Continuing Education Activity
Melanoma is a malignant tumor produced from malignant transformation of melanocytes. This can be sporadic or arise from a preexisting premalignant lesion. Due to the fact that melanocytes are of neural crest origin, melanomas can arise in other locations where neural crest cells are present, including the brain and gastrointestinal tract. Approximately 10 to 25% of melanomas are found in the head and neck region. This activity reviews the pathophysiology and causes of melanoma and highlights the role of the interprofessional team in its management.
Objectives:
- Review the different types of melanoma.
- Describe the pathophysiology of melanoma.
- Summarize the treatment options for melanoma.
- Outline the importance of improving care coordination among interprofessional team members to improve outcomes for patients affected by melanoma.
Introduction
Melanoma is a malignant tumor produced from malignant transformation of melanocytes. This can be sporadic or arise from a preexisting premalignant lesion. Due to the fact that melanocytes are of neural crest origin, melanomas can arise in other locations where neural crest cells are present, including the brain and gastrointestinal tract. Approximately 10% to 25% of melanomas are found in the head and neck region. The most common sites are the occipital scalp and skin of the cheek. Common locations are face (40 to 60%), scalp (14 to 49%), neck (20 to 29%), and ear (8 to 11%). Location of the lesion is of particular importance when addressing melanoma in the head and neck as some locations had a worse prognosis. Additionally, the location requires different management compared to other parts of the body.[1][2][3][4]
Etiology
Melanoma Risks
- Ultraviolet (UV) light exposure: Frequent and intermittent exposure to sunlight appears to be the highest risk factor for cutaneous melanoma. UV light causes photochemical reactions and damage in DNA, leading to pyrimidine dimers (cytosine and thymine). UVB light (290 to 310 nanometers) is attributed to more potent damage of DNA. Although, in natural sunlight, UVA (320 to 400 nanometers) is present more so than UVB. However, UVA can also cause oxidative DNA damage. UVA is said to mutate DNA via non-NDA endogenous sensitizers. Sunscreen and sunblock have SPF ratings, but SPF ratings only consider UVB light. There are products present that block UVA. Recent evidence has shown that the risk of melanoma is higher in people using sunscreen because most work on UVB. Tanning beds produce mostly UVA radiation, and some have varying amounts of UVB radiation. Frequent use of tanning beds shows an increased risk of malignant melanoma. Those living in low latitudes with increased sun exposure over a lifetime have an increased risk.
- Personal characteristics: Skin type (Fitzpatrick type I and II), blonde or red hair, and blue eyes have a higher risk. The presence of more melanocytic nevi increases risk.
- Family history of melanoma or pancreatic cancer may suggest a deletion mutation in p16.
- Socioeconomic status: Lower socioeconomic status may be linked to more advanced disease at the time of detection. This may be due to a decreased knowledge of the diseases and decreased perception of risk.
- Large congenital nevi (greater than 20 cm)
- Atypical mole syndrome (formerly termed B-K mole syndrome, dysplastic nevus syndrome, familial atypical multiple mole melanomas/familial dysplastic mole syndrome, autosomal dominant) The result of a genetic defect of CDKN2A gene that encodes for p16 (a transcription factor involved in apoptosis) and p14ARF. This gene is located on chromosome 9p21. There is a higher risk of melanoma depending on the number of family members affected. The risk is nearly 100% with relatives having melanoma or dysplastic nevi.
- Xeroderma pigmentosa (XP, autosomal recessive), a disorder of nucleotide excision repair, cannot repair DNA damage of thymine dimers. XP has an increased risk of all skin cancers.
Epidemiology
Melanoma has the highest incidence in areas with populations with fair skin and in locations with lots of sun exposure. Melanoma is the sixth most common cancer in the United States. Melanoma has been noted to be the fifth most common malignancy in men and seventh most common in women overall. Melanoma is the most common cancer in Caucasian women age 25 to 29 and the second most common cancer of women age 30 to 34. Melanoma is found more commonly in whites than Asians or blacks. In the United States, during 2010, approximately 68,000 new cases of malignant melanoma were diagnosed with 8700 deaths from melanoma. Approximately 30% of melanomas are located within the head and neck. Recently, increases in incidence have been noted at 5% per year and mortality at 2% per year. This increase is faster than any other cancer except lung cancer in women. Melanoma accounts for the third-highest number of deaths across all cancers.
Pathophysiology
Melanomas are malignant tumors of melanocytes, which are derived from neural crest cells. Melanoma can be sporadic or due to a transformation of a premalignant lesion. Upward of 80% of melanomas arise from preexisting lesions. A melanoma that develops in healthy skin is said to arise de novo (without evidence of a precursor lesion). These are often attributed to solar radiation. However, melanoma can occur in areas such as the palms, soles, and perineum.[5][6][7][8][9]
Precursor lesions of melanoma include the following nevi:
- Congenital nevi: Large congenital nevi (greater than 20 cm) have approximately a 10% lifetime risk of developing melanoma
- Dysplastic nevi: Usually larger than normal moles, with irregular/indistinct borders. Dysplastic nevi should be watched closely or removed if there is a high suspicion of melanoma or if malignant characteristics of the lesion evolve.
In sporadic melanoma, a number of pathways have been identified which are targets of therapy. Pathways of great interest are the RAS-RAF-MEK-ERK pathway, PI3K/PTEN, and c-Kit pathways.
In the RAS-RAF-MEK-ERK pathway, roughly up to 80% of melanomas have activating mutations affecting the structure of the b-type rapidly accelerated fibrosarcoma (BRAF) protein. Activating nuclear receptors are the most common RAS family mutations. This results in signaling through the RAF to MAP kinase pathway. BRAF inhibits being studied in the treatment of melanoma. Inhibition of BRAF/MEK in patients with BRAF V600 (Most often a valine to glutamic acid)-mutated melanoma has resulted in improved survival. This is the most common mutation in melanoma. However, it should be noted that having the BRAF mutation alone is not sufficient to develop melanoma; it requires p53 inactivation as well.
The PI3K/PTEN is an important pathway. Activation is associated with cell proliferation and malignant transformation. Most commonly observed changes include activating mutations in PI3K, silencing of PTEN, and amplification of AKT. The PI3K-AKT pathway may be a crucial target for combination therapy as it plays a significant role in BRAF-/MEK-inhibitor resistance in patients with melanoma.
The c-KIT pathway is another targeted area. Mutations in this area are more commonly found in acral and mucosal melanoma, melanomas unrelated to sun exposure. KIT is a transmembrane tyrosine kinase receptor that is expressed on hematopoietic progenitor cells, mast cells, melanocytes, primordial germ cells, and interstitial cells of Cajal. Activating mutations and amplifications cause activation of growth and proliferation pathways. Drugs such as imatinib focus on this pathway.
Major Types of Melanoma
There are 4 subtypes of melanoma: superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma, and acral lentiginous melanoma.
- Superficial spreading melanoma is the most common, comprising approximately 70% of melanomas. These have an initial radial growth phase with progression to a vertical growth phase. Often, ulceration or bleeding is accompanying the lesion. The lesions average 2 cm in diameter when diagnosed.
- Nodular melanoma is the second most common melanoma, but most commonly on the head and neck. It accounts for 15 to 30% of all melanoma. There is a very aggressive (rapid vertical phase), which may present on non-sun-exposed areas (de novo). These tumors are blue-black but may lack pigment. They often have a poor prognosis.
- Lentigo maligna melanomas represent 4 to 10% of melanomas. Lentigo maligna becomes lentigo maligna melanoma once it invades into the papillary dermis. There is often a prolonged radial growth of years to decades.
- Acral lentiginous melanoma constitutes 2 to 8% of melanomas in whites and 35% to 60% of them in dark-skinned individuals. Most common among African Americans, Latin Americans, Native Americans, and Japanese.
Additional Subtypes
- Desmoplastic is a rarer variant, but it is often found in the head and neck compared to the rest of the body. There is a high affinity for a perineural spread and a low rate of lymphatic metastases. It is often non-pigmented (amelanotic). Treatment is generally with wide excision and adjuvant radiotherapy.
- Mucosal melanoma accounts for less than 1% of melanomas. However, approximately 10% of head and neck melanomas are mucosal. This subtype has a poor prognosis. The most common sites in order are nasal, paranasal sinuses, oral cavity, and nasopharynx. The best prognostic locations are nasal and oral cavity, with paranasal sinus having the worst prognosis.
