Introduction
Posterior vitreous detachment (PVD) is a common occurrence in old age. It is defined as the separation of the cortical vitreous from the neurosensory layer of the retina. The posterior vitreous detachment was first narrated histopathologically by Muller in 1856 and clinically by Briere in 1875, but it was not explored thoroughly until 1914.
Vitreous humor is a gel-like substance that is present amid the lens and the retina. The composition of vitreous humor includes water (98%), type 2 collagen, and hyaluronic acid.[1][2] It is surrounded by a translucent membrane called the hyaloid membrane. The vitreous is completely attached to the retina in the early period of life.
Posterior vitreous detachment is the most common cause of primary symptomatic floaters. Floaters are small cobweb shaped particles emerging from a compact collagen matrix of the posterior vitreous cortex.[3] A rapid increase in the numbers of floaters with sudden onset of photopsia (flashes) needs immediate ophthalmic care.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The primary cause of posterior vitreous detachment is the old age. Normally in a young, healthy individual, the vitreous is adherent to the internal limiting membrane of the retina. The adherence of the vitreous is strongest at the vitreous base.[4] Other areas of vitreoretinal adhesion include optic disc margins, macula, and peripheral blood vessels.
As the person ages, the gel-like consistency of vitreous degenerates and undergoes the phenomenon of synchysis and syneresis. Vitreous degeneration begins with the stage of vitreous liquefaction, which is called synchysis.[5] Syneresis is the aggregation of the collagen fibrils leading to the collapse of the vitreous.[6] This event produces thick bundles of collagen fibrils that float in vitreous and give rise to floaters (myodesopsia) in the eye. Vitreous degeneration also provokes weakening of the vitreoretinal adhesion, which may result in posterior vitreous detachment.[7]
The process of a posterior vitreous detachment is spontaneous, but it can be brought about by events such as cataract surgery, trauma, uveitis, panretinal photocoagulation, and laser capsulotomy. About 8%-22% of patients with acute symptomatic PVD have retinal tears at the initial examination.[8][9] In such cases, retinal tears are usually present at or soon after the onset of symptoms. Retinal tears or breaks are usually seen in the superotemporal quadrant of the retina.[10] A retinal break may have different morphologies, including U-tears (horseshoe), operculated tears, or retinal holes. In about 2-5% of the patients diagnosed with acute PVD without any retinal break on the first examination, retinal breaks (new or missed) are observed on the follow-up visit.[11]
The risk of retinal tears is more in PVD associated with vitreous hemorrhage than in PVD without vitreous hemorrhage.[8] It is noted that about 50%-70% of the patients with PVD complicated by vitreous hemorrhage have retinal tears. In contrast, only 7%-12% of the patients with PVD without vitreous hemorrhage present with a retinal tear. Moreover, patients with acute PVD associated with retinal tears are seven times more likely to present with vitreous pigments or granules than are those without a retinal tear.[12]
The most important risk factors for PVD include:
- Old age: The incidence of posterior vitreous detachment after 50 years of age is 53%, and between ages 66 to 86 years is 66%.[13][14] Postmortem studies revealed that PVD was present in 27% of eyes by the seventh decade and 63% of the eyes by the eighth decade of life.[7]
- Female gender: The progression of a posterior vitreous detachment is faster in women than in men at age 60 or more. This implies that the macular pathologies linked with posterior vitreous detachment occur at a younger age in females.[15][16]
- Myopia: The incidence of posterior vitreous detachment depends on the axial length of the eyeball. The eyes with axial length more than 30 mm have a greater chance of developing posterior vitreous detachment than eyes with axial length less than 29 mm.[17]
- Underlying diseases like retinitis pigmentosa and sticklers syndrome.[18][19]
- Menopause: Post-menopausal female patients may be more prone to develop posterior vitreous detachment because of a lack of estrogen. Estrogen may have a protective effect against PVD in premenopausal females.[15]
- Vitamin B6: Vitamin B6 has an anti-estrogen effect. A higher intake of vitamin B6 may increase the incidence of posterior vitreous detachment in females.[15]
- Inflammation: Long-standing inflammation involves cellular proliferation and, eventually, fibrosis. Fibrosis of the vitreous cause traction over the retina resulting in posterior vitreous detachments or retinal breaks.[20]
- Trauma: Posterior vitreous detachment occurs as a consequence of penetrating injury to the eye. It was noted as separation at the level of the internal limiting membrane or as a cleavage within the vitreous in a study.[21]
- Ocular surgery: Various vitreous modifications occur during ocular surgeries, including cataract extraction and intraocular lens placement, LASIK (laser in situ keratomileusis), and others. These modifications may be involved in the development of postoperative posterior vitreous detachment.[22] The vitreous modifications after cataract surgery include a higher viscosity of vitreous near the retina compared to anterior hyaloid (opposite to normal condition), altered vitreous fluid proteome, destabilized vitreous body, anomalous PVD, and rheological changes of vitreous without clear separation of vitreous and retina.[22] Other predispositions include posterior capsular tear, aggressive surgical maneuvers, vitreous loss, laser capsulotomy, and myopia.[22]
- Retinal laser[23]
- Retinal cryo[24]
- The vitreous gel contains various angiogenic factors. These factors are responsible for neovascularization by endothelial cell proliferation. The posterior hyaloid face acts as a scaffold for the growth of the retinal or optic disc new vessels. So the presence of a complete PVD may prevent the process of neovascularization and protects the eye from the progression of proliferative diabetic retinopathy.[25]
Epidemiology
The statistics on the prevalence of posterior vitreous detachment are largely lacking. The data collected from hospital-based and post mortem studies suggest age is an important factor for the development of posterior vitreous detachment.
History and Physical
In most individuals, the early stages of posterior vitreous detachment are asymptomatic and not detected clinically until the separation of vitreous from optic disc margins produces symptoms.[26]
The patient mostly presents with symptoms of flashes of light (photopsia) and floaters (myodesopsia). Nineteen percent of patients presenting with flashes or floaters alone have posterior vitreous detachment.[27] Flashes of light are typically quick and located in the temporal quadrant. They are induced upon the movement of the head or eye and are more noticeable in a dim environment.[28] Floaters are small, mobile vitreous particles evident against a bright background. 67% of patients with posterior vitreous detachment complain of blurring of vision.[8] The blurring of vision may occur due to the vitreous hemorrhage resulting from the retinal breaks or ample floaters crowding the visual field.
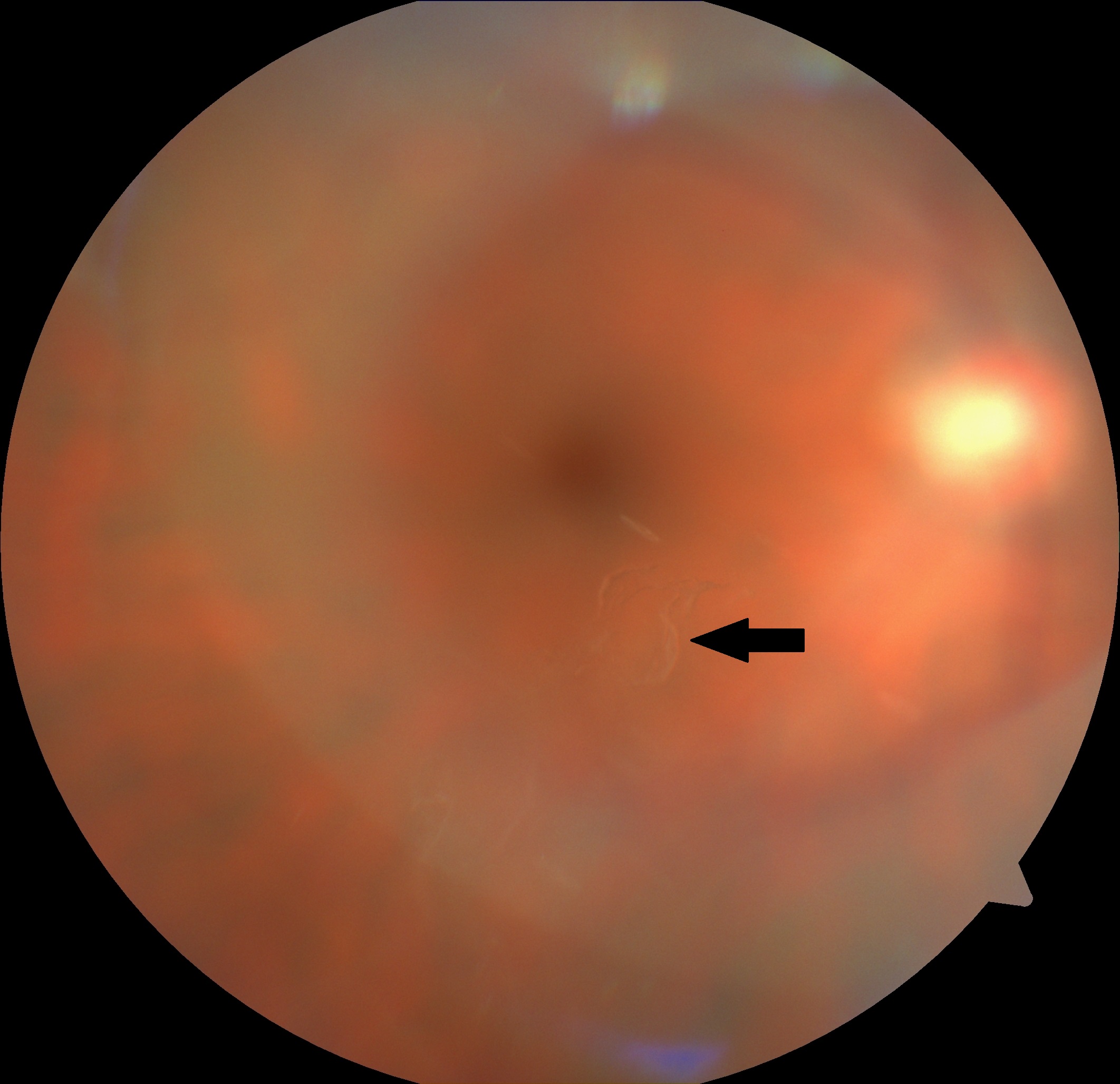
Patients with symptoms of PVD need to be assessed carefully. Complete retinal examination, including visualization of ora Serrata for 360 degrees (by scleral indentation while using indirect ophthalmoscopy) along with slit-lamp biomicroscopy, should be performed. The detailed examination reveals a detached posterior hyaloid membrane. It appears like a crumpled translucent membrane in mid vitreous. The diagnosis of complete PVD is usually made on the basis of the presence of the Weiss ring.[29] It is a ring of glial tissue seen attached to the posterior hyaloid anterior to the optic disc. Weiss ring has a diameter of about 1.5 mm.[30] The presence of red cells and pigments granules (Shafer sign or tobacco dust) in the anterior vitreous suggest the presence of retinal tears along with posterior vitreous detachment. Such patients require detailed retinal examination and should be referred to the specialist.[31]
Anomalous PVD (APVD) is the condition in which the liquefaction of vitreous humor is more than the vitreoretinal dehiscence. The consequences of APVD vary with the site of its presence as follows[32]:
- At retinal periphery: causes retinal tears
- At macula: causes vitreomacular traction, macular pucker, or macular hole
- At optic disc or retina: leads to vitreopapillary traction and plays a crucial role in neovascularization of optic disc and retina
Evaluation
The fundamental diagnostic procedure in the assessment of acute posterior vitreous detachment is binocular indirect ophthalmoscopy and three-mirror contact lens biomicroscopy. Imaging-based diagnosis of a PVD traditionally has relied on dynamic B-scan ultrasonography. More recently, optical coherence tomography (OCT) has been added as an attempt to establish the diagnosis of posterior vitreous detachment.[33]
Slit-lamp Biomicroscopy
Before going for a slit lamp biomicroscopy, the pupils of the patient are fully dilated using mydriatic agents. It aids in obtaining a wide illumination angle for various segments of the vitreous. Posterior vitreous detachment has been categorized as:[34]
- Complete (C-PVD) or
- Partial (P-PVD)
In complete PVD, there is no attachment of the separated posterior vitreous cortex at or beyond the globe equator. It can be easily outlined up to the vitreous base. C-PVD can be:
- Collapsed or
- Without a collapse[34]
In complete PVD with collapse, the posterior hyaloid membrane is loose, highly detached, and easily detectable. In this case, a peripapillary glial band is seen on slit-lamp biomicroscopy. However, in the case of complete PVD without collapse, the posterior hyaloid membrane is detached slightly and can only be traced in front of the retina.[34]
In partial PVD, some vitreoretinal adherence can be identified at or posterior to the globe equator. The P-PVD is associated with
- The shrinkage of the posterior hyaloid membrane in some cases and
- Without shrinkage of the posterior hyaloid membrane in others
The vitreous gel is seen adherent to the macula by a pre-macular opening in the posterior hyaloid membrane in few cases of partial PVD without shrinkage.[34]
B-Scan Ultrasonography
Ultrasonography (US) has been used traditionally to find out the condition of the vitreous gel. It also helps to check the presence and extent of the posterior vitreous detachment. The principle of the B-scan US is that strong echoes are generated by acoustic interfaces present at the junctions of media. These echoes are of different sound velocities and density. The greater the difference in the density between the two media, the more noticeable is the echo.
