[1]
Wu ML, Yang CC, Ger J, Tsai WJ, Deng JF. Acute hydