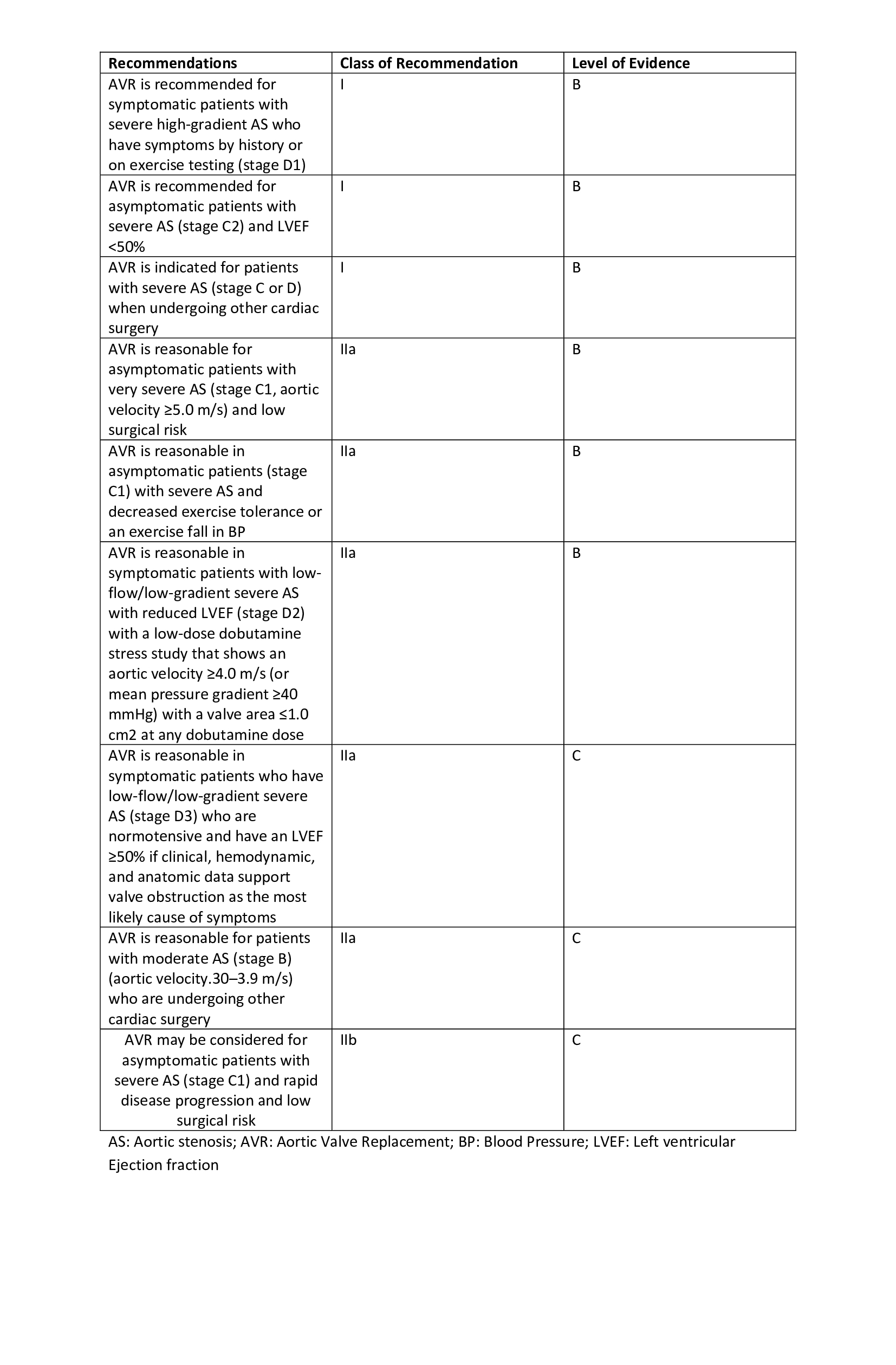
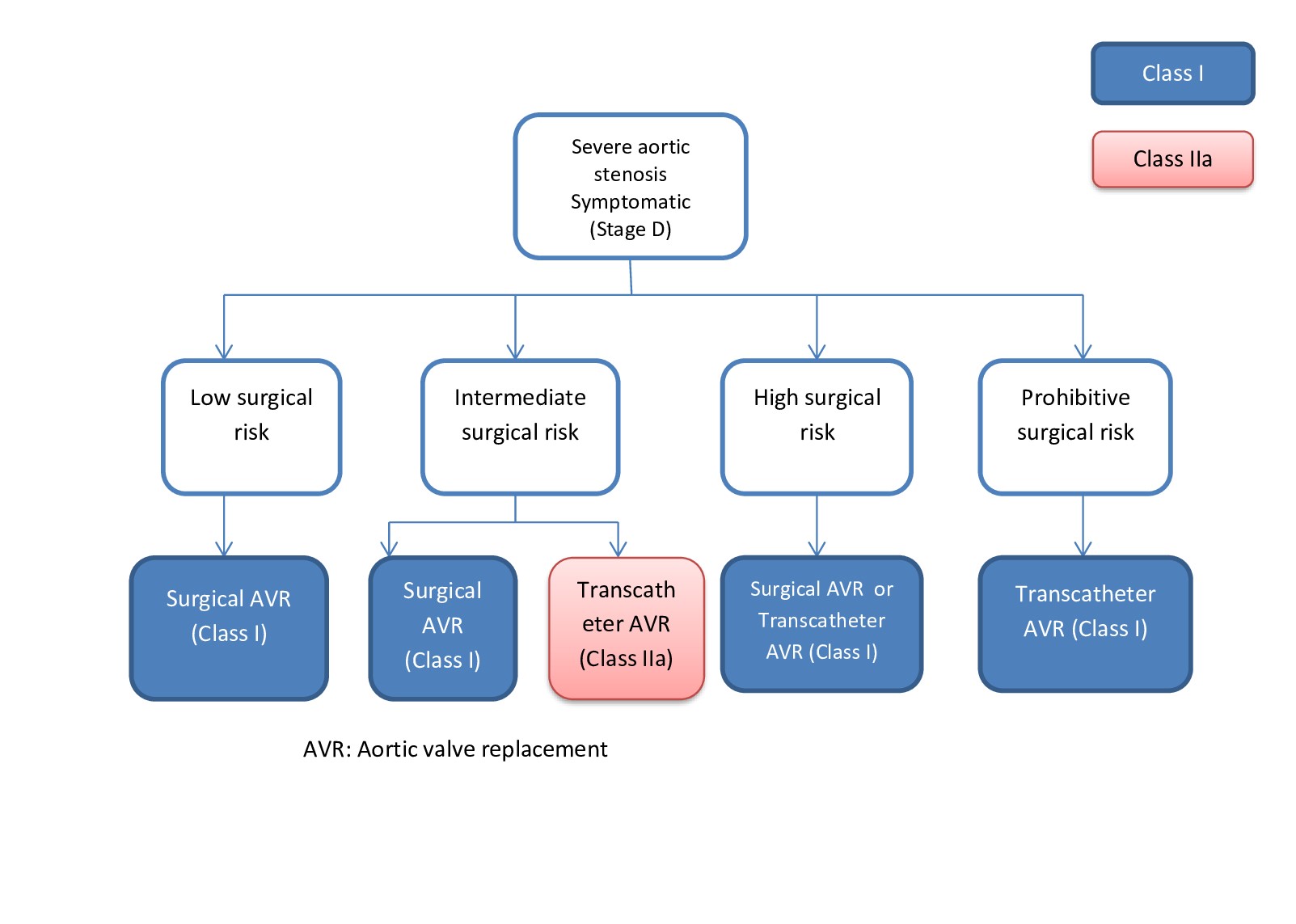
[1]
Zoltowska DM, Agrawal Y, Patel N, Sareen N, Kalavakunta JK, Gupta V, Halabi A. Association Between Pulmonary Hypertension and Transcatheter Aortic Valve Replacement: Analysis of a Nationwide Inpatient Sample Database. Reviews on recent clinical trials. 2019:14(1):56-60. doi: 10.2174/1574887113666181120113034. Epub
[PubMed PMID: 30457054]
[2]
Iantorno M, Ben-Dor I, Rogers T, Gajanana D, Attaran S, Buchanan KD, Satler LF, Shults CC, Thourani VH, Waksman R. Emergent valve-in-valve transcatheter aortic valve replacement in patient with acute aortic regurgitation and cardiogenic shock with preoperative extracorporeal membrane oxygenator: A case report and review of the literature. Cardiovascular revascularization medicine : including molecular interventions. 2018 Dec:19(8S):68-70. doi: 10.1016/j.carrev.2018.11.007. Epub 2018 Nov 12
[PubMed PMID: 30455139]
Level 3 (low-level) evidence
[3]
Henn MC, Zajarias A, Quader N, Sintek M, Lasala JM, Koogler K, Damiano MS, Kachroo P, Miller DC, King CR, Melby SJ, Moon MR, Damiano RJ Jr, Maniar HS. Observed to expected 30-day mortality as a benchmark for transcatheter aortic valve replacement. The Journal of thoracic and cardiovascular surgery. 2019 Mar:157(3):874-882.e8. doi: 10.1016/j.jtcvs.2018.06.097. Epub 2018 Jul 27
[PubMed PMID: 30454980]
[4]
Wilson R, McNabney C, Weir-McCall JR, Sellers S, Blanke P, Leipsic JA. Transcatheter Aortic and Mitral Valve Replacements. Radiologic clinics of North America. 2019 Jan:57(1):165-178. doi: 10.1016/j.rcl.2018.08.001. Epub 2018 Oct 31
[PubMed PMID: 30454811]
[5]
Lateef N, Khan MS, Deo SV, Yamani N, Riaz H, Virk HUH, Khan SU, Hedrick DP, Kanaan A, Reed GW, Krishnaswamy A, Puri R, Kapadia SR, Kalra A. Meta-Analysis Comparing Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation With Versus Without Percutaneous Coronary Intervention. The American journal of cardiology. 2019 Dec 1:124(11):1757-1764. doi: 10.1016/j.amjcard.2019.08.024. Epub 2019 Sep 26
[PubMed PMID: 31575422]
Level 1 (high-level) evidence
[6]
Edelman JJ, Thourani VH. Transcatheter aortic valve replacement and surgical aortic valve replacement: Both excellent therapies. The Journal of thoracic and cardiovascular surgery. 2018 Dec:156(6):2135-2137. doi: 10.1016/j.jtcvs.2018.07.065. Epub
[PubMed PMID: 30449571]
[7]
Gillam LD, Marcoff L. Echocardiographic Assessment of Transcatheter Aortic Valve Hemodynamics: More Tools for the Toolbox. JACC. Cardiovascular imaging. 2019 Jan:12(1):35-37. doi: 10.1016/j.jcmg.2018.05.015. Epub 2018 Nov 15
[PubMed PMID: 30448141]
[8]
Chetcuti SJ, Deeb GM, Popma JJ, Yakubov SJ, Grossman PM, Patel HJ, Casale A, Dauerman HL, Resar JR, Boulware MJ, Dries-Devlin JL, Li S, Oh JK, Reardon MJ. Self-Expanding Transcatheter Aortic Valve Replacement in Patients With Low-Gradient Aortic Stenosis. JACC. Cardiovascular imaging. 2019 Jan:12(1):67-80. doi: 10.1016/j.jcmg.2018.07.028. Epub 2018 Nov 15
[PubMed PMID: 30448116]
[9]
Coughlan JJ, Kiernan T, Mylotte D, Arnous S. Annular Rupture During Transcatheter Aortic Valve Implantation: Predictors, Management and Outcomes. Interventional cardiology (London, England). 2018 Sep:13(3):140-144. doi: 10.15420/icr.2018.20.2. Epub
[PubMed PMID: 30443272]
[10]
Ando T, Adegbala O, Villablanca PA, Briasoulis A, Takagi H, Grines CL, Schreiber T, Nazif T, Kodali S, Afonso L. In-hospital outcomes of transcatheter versus surgical aortic valve replacement in non-teaching hospitals. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2019 Apr 1:93(5):954-962. doi: 10.1002/ccd.27968. Epub 2018 Nov 8