Optical Coherence Tomography (OCT)
Optical coherence tomography (OCT), a non-invasive ocular imaging tool, appeared in 1991.[35] It operates by producing a false-color image of the tissue structures, based on the intensity of the reflected light. OCT provides morphology and thickness analysis of the examined tissue. In the case of posterior vitreous detachment, OCT shows the separation of posterior vitreous face and retina. OCT has ascendancy over slit-lamp biomicroscopy, and B scan US as it can identify a shallow PVD. Whereas slit-lamp biomicroscopy and B scan US fail to identify shallow PVD. OCT has shown that PVD usually starts as a vitreous retinal detachment around the fovea.[30]
OCT categorizes PVD into five stages:[36]
Stage 0: is characterized by the nonexistence of PVD.
Stage 1: is characterized by focal perifoveal PVD in three or less than three quadrants. In this stage, there is a persistent attachment of the vitreous cortex at the fovea, optic nerve head, and mid-peripheral retina.
Stage 2: is the same as stage 1 but with perifoveal PVD in all four quadrants of the retina.
Stage 3: in this stage, the vitreous cortex is not attached to the level of the fovea. However, adherence to the optic nerve head and mid-peripheral retina persist.
Stage 4: is characterized by complete PVD along with a prominent Weiss ring on slit-lamp biomicroscopy.
OCT showing an oblique foveal vitreoretinal attachment without abnormality in the foveal contour is expressed by a term, stage 0 macular hole (vitreomacular adhesion).[37] These findings are picked up on OCT before the appearance of the clinical changes and have a normal biomicroscopic appearance.
Treatment / Management
Acute symptomatic posterior vitreous detachment without vitreous hemorrhage and peripheral retinal breaks should be followed up at 2-4 weeks for precise peripheral retinal examination with scleral indentation. A patient complaining of floaters is conservatively managed. Patients are reassured that adaptation will develop to the visual symptoms overtime, or the floaters may resolve. However, in many cases, floaters may persist beyond six months to one year. The vitreous hemorrhage associated with acute PVD is usually mild, with a blob of hemorrhage just in front of the posterior pole. There may be intraretinal hemorrhage near the optic disc. Usually, a circular circumferential attachment of the vitreous to the retina is noted around the equator with some preretinal bleed settled inferiorly just behind the posterior vitreous face. Most associated breaks lie in the superior retina. In case of vitreous hemorrhage precluding the complete examination, the patient is advised a propped up position, bed rest, and bilateral eye patching is an option in such cases.[38] Vitreous hemorrhage obscuring the fundal view should be evaluated by ultrasonography to rule out the presence of retinal breaks and other retinal conditions.[39]
In cases with highly symptomatic floaters that are clinically significant and persistent and impact the quality of life, the interventional options are as follows.
Vitrectomy
The psychological implications of floaters may be huge, with some patients even having suicidal thoughts due to floaters.[40] Pars plana vitrectomy is a successful treatment modality for the management of vitreous floaters associated with PVD.[41] Pars plana vitrectomy alleviates the symptoms of floaters to a great extent resulting in a clear visual field. However, it is associated with many complications.[42] Major complications include cataract formation, postoperative retinal detachment, and cystoid macular edema, possibly resulting in permanent vision loss.[43][44] So postoperative visual prognosis needs to be weighed out with the preoperative symptoms of floaters before opting for vitrectomy.(B2)
Nd YAG Laser Vitreolysis
Nd: YAG laser fires short, strong pulses and builds energy to vaporize the vitreous opacities to plasma. This is done by increasing the regional temperature to above 1000 Kelvin (726.85°C) at a confined spot.[45][46] In this way, the large floaters are broken down into smaller and less noticeable ones.(B2)
Vitreolysis by Drugs
Vitreolytic agents are classified as enzymatic or non-enzymatic agents. Mostly the enzymatic agents are used. They include tissue plasminogen activator (tPA), plasmin, microplasmin, nattokinase, chondroitinase, and hyaluronidase.[24] Non-enzymatic agents involve the use of urea and arginine-glycine-aspartate peptide.[47] Ocriplasmin has shown a success rate of 78% in clinical practice.[48] Ocriplasmin has proteolytic activity. It dissolves the protein component (collagen, laminin, fibronectin) of the vitreous which is responsible for the vitreomacular adhesion. The effective dose of ocriplasmin is 125 mcg intravitreal injection.[49] The aim of the use of these agents is to induce liquefaction of vitreous gel and to cause complete dehiscence of vitreous from the retina.(B3)
Management guidelines for posterior vitreous detachment associated with retinal tears depend on the type of tear. Operculated tears (symptomatic or asymptomatic) may not need treatment.[12] Whereas at least 50% of acute symptomatic U-tears with persistent vitreoretinal traction lead to clinical retinal detachment if not treated and need prompt management.[12] According to the preferred practice pattern by the American Academy of Ophthalmology (AAO), other lesions that need treatment include acute symptomatic retinal dialysis and traumatic retinal breaks.[12] In this case, the treatment options include:
- Laser Retinopexy: This procedure is performed under topical anesthesia. Two to three rows of confluent burns are applied around the lesion.
- Cryo-retinopexy: This procedure is performed under regional or subconjunctival anesthesia. If the lesion is beyond the equator, a subconjunctival incision may be given in order to get clear access. Using a binocular indirect ophthalmoscope, the lesion is carefully indented, and the cryoprobe is activated until the whitening of the retina is seen. The probe should not be removed from the operated site until thawing occurs (2-3s), As this might lead to subretinal hemorrhage.[50] The lesion is surrounded by a single confluent row of applications. (B3)
After laser or cryo-retinopexy, the patient should be advised to take rest and avoid strenuous exercise to ensure proper adhesion of the tear. Laser retinopexy is superior to cryo-retinopexy as it is more precise and causes less collateral retinal damage.[51] The risk of epiretinal membrane formation is considerably less with laser retinopexy than cryo-retinopexy.[52][53] On the other hand, cryotherapy is preferred in eyes with the hazy cornea and small pupils.(B2)
Differential Diagnosis
Causes of Photopsia other than posterior vitreous detachment include the following:
- A retinal tear or retinal detachment
- Migraine with aura (classic)
- Migraine headache without aura
- Posterior uveitis (multiple evanescent white dot syndrome, acute idiopathic blind spot enlargement syndrome, acute posterior multifocal placoid pigment epitheliopathy, acute zonal occult outer retinopathy, multifocal choroiditis, and panuveitis, Birdshot retino-choroiditis) [54]
- Both early and the late stage of retinitis pigmentosa [55]
Causes of floaters other than posterior vitreous detachment include the following:
- Vitreous hemorrhage due to any cause including retinal tear or retinal detachment, proliferative diabetic retinopathy
- Vitreous exudates in posterior uveitis, endophthalmitis
- Vitreous pigments
- Vitreous amyloidosis
- Intravitreal injection of drugs
Prognosis
Uncomplicated posterior vitreous detachment usually has a good visual prognosis, whereas the prognosis of anomalous PVD depends on the cause and the complications associated with it.
Complications
Various deleterious effects upon retina as well as vitreous occur as a result of abnormal traction at the vitreoretinal interface. The traction over the retina can result in a macular pucker, macular hole, or complicated proliferative diabetic vitreoretinopathy.[32]
Important complications of posterior vitreous detachment are listed below:
- Retinal breaks/tears
- Retinal detachment
- Vitreous hemorrhage
- Retinal hemorrhage
- Cystoid macular edema
- Macular hole
Deterrence and Patient Education
Patients should be counseled according to the severity of the disease. In the case of mild floaters, the patient should be assured that in most cases, these floaters settle on their own and become less noticeable. Patients should be given hand-written instructions emphasizing the need to re-consult if new symptoms like a sudden increase in floaters/flashes, vision loss, and peripheral loss of vision appear.
Enhancing Healthcare Team Outcomes
The diagnosis and management of posterior vitreous detachment are crucial. The patient presenting with complaints of sudden onset floaters or photopsia should be managed by a team, including an optometrist, general ophthalmologist, experienced vitreoretinal expert, and ophthalmology nurses. The nurses participate in patients' education, counseling, and follow up, informing the ophthalmologist of any issues. This collaborative approach can ensure optimal patient outcomes.