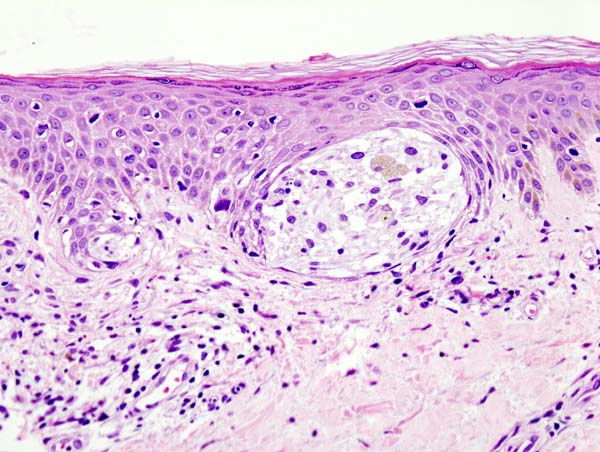
Histopathology
Clark’s Level
- Measures the level of invasion
- I: melanoma cells are in the epidermis only (melanoma in situ)
- II: melanoma cells invade through the basal layer and are in the papillary dermis
- III: melanoma cells fill papillary dermis
- IV: melanoma cells involve the reticular dermis
- V: melanoma cells involve the subcutaneous fat
Breslow Thickness
- Measures depth of invasion in mm
- Measures of tumor thickness
- Performed on 10x magnification from stratum granulosum to the deepest invasive tumor cell
- When ulceration is present, Breslow is measured from the bottom of the ulceration
- Breslow less than 1 mm: Thin melanoma
- Breslow greater than 1 mm usually require sentinel lymph node biopsy
Melanocytic Markers
S100
- A common marker for neural tissue. Acidic protein in the nucleus and cytoplasm
MART-1 (MELAN-A)
- Most sensitive melanocytic marker
- Can stain pseudonests in lichenoid actinic keratosis in sun-damaged skin
MITF-1
- Nuclear stain, positive in melanocytes, mast cells, and osteoclasts
HMB-45
- Recognizes melanosomal glycoprotein gp100
- Blue nevi stain with HMB-45
Special Considerations: Desmoplastic melanoma
- S100: Must be performed
- MART-1 is usually negative
History and Physical
Head and Neck Points