[PubMed PMID: 30408309]
[11]
Rodés-Cabau J, Sacco RL. Neurological Complications Following Aortic Valve Replacement: TAVR Better Than SAVR, But Room for Improvement. Journal of the American College of Cardiology. 2018 Oct 30:72(18):2120-2122. doi: 10.1016/j.jacc.2018.06.080. Epub
[PubMed PMID: 30360821]
[12]
Chen S, Redfors B, Ben-Yehuda O, Crowley A, Greason KL, Alu MC, Finn MT, Vahl TP, Nazif T, Thourani VH, Suri RM, Svensson L, Webb JG, Kodali SK, Leon MB. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Prior Cardiac Surgery in the Randomized PARTNER 2A Trial. JACC. Cardiovascular interventions. 2018 Nov 12:11(21):2207-2216. doi: 10.1016/j.jcin.2018.08.006. Epub 2018 Aug 28
[PubMed PMID: 30409278]
Level 1 (high-level) evidence
[13]
Spaziano M, Lefèvre T, Romano M, Eltchaninoff H, Leprince P, Motreff P, Iung B, Van Belle E, Koning R, Verhoye JP, Gilard M, Garot P, Hovasse T, Le Breton H, Chevalier B. Transcatheter Aortic Valve Replacement in the Catheterization Laboratory Versus Hybrid Operating Room: Insights From the FRANCE TAVI Registry. JACC. Cardiovascular interventions. 2018 Nov 12:11(21):2195-2203. doi: 10.1016/j.jcin.2018.06.043. Epub
[PubMed PMID: 30409276]
[14]
Egger F, Zweiker D, Freynhofer MK, Löffler V, Rohla M, Geppert A, Farhan S, Vogel B, Falkensammer J, Kastner J, Pichler P, Vock P, Lamm G, Luha O, Schmidt A, Scherr D, Hammerer M, Hoppe UC, Maurer E, Grund M, Lambert T, Tkalec W, Sturmberger T, Zeindlhofer E, Grabenwöger M, Huber K, Austrian TAVI Group. Impact of On-Site Cardiac Surgery on Clinical Outcomes After Transfemoral Transcatheter Aortic Valve Replacement. JACC. Cardiovascular interventions. 2018 Nov 12:11(21):2160-2167. doi: 10.1016/j.jcin.2018.07.015. Epub
[PubMed PMID: 30409272]
Level 2 (mid-level) evidence