Media
(Click Image to Enlarge)
References
Linsenmayer TF,Gibney E,Little CD, Type II collagen in the early embryonic chick cornea and vitreous: immunoradiochemical evidence. Experimental eye research. 1982 Mar; [PubMed PMID: 7067745]
Level 3 (low-level) evidenceScott JE, The chemical morphology of the vitreous. Eye (London, England). 1992; [PubMed PMID: 1289129]
Milston R,Madigan MC,Sebag J, Vitreous floaters: Etiology, diagnostics, and management. Survey of ophthalmology. 2016 Mar-Apr; [PubMed PMID: 26679984]
Level 3 (low-level) evidenceJohnson MW, Posterior vitreous detachment: evolution and complications of its early stages. American journal of ophthalmology. 2010 Mar; [PubMed PMID: 20172065]
Nuzzi R,Marchese A,Gulino GR,Versino E,Ghigo D, Influence of posterior vitreous detachment and type of intraocular lens on lipid peroxidation in the human vitreous. Molecular vision. 2015 [PubMed PMID: 26396488]
Le Goff MM,Bishop PN, Adult vitreous structure and postnatal changes. Eye (London, England). 2008 Oct [PubMed PMID: 18309340]
Foos RY,Wheeler NC, Vitreoretinal juncture. Synchysis senilis and posterior vitreous detachment. Ophthalmology. 1982 Dec; [PubMed PMID: 7162795]
Bond-Taylor M,Jakobsson G,Zetterberg M, Posterior vitreous detachment - prevalence of and risk factors for retinal tears. Clinical ophthalmology (Auckland, N.Z.). 2017 [PubMed PMID: 29075095]
Coffee RE,Westfall AC,Davis GH,Mieler WF,Holz ER, Symptomatic posterior vitreous detachment and the incidence of delayed retinal breaks: case series and meta-analysis. American journal of ophthalmology. 2007 Sep [PubMed PMID: 17583667]
Level 2 (mid-level) evidenceShunmugam M,Shah AN,Hysi PG,Williamson TH, The pattern and distribution of retinal breaks in eyes with rhegmatogenous retinal detachment. American journal of ophthalmology. 2014 Jan [PubMed PMID: 24200230]
Protein-sugar interactions: preparation, purification, and properties of rabbit antibodies against di-N-acetylchitobiose., Kieda CM,Delmotte FM,Monsigny ML,, Proceedings of the National Academy of Sciences of the United States of America, 1977 Jan [PubMed PMID: 15824220]
Flaxel CJ,Adelman RA,Bailey ST,Fawzi A,Lim JI,Vemulakonda GA,Ying GS, Posterior Vitreous Detachment, Retinal Breaks, and Lattice Degeneration Preferred Practice Pattern®. Ophthalmology. 2020 Jan [PubMed PMID: 31757500]
Yonemoto J,Ideta H,Sasaki K,Tanaka S,Hirose A,Oka C, The age of onset of posterior vitreous detachment. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1994 Feb; [PubMed PMID: 8157177]
FAVRE M,GOLDMANN H, [Genesis of posterior vitreus body detachment]. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1956 Aug; [PubMed PMID: 13378828]
Chuo JY,Lee TY,Hollands H,Morris AH,Reyes RC,Rossiter JD,Meredith SP,Maberley DA, Risk factors for posterior vitreous detachment: a case-control study. American journal of ophthalmology. 2006 Dec; [PubMed PMID: 17157578]
Level 2 (mid-level) evidenceHayashi K,Sato T,Manabe SI,Hirata A, Sex-Related Differences in the Progression of Posterior Vitreous Detachment with Age. Ophthalmology. Retina. 2019 Mar [PubMed PMID: 31014700]
Morita H,Funata M,Tokoro T, A clinical study of the development of posterior vitreous detachment in high myopia. Retina (Philadelphia, Pa.). 1995; [PubMed PMID: 7624598]
Hikichi T,Akiba J,Trempe CL, Prevalence of posterior vitreous detachment in retinitis pigmentosa. Ophthalmic surgery. 1995 Jan-Feb [PubMed PMID: 7746622]
Ronan SM,Tran-Viet KN,Burner EL,Metlapally R,Toth CA,Young TL, Mutational hot spot potential of a novel base pair mutation of the CSPG2 gene in a family with Wagner syndrome. Archives of ophthalmology (Chicago, Ill. : 1960). 2009 Nov [PubMed PMID: 19901218]
Hogan MJ, Inflammation and its effect on the vitreous. Transactions of the ophthalmological societies of the United Kingdom. 1975 [PubMed PMID: 1066854]
