Introduction
The oculomotor nerve (the third cranial nerve; CN III) has three main motor functions:
- Innervation to the pupil and lens (autonomic, parasympathetic)
- Innervation to the upper eyelid (somatic)
- Innervation of the eye muscles that allow for visual tracking and gaze fixation (somatic)
Like all other nerve fibers in the human body, the oculomotor nerve can become impaired in disease states which can lead to lifelong impairment in normal vision. Dysfunction can also be indicative of more serious underlying diseases, such as an aneurysm or a neoplasm.
Structure and Function
The oculomotor nerve originates from 2 nuclei in the midbrain[1]:
- Oculomotor nucleus
- Accessory parasympathetic nucleus (Edinger-Westphal nucleus)
The oculomotor nerve exits the brainstem near midline at the base of the midbrain just caudal to the mammillary bodies. It passes through the cavernous sinus and proceeds through the supraorbital fissure to reach the orbit of the eye (Figure 1).
The third cranial nerve has both somatic and autonomic fibers. Somatic (voluntary) nerve fibers are bundled deep inside the nerve, while the autonomic (involuntary) fibers surround the somatic fibers around the outside of the nerve. Knowing the spatial layout of these fibers will help one understand the various forms of presentation in third nerve palsies.
Somatic (voluntary) functions of the oculomotor nerve include elevation of the upper eyelid via innervation of the levator palpebrae superioris muscle. Other essential functions include coordination of eye muscles for visual tracking and gaze fixation. These functions of eye movement occur through innervation of four eye muscles:
- Superior rectus muscle - elevates the eye while looking straight ahead (primary position)
- Medial rectus muscle - adducts the eye from a primary position
- Inferior rectus muscle - moves the eye down from a primary position
- Inferior oblique muscle - elevates the eye when the eye is adducted from a primary position
There are two primary functions of the autonomic parasympathetic (involuntary) oculomotor nerve. It constricts the pupil (miosis) by innervating the smooth muscle (sphincter pupillae) near the pupil. It also innervates the ciliary muscles. These muscles connect the iris to the choroid. Contraction of the muscle alters the curvature of the lens which allows individuals to focus the lens on near objects (see Figure. Oculomotor Nerve Palsy).[2]
Embryology
The oculomotor nerve and its associated cranial nerve nuclei exist within the midbrain. The midbrain develops from the mesencephalon. Neuroblasts from the basal plates develop into the tegmentum. The tegmentum includes cranial nerves III and IV, Edinger-Westphal nuclei, oculomotor nuclei, trochlear nuclei, red nuclei, and reticular nuclei.
Cranial nerve III has somatic and autonomic functions. Somatic nerves are homologous with ventral roots of spinal nerves. They originate from the basal plate and innervates the superior rectus, inferior rectus, medial rectus, and inferior oblique muscles. These muscles derive from the first preoptic myotome.
Most research about the development of parasympathetic nerves and the ciliary ganglion has been on chickens. The parasympathetic nerve supply of the oculomotor nerves develops from the caudal midbrain and rostral hindbrain neural crest cells. Some cells may also originate from the ectodermal placode caudal to the eye.[3][4] These cells migrate ventrolaterally and rostrally toward the optic vesicle. As the neurons in the ciliary ganglion differentiate, they extend axons to innervate the blood vessels of the choroid plexus as well as the striated muscles of the iris and ciliary body.[5]
Blood Supply and Lymphatics
The somatic and autonomic components of the oculomotor nerve have differentiated vascular supplies. The vasa vasorum supplies the inner somatic (voluntary) nerve fibers while pia mater blood vessels supply the outer autonomic nerve fibers.[2]
Lymphatic drainage of the orbit of the eye is not yet well understood. There is morphological evidence for lymphatic structures within the lacrimal gland and arachnoid; however, distinctive lymphatic structures within other regions of the orbit are currently unknown.[6]
Both the oculomotor nucleus and Edinger-Westphal nucleus are both located in the medial midbrain, which is supplied by the paramedian branches of the upper basilar artery and the proximal posterior cerebral artery.
Nerves
The oculomotor nerve helps to adjust and coordinate eye position during movement. Several movements assist with this process: saccades, smooth pursuit, fixation, accommodation, vestibulo-ocular reflex, and optokinetic reflex.
Saccades are rapid, jerky motions of the eye. This type of motion typically occurs when moving a gaze between objects. When tracking an object, we use smooth pursuit to keep our eyes focused on an object as it moves. When we want to stare at an object, we fixate on that object. Control of each of these actions is by either vestibule-ocular or optokinetic reflexes.
The vestibule-ocular reflex adjusts eye position during fast movements of the head. Head motion activates cells within the semicircular canals. Information about the motion is transmitted to the ipsilateral vestibular nucleus and forwarded to the oculomotor nucleus. Therefore, if the head moves to the left, the eyes move to the right to keep the gaze steady.
The optokinetic reflex adjusts eye position in response to changes in the visual field. This reflex initiates from visual information. Information about the visual field is transmitted from the parieto-occipital eye field through the pontine nuclei to the vestibulo-cerebellum. It then moves through the vestibular nuclei to the oculomotor nucleus where eye movement initiates. This reflex allows the eyes to follow large objects in the visual field.