In melanoma of the head and neck, a thorough examination is warranted as several important considerations are different from management elsewhere. Head and neck melanoma have a worse prognosis than other sites; location of the lesion has a significant impact on prognosis. Scalp location has the worst prognosis, followed by ear, cheek, and neck, all in respected order. Additionally, management and resection take place at different levels.
Lymph Nodes
A thorough examination of lymph nodes at all levels of the neck should be emphasized. Additionally, the parotid gland should be examined, as there are parotid lymph nodes where the head drains. Parotid gland examination is particularly important for lesions on the anterior scalp, temple, and cheek. For lesions located on the posterior scalp and retroauricular, occipital lymph nodes should be examined thoroughly.
General Melanoma Physical Examination Information
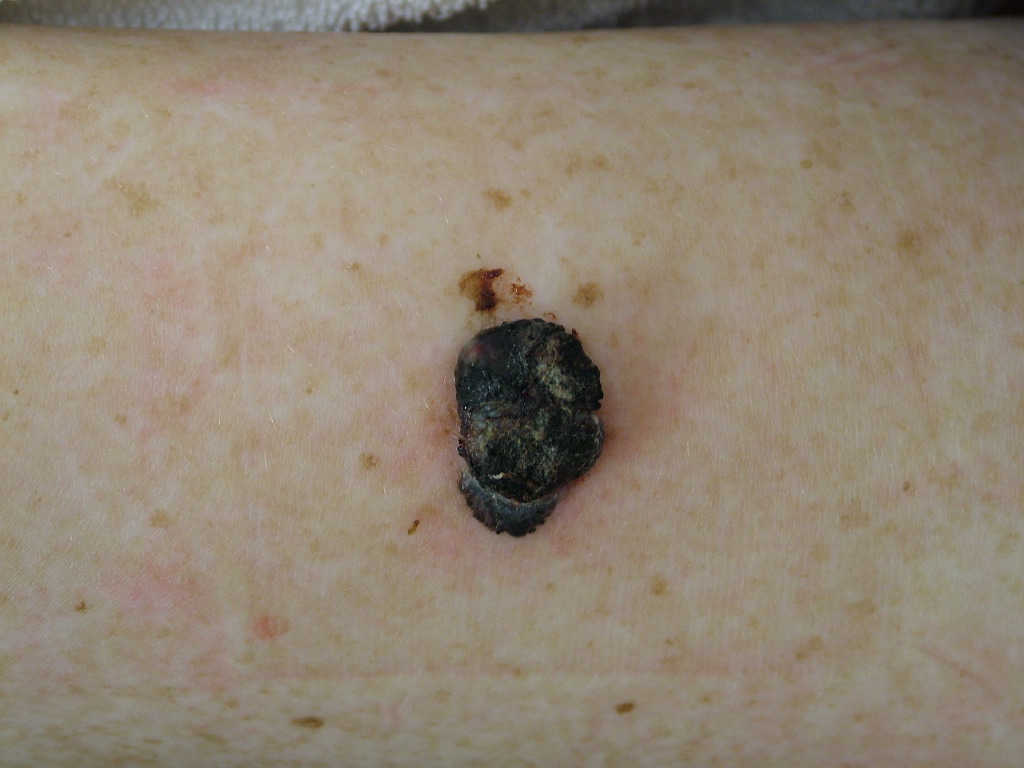
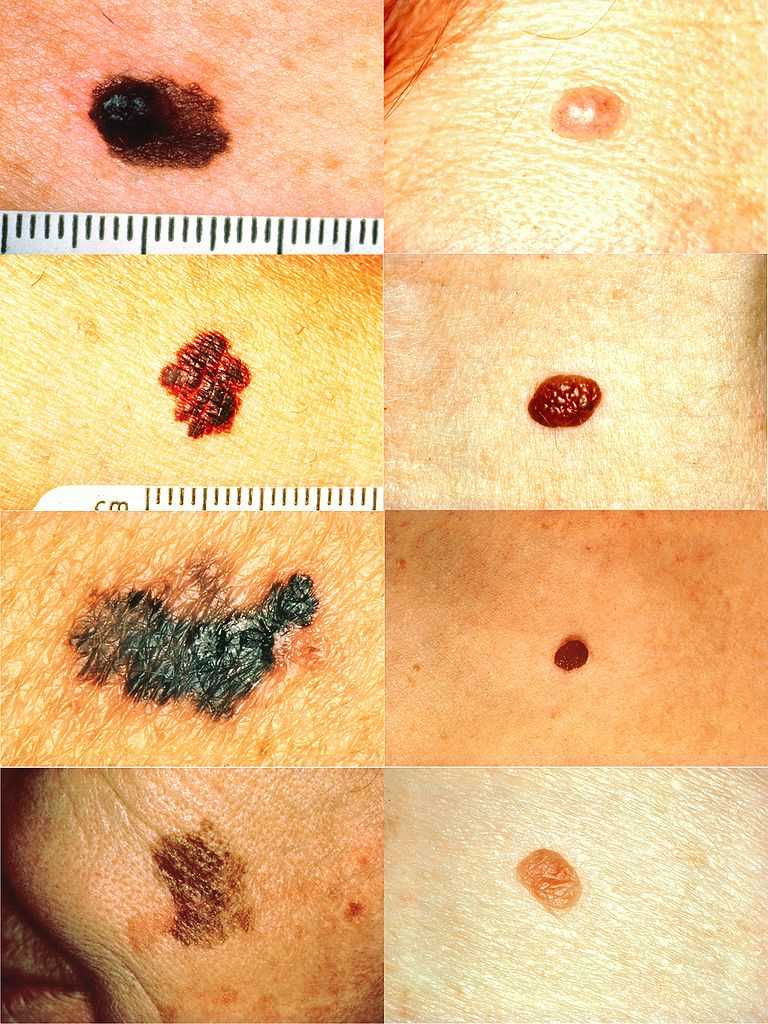
When considering melanoma in physical examination, patients may have a new lesion or existing mole with changing characteristics. However, amelanotic melanoma will not have typical dark pigment from melanin. Amelanotic melanoma may be pink, red, purple, or normal skin color. The characteristics of melanoma are commonly known by the acronym ABCDE and include the following:
A: Asymmetry
B: Irregular border
C: Color variations, especially red, white, and blue tones in a brown or black lesion
D: Diameter greater than 6 mm
E: Evolution of the mole(s) has become the most important factor to consider when it comes to diagnosing a melanoma if a mole has recently changed in color and/or size or if there any signs of regression.