Hsu HT,Patterson R,Ryan SJ, Traumatic posterior vitreous detachment: scanning electron microscopy of an experimental model in the monkey eye. Scanning electron microscopy. 1984 [PubMed PMID: 6438790]
Level 3 (low-level) evidenceCoppé AM,Lapucci G, Posterior vitreous detachment and retinal detachment following cataract extraction. Current opinion in ophthalmology. 2008 May [PubMed PMID: 18408500]
Level 3 (low-level) evidenceSebag J,Buzney SM,Belyea DA,Kado M,McMeel JW,Trempe CL, Posterior vitreous detachment following panretinal laser photocoagulation. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1990 [PubMed PMID: 2311946]
Hesse L,Nebeling B,Schroeder B,Heller G,Kroll P, Induction of posterior vitreous detachment in rabbits by intravitreal injection of tissue plasminogen activator following cryopexy. Experimental eye research. 2000 Jan; [PubMed PMID: 10644418]
Level 3 (low-level) evidenceHendrikse F,Yeo KT, [Role of the vitreous body in diabetic retinopathy]. Klinische Monatsblatter fur Augenheilkunde. 1993 Nov [PubMed PMID: 8114473]
Romano MR,Comune C,Ferrara M,Cennamo G,De Cillà S,Toto L,Cennamo G, Retinal Changes Induced by Epiretinal Tangential Forces. Journal of ophthalmology. 2015; [PubMed PMID: 26421183]
Rejection by syngeneic mice of cell variants obtained by mutagenesis of a malignant teratocarcinoma cell line., Boon T,Kellermann O,, Proceedings of the National Academy of Sciences of the United States of America, 1977 Jan [PubMed PMID: 8944097]
Level 2 (mid-level) evidenceBrown GC,Brown MM,Fischer DH, Photopsias: A Key to Diagnosis. Ophthalmology. 2015 Oct [PubMed PMID: 26249730]
Akiba J,Ishiko S,Yoshida A, Variations of Weiss's ring. Retina (Philadelphia, Pa.). 2001; [PubMed PMID: 11421014]
Johnson MW, Perifoveal vitreous detachment and its macular complications. Transactions of the American Ophthalmological Society. 2005 [PubMed PMID: 17057817]
Level 2 (mid-level) evidenceTanner V,Harle D,Tan J,Foote B,Williamson TH,Chignell AH, Acute posterior vitreous detachment: the predictive value of vitreous pigment and symptomatology. The British journal of ophthalmology. 2000 Nov [PubMed PMID: 11049952]
Sebag J, Anomalous posterior vitreous detachment: a unifying concept in vitreo-retinal disease. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2004 Aug; [PubMed PMID: 15309558]
Level 3 (low-level) evidenceFincham GS,James S,Spickett C,Hollingshead M,Thrasivoulou C,Poulson AV,McNinch A,Richards A,Snead D,Limb GA,Snead MP, Posterior Vitreous Detachment and the Posterior Hyaloid Membrane. Ophthalmology. 2018 Feb; [PubMed PMID: 28867131]
Kakehashi A,Takezawa M,Akiba J, Classification of posterior vitreous detachment. Clinical ophthalmology (Auckland, N.Z.). 2014 [PubMed PMID: 24376338]
Huang D,Swanson EA,Lin CP,Schuman JS,Stinson WG,Chang W,Hee MR,Flotte T,Gregory K,Puliafito CA, Optical coherence tomography. Science (New York, N.Y.). 1991 Nov 22; [PubMed PMID: 1957169]
Uchino E,Uemura A,Ohba N, Initial stages of posterior vitreous detachment in healthy eyes of older persons evaluated by optical coherence tomography. Archives of ophthalmology (Chicago, Ill. : 1960). 2001 Oct [PubMed PMID: 11594947]
Chan A,Duker JS,Schuman JS,Fujimoto JG, Stage 0 macular holes: observations by optical coherence tomography. Ophthalmology. 2004 Nov [PubMed PMID: 15522368]
Level 3 (low-level) evidencevan Etten PG,van Overdam KA,Reyniers R,Veckeneer M,Faridpooya K,Wubbels RJ,Manning S,La Heij EC,van Meurs JC, STRICT POSTURING WITH OR WITHOUT BILATERAL PATCHING FOR POSTERIOR VITREOUS DETACHMENT-RELATED VITREOUS HEMORRHAGE. Retina (Philadelphia, Pa.). 2020 Jun [PubMed PMID: 31136460]
Sandinha MT,Kotagiri AK,Owen RI,Geenen C,Steel DH, Accuracy of B-scan ultrasonography in acute fundus obscuring vitreous hemorrhage using a standardized scanning protocol and a dedicated ophthalmic ultrasonographer. Clinical ophthalmology (Auckland, N.Z.). 2017 [PubMed PMID: 28794614]
Kim YK,Moon SY,Yim KM,Seong SJ,Hwang JY,Park SP, Psychological Distress in Patients with Symptomatic Vitreous Floaters. Journal of ophthalmology. 2017 [PubMed PMID: 29375909]
Henry CR,Smiddy WE,Flynn HW Jr, Pars plana vitrectomy for vitreous floaters: is there such a thing as minimally invasive vitreoretinal surgery? Retina (Philadelphia, Pa.). 2014 Jun; [PubMed PMID: 24589875]
Tripathy K, Is Floaterectomy Worth the Risks? Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2017 May-Jun [PubMed PMID: 28558177]
de Nie KF,Crama N,Tilanus MA,Klevering BJ,Boon CJ, Pars plana vitrectomy for disturbing primary vitreous floaters: clinical outcome and patient satisfaction. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2013 May; [PubMed PMID: 23250478]
Level 2 (mid-level) evidenceSchulz-Key S,Carlsson JO,Crafoord S, Longterm follow-up of pars plana vitrectomy for vitreous floaters: complications, outcomes and patient satisfaction. Acta ophthalmologica. 2011 Mar; [PubMed PMID: 19860781]
Level 2 (mid-level) evidenceErratum: Borderud SP, Li Y, Burkhalter JE, Sheffer CE and Ostroff JS. Electronic cigarette use among patients with cancer: Characteristics of electronic cigarette users and their smoking cessation outcomes. Cancer. doi: 10.1002/ cncr.28811. Cancer. 2015 Mar 1; [PubMed PMID: 25855820]
Delaney YM,Oyinloye A,Benjamin L, Nd:YAG vitreolysis and pars plana vitrectomy: surgical treatment for vitreous floaters. Eye (London, England). 2002 Jan; [PubMed PMID: 11913884]
Level 2 (mid-level) evidenceSebag J, Pharmacologic vitreolysis--premise and promise of the first decade. Retina (Philadelphia, Pa.). 2009 Jul-Aug; [PubMed PMID: 19584647]
Khoshnevis M,Sebag J, Pharmacologic vitreolysis with ocriplasmin: rationale for use and therapeutic potential in vitreo-retinal disorders. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2015 Apr [PubMed PMID: 25812991]
Level 3 (low-level) evidenceSelective and accurate transcription of the Xenopus laevis 5S RNA genes in isolated chromatin by purified RNA polymerase III., Parker CS,Roeder RG,, Proceedings of the National Academy of Sciences of the United States of America, 1977 Jan [PubMed PMID: 24062204]
Takayama K,Enoki T,Kojima T,Ishikawa S,Takeuchi M, Treatment of peripheral exudative hemorrhagic chorioretinopathy by intravitreal injections of ranibizumab. Clinical ophthalmology (Auckland, N.Z.). 2012 [PubMed PMID: 22791965]
Level 3 (low-level) evidenceEmsley E,Steptoe PJ,Cazabon S, Management of a rhegmatogenous retinal detachment in a low-resource setting: treatment options when there is no vitreoretinal surgeon. BMJ case reports. 2018 Mar 28 [PubMed PMID: 29592990]
Level 3 (low-level) evidenceChalam KV,Murthy RK,Gupta SK,Khetpal V, Prophylactic circumferential intraoperative laser retinopexy decreases the risk of retinal detachment after macular hole surgery. European journal of ophthalmology. 2012 Sep-Oct; [PubMed PMID: 22344467]
Level 2 (mid-level) evidenceParolini B,Prigione G,Romanelli F,Cereda MG,Sartore M,Pertile G, Postoperative complications and intraocular pressure in 943 consecutive cases of 23-gauge transconjunctival pars plana vitrectomy with 1-year follow-up. Retina (Philadelphia, Pa.). 2010 Jan; [PubMed PMID: 19816241]
Level 2 (mid-level) evidenceSudharshan S,Ganesh SK,Biswas J, Current approach in the diagnosis and management of posterior uveitis. Indian journal of ophthalmology. 2010 Jan-Feb [PubMed PMID: 20029144]
Bittner AK,Diener-West M,Dagnelie G, A survey of photopsias in self-reported retinitis pigmentosa: location of photopsias is related to disease severity. Retina (Philadelphia, Pa.). 2009 Nov-Dec [PubMed PMID: 19730162]
Level 3 (low-level) evidence