The last action is accommodation. This action helps to keep the gaze focused when both vestibular and visual stimuli change. It requires the interaction of circuits between the vestibule-ocular and optokinetic reflexes.[7][8]
Deficits within the peripheral or central vestibular system will result in an inappropriate quick gaze deviation movements of the eyes “nystagmus.” The direction of the fast phase of the nystagmus can assist clinicians in the diagnosis of a patient’s pathology.
Muscles
The oculomotor nerve controls several muscles:
- Levator palpebrae superioris - raises the upper eyelid
- Superior rectus muscle - rotates the eyeball backward, "looking up"
- Medial rectus muscle - adducts the eye, "looking towards your nose"
- Inferior rectus muscle - rotates the eyeball forwards, "looking down"
- Inferior oblique muscle - rotates the eyeball backward when the eye is adducted
- Ciliary muscle - controls lens shape to focus on up-close objects
- Sphincter pupillae - constricts the pupil
Control of other eye muscles is by the trochlear (CN IV) and abducens (CN VI) nerves.
The trochlear nerve (4th cranial nerve) controls:
- Superior oblique muscle - rotates the eyeball forward when the eye is adducted
The abducens nerve (6th cranial nerve) controls:
- Lateral rectus muscle - abducts the eye, "looking towards your ear on the same side"
Physiologic Variants
Congenital oculomotor palsy is a rare condition that is typically caused by intracranial pressure at the junction between the posterior communicating artery, and the internal carotid artery causes a local compression of the oculomotor nerve. Symptoms of this disorder include a unilaterally fixed pupil size that is unreactive to light. Additional symptoms include unilateral ptosis and decreased visual acuity.
While oculomotor palsy is not life-threatening, the presence at birth may be a sign of other congenital malformations (e.g., PHACE syndrome, type 2 neurofibromatosis, Klippel-Trenaunay syndrome, etc.). Even without other neurological signs, clinicians should take care to examine these patients for more significant malformations.[9]
Surgical Considerations
One of the main goals of intracranial neurosurgery should be to avoid postsurgical complications. A dilated pupil and ptosis is the most common clinical presentation of oculomotor nerve palsy post-neurosurgery, usually after clipping of an intracranial aneurysm.[10] Patients who undergo open surgery or a minimally invasive technique on basilar artery aneurysms may develop a third nerve palsy after surgery, although this complication has been shown to be transient.[11]
Clinical Significance
Recognizing oculomotor nerve dysfunction (oculomotor nerve palsy) is crucial because it can be a sign of underlying or uncontrolled chronic disease states. It is an essential clinical symptom that can be life-saving in certain circumstances.
Pathophysiology
The cause of oculomotor nerve palsy is most commonly by microvascular diseases including diabetes, hypertension, compression from an intracranial neoplasm or an aneurysm, or trauma.[10]
The microvascular disease can affect the vasa vasorum. In these instances, the fibers innervating the upper eyelid and most of the extraocular muscles become ischemic. Compression of the oculomotor nerve can also occur via an aneurysm, intracranial neoplasm, or uncal herniation. These ischemic events can affect the outer pia blood vessels supplying the fibers to the pupil and lens and eventually the inner vasa vasorum.
Clinical Signs
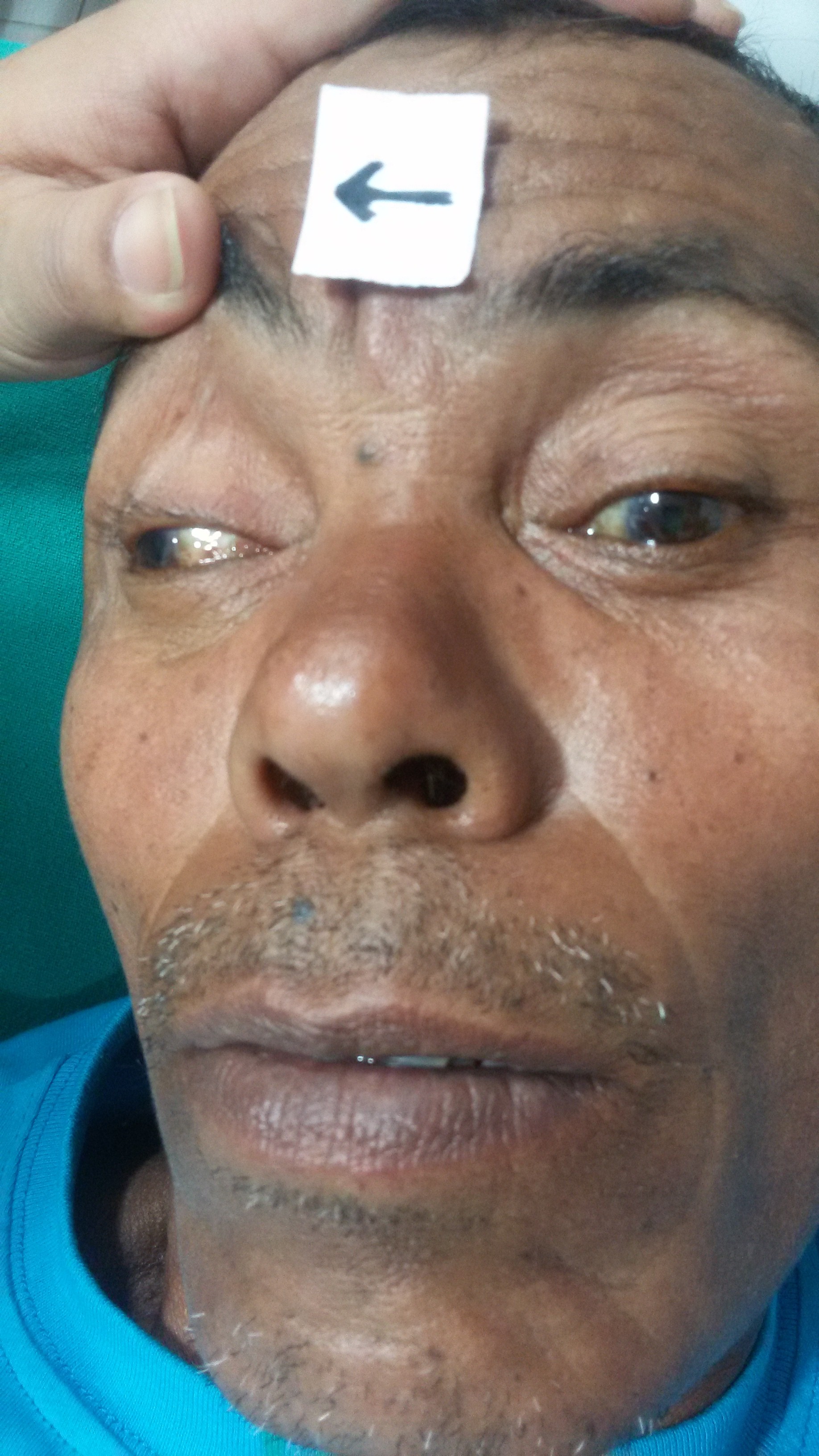