Physical examination for aggressive/advanced disease should be considered, including ulceration, nodularity, and satellite lesions.
Evaluation
In head and neck melanoma, all suspicious lesions start with a biopsy with a method that will give a definitive diagnosis and depth of invasion. A shave biopsy should never be used for the evaluation of melanoma. Depth is needed in the evaluation of melanoma; therefore, an excisional biopsy should be performed with 2 mm margins.
Biopsy Techniques
Sometimes lymphoscintigraphy, sentinel lymph node mapping, is utilized in the evaluation of malignant melanoma. This may lead to one using different biopsy techniques. However, excisional biopsy is the most commonly used method. If lymphoscintigraphy is planned, an excisional biopsy may disrupt lymphatic drainage, whereas an incisional/punch will not.
Excisional biopsy: This is an acceptable method with small lesions and used with 1mm to 2mm margins. If pathological examination results in malignant melanoma, then wide excision is necessary.
Incisional/punch biopsy: Through the thickest portion of the tumor. This allows for depth to be examined, does not disrupt lymphatic drainage, does not disrupt the borders. Often controversial, depending on the source.
Baseline Laboratory
- Complete blood count (CBC)
- Complete chemistry panel including total protein, albumin, liver transaminases, lactate dehydrogenase)
- Note lactate dehydrogenase has been used in staging workup for melanoma of the head and neck.
Radiological Imaging
Chest X-ray
- Chest x-ray is usually the only imaging obtained in a melanoma workup unless physical examination or laboratory results raise concern or suspicion for distant metastases.
CT with Intravenous (IV) Contrast
- CT of the head and neck with intravenous contrast is used to evaluate local disease.
Metastatic Work-Up
Metastatic workup should be considered in patients that have melanomas greater than 4 mm, ulceration, satellite lesions, or any recurrent lesion.
Metastatic workup is required for patients with regional disease (any patient with laboratory and clinical exam)
- CT chest, abdomen, and pelvis with IV contrast
- MRI of the brain
- PET CT has been shown to be highly sensitive
Treatment / Management
Excisional Surgery
Excisional margins for melanoma depend on the thickness of the tumor (Breslow). There has been a debate on the margins to use.[10][11][12][13]
Most Widely Accepted Margins
1-cm margin
- Melanoma tumors less than 1 mm thickness
Greater than a 1-cm margin
- Melanoma tumor 1 to 2 mm thickness
2-cm margin
- Melanoma tumor greater than 2 mm thickness
- Ulcerated
Unique Depth of Resection for Lesions in Specific Areas of the Head and Neck
Face
- Resect to the level of muscles of facial expression (facial mimetic muscles)
Lesions Overlying the Parotid Gland
- Resect down to masseteric fascia (parotideomasseteric fascia). This fascia covers the masseter muscle. It is attached to the lower border of the zygomatic arch, and behind, it invests the parotid gland proceeding into the parotid fascia. However, if the thickness of the melanoma extends into this region, a superficial parotidectomy is performed.
Scalp
- Resect to the calvarial periosteum
Ear
- Auricle: Partial or total auriculectomy depending on the size of tumor and presence of satellite lesions
- Ear canal: Lesions in the ear canal may require temporal bone resection
Sentinel Node Biopsy and Neck Dissection
Finite regions of skin have lymphatic drainage to an initial node within a nodal basin. This is known as the sentinel node (SLN). A tool to characterize the regional nodal basin in patients with cutaneous melanoma of the head and neck is the sentinel node biopsy. The frequency of SLN metastasis increases with increasing tumor thickness and other adverse clinicopathological prognostic factors.
- Status is highly predictive of overall and disease-free survival
- Sentinel node biopsy has largely replaced elective neck dissection
- Sentinel node tumor burden is considered a regional disease prognostic factor that should be collected for all patients with positive sentinel nodes but is not used to determine N-category groupings
- Lymphoscintigraphy is performed 1 to 24 hours before SLN biopsy. A SPECT/CT is performed to map the lymph nodes draining the site.
- SLNs that are biopsied are sent for H and E staining. SLN also undergoes immunohistochemistry (IHC) evaluation utilizing S-100, MART-1 (Melana-A), and HMB-45.
- Neck dissection is reserved for those with positive lymph nodes. A neck dissection is necessary for those with positive SLN or metastatic melanoma.
- For stage III melanoma of the head and neck, the standard of care involves a neck dissection (selective versus modified). Superficial parotidectomy is for areas that drain into the parotid basin: temple, forehead, and cheek. For those that undergo neck dissection for retroauricular melanoma, posterior ear, or posterior scale, nodes from the suboccipital and postauricular must be included.
- Negative sentinel lymph node must be watched closely for recurrent disease
Indications for SLN Biopsy
- Localized stage only
- Breslow depth greater than or equal to 1 mm
- Breslow depth 0.75 to 1 mm (T1) in the presence of adverse prognostic variables:
- Tumor extension to deep margin: ulceration
- Lymphovascular invasion
- Extensive regression to 1.0 mm
- Young age
- High mitotic rate (greater than or equal to 1 mm
- Clark level IV or V (reticular dermis or deeper)
Parotidectomy
A superficial parotidectomy is required in patients with melanoma lesions on the anterior scalp, facial, temple, or ear with evidence of regional disease.
Melanoma on the chin or neck does not require a parotidectomy.
Medical Oncology
Agents that are used in the medical management of melanoma include the following:
- High dose interleukin-2
- Interferon alfa
- Pegylated interferon alfa-2b
- Granulocyte-macrophage colony-stimulating factor (GM-CSF)
- Dacarbazine
- Temozolomide
- Cisplatin, vinblastine, and dacarbazine (CVD)
- Cisplatin, dacarbazine, carmustine, and tamoxifen
- Carboplatin and paclitaxel
- Trametinib
Immunomodulators
- Ipilimumab: A monoclonal antibody that targets CTLA-4
Programmed Cell Death Protein-1 (PD-1) Inhibitors
Molecularly targeted therapy approved by FDA for the treatment of BRAF mutated melanoma
BRAF Inhibitors
MEK Inhibitors
BRAF and MEK Inhibitor Combinations
- Dabrafenib + Trametinib
- Vemurafenib + Cobimetinib
Radiation Therapy
Melanomas are radioresistant. Radiation therapy is used as adjuvant therapy for multiple positive nodes or macroscopic extranodal extension. It is used as primary therapy for elderly and nonsurgical patients. Radiation therapy is indicated for patients with brain metastasis (stereotactic radiosurgery). Radiation is generally reserved for palliation.[14]
Differential Diagnosis
- Benign melanocytic lesions (blue nevus, Mongolian spots, melanocytic nevus,
- Seborrheic keratosis
- Melanoma
Staging
Staging is carefully performed utilizing the most up-to-date American Joint Committee on Cancer (AJCC) Eighth Edition Guidelines for Melanoma. Important factors determining the stage of cancer and communicating this information are TMN criteria (T, tumor mass; N, lymph nodes; M, metastases).
Prognosis
Prognostic Factors for Melanomas in General
Location in the head and neck has an impact on prognosis as melanoma in the head and neck has a worse prognosis than other sites of the body. The scalp has the worst prognosis.
Prognosis depends on the disease stage at diagnosis.
Stage I Disease
- Five-year survival rate of greater than 90%
Stage II Disease
- Five-year survival rate ranging from 45 to 77%
Stage III Disease
- Five-year survival rate ranging from 27 to 70%
Metastatic Disease
- Five-year survival rate of less than 20%
Postoperative and Rehabilitation Care
Patients will need very close follow-up physical examinations after a diagnosis of melanoma from a surgical and dermatologic standpoint.
Deterrence and Patient Education
Patients should be advised to avoid sun exposure and take proper precautions after a melanoma diagnosis. Sun exposure increases the risk of developing melanoma.
Enhancing Healthcare Team Outcomes
Melanoma involves many practitioners and the location of the tumor impacts management. Dermatology, surgical oncology, otolaryngology (head and neck) surgery, hematology, and oncology. The more advanced disease will likely need consults in gastroenterology, pulmonology, and neurology.
Approximately 25% of melanoma occurs on the head and neck. It is important to understand that melanoma of the head and neck is a complex disease where management differs to elsewhere body and that location can impact prognosis. Due to the aggressive nature of head and neck melanoma, it should be treated aggressively. A shave biopsy should never be performed on a pigmented lesion that is suspicious for melanoma as depth is necessary for the evaluation. There are many molecular pathways shown to play a role. The 2 most widely accepted prognostic factors of melanoma are Breslow depth greater than 1 mm and ulceration.[15]