munakomi S. Anatomy, Head and Neck, Nasal Cavity. StatPearls. 2024 Jan:(): [PubMed PMID: 31334952]
Most SP, Rudy SF. Septoplasty: Basic and Advanced Techniques. Facial plastic surgery clinics of North America. 2017 May:25(2):161-169. doi: 10.1016/j.fsc.2016.12.002. Epub 2017 Feb 21 [PubMed PMID: 28340647]
Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2004 Feb:130(2):157-63 [PubMed PMID: 14990910]
Level 1 (high-level) evidenceFettman N, Sanford T, Sindwani R. Surgical management of the deviated septum: techniques in septoplasty. Otolaryngologic clinics of North America. 2009 Apr:42(2):241-52, viii. doi: 10.1016/j.otc.2009.01.005. Epub [PubMed PMID: 19328889]
Sautter NB, Smith TL. Endoscopic septoplasty. Otolaryngologic clinics of North America. 2009 Apr:42(2):253-60, viii. doi: 10.1016/j.otc.2009.01.010. Epub [PubMed PMID: 19328890]
Slavin SA, Goldwyn RM. The cocaine user: the potential problem patient for rhinoplasty. Plastic and reconstructive surgery. 1990 Sep:86(3):436-42 [PubMed PMID: 2385660]
Level 3 (low-level) evidenceAndrews JE, Jones NN, Moody MP, Vincent AG, Teixeira JC, Thomas RF, Hohman MH. Nasoseptal Surgery Outcomes in Smokers and Nonsmokers. Facial plastic surgery & aesthetic medicine. 2021 Jul-Aug:23(4):283-288. doi: 10.1089/fpsam.2020.0349. Epub 2020 Aug 26 [PubMed PMID: 32856954]
Khan N, Rashid M, Khan I, Ur Rehman Sarwar S, Ur Rashid H, Khurshid M, Khalid Choudry U, Fatima N. Satisfaction in Patients After Rhinoplasty Using the Rhinoplasty Outcome Evaluation Questionnaire. Cureus. 2019 Jul 30:11(7):e5283. doi: 10.7759/cureus.5283. Epub 2019 Jul 30 [PubMed PMID: 31576273]
Champagne C, Régloix SB, Genestier L, Crambert A, Maurin O, Pons Y. Endoscopic septoplasty: Learning curve. European annals of otorhinolaryngology, head and neck diseases. 2016 Jun:133(3):167-70. doi: 10.1016/j.anorl.2016.01.002. Epub 2016 Feb 15 [PubMed PMID: 26898762]
Gubisch W. Extracorporeal septoplasty for the markedly deviated septum. Archives of facial plastic surgery. 2005 Jul-Aug:7(4):218-26 [PubMed PMID: 16027341]
Level 2 (mid-level) evidenceMcGrath M, Bell E, Locketz GD, Becker DG. Review and update on extracorporeal septoplasty. Current opinion in otolaryngology & head and neck surgery. 2019 Feb:27(1):1-6. doi: 10.1097/MOO.0000000000000509. Epub [PubMed PMID: 30507685]
Level 3 (low-level) evidenceSong HM, Lee BJ, Jang YJ. Processed costal cartilage homograft in rhinoplasty: the Asan Medical Center experience. Archives of otolaryngology--head & neck surgery. 2008 May:134(5):485-9. doi: 10.1001/archotol.134.5.485. Epub [PubMed PMID: 18490568]
Level 2 (mid-level) evidenceDąbrowska-Bień J, Skarżyński PH, Gwizdalska I, Łazęcka K, Skarżyński H. Complications in septoplasty based on a large group of 5639 patients. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2018 Jul:275(7):1789-1794. doi: 10.1007/s00405-018-4990-8. Epub 2018 May 16 [PubMed PMID: 29770875]
Tsang CLN, Nguyen T, Sivesind T, Cervin A. Long-term patient-related outcome measures of septoplasty: a systematic review. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2018 May:275(5):1039-1048. doi: 10.1007/s00405-018-4874-y. Epub 2018 Jan 13 [PubMed PMID: 29332171]
Level 1 (high-level) evidenceStandlee AG, Hohman MH. Evaluating the Effect of Spreader Grafting on Nasal Obstruction Using the NOSE Scale. The Annals of otology, rhinology, and laryngology. 2017 Mar:126(3):219-223. doi: 10.1177/0003489416685320. Epub 2017 Jan 5 [PubMed PMID: 28056521]
Jessen M, Ivarsson A, Malm L. Nasal airway resistance and symptoms after functional septoplasty: comparison of findings at 9 months and 9 years. Clinical otolaryngology and allied sciences. 1989 Jun:14(3):231-4 [PubMed PMID: 2743612]
Sundh C, Sunnergren O. Long-term symptom relief after septoplasty. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2015 Oct:272(10):2871-5. doi: 10.1007/s00405-014-3406-7. Epub 2014 Nov 29 [PubMed PMID: 25432640]