- Ptosis (droopy eyelid) - due to paralysis of levator palpebrae superioris
- "Down and out" positioning of the affected eye
- Position caused by unopposed action of:
- lateral rectus muscle (eye abduction action)
- innervation = abducens nerve (VI)
- superior oblique muscle (Adduct and move eye down)
- innervation = trochlear nerve (IV)
- Diplopia ("seeing double") - due to a misalignment of the eyes (ptosis may block the vision in one eye, resulting in the absence of diplopia)
- Dilated pupil - caused by lack of parasympathetic innervation
- Abnormal swinging flashlight test - if parasympathetic nerve fibers are affected, shining a light into EITHER eye will NOT result in pupillary constriction of the affected eye
- Inability to accommodate - this may be difficult to assess in an older patient because their lens may already be hard and difficult to change shape due to a normal part of the aging process
Labs and Imaging
Evaluation should base its conclusions on the presence or absence of pupil involvement.
If the pupil is spared, obtain:
- Blood pressure measurement
- HbA1C
- Complete blood count
- Erythrocyte sedimentation rate
If the pupil is involved, compression from the outside should be suspected, and one should obtain[2]:
- Prompt referral to a neuro-ophthalmologist
- Magnetic resonance imaging (MRI)
- Computed tomogram (CT) angiography (if an aneurysm suspected)
Conservative Management
Treatment is conservative in the acute phase.
If diplopia is present and distressful, an eye patch, opaque contact lens, or a blurred lens in glasses covering the affected eye can be helpful.
Botulinum toxin can be useful in the acute setting of partial third nerve palsy with horizontal deviation; best used with paralysis of only the medial rectus. With injection into the overcompensating lateral rectus in these cases, horizontal deviation in the primary position is correctable. If there is mild vertical deviation remaining, prisms in glasses may be of some help. Botulinum toxin should be avoided in vertical muscle imbalance due to the high rate of complications.[12]
Most patients (63%) with an acquired third nerve palsy recover completely with conservative management. Patients with microvascular etiology having the greatest chance of full recovery.[10]
Surgical management
Minimal or no improvement after 6 months of conservative therapy warrants strabismus surgery. Strabismus surgery involves fixing the malalignment of the extraocular muscles. Surgery usually involves a combination of a recession (cutting the muscle from the eye and reattaching it posterior to the initial position to WEAKEN the muscle's function) and resection (removing a section of the muscle to shorten it, STRENGTHENING the muscle's function). Depending on the pair of muscles involved, the surgeon will typically recess the contracted muscle while resecting on the antagonist muscle.[12]
If the pupil is involved, patients should undergo a thorough evaluation by a neurologist.[2]
Other Issues
As individuals age, it is common for individuals to lose the ability to focus within the near range (presbyopia). In these individuals, the ciliary muscle contracts when stimulated. However, the muscle's physical characteristics will show differences that correlate with the presbyopia deficit. Researchers report that in such cases, the muscle is shorter and the internal apical edge moves forward. These results suggest that changes in the muscle, and not changes in the innervation of the muscle, may account for the presbyopia.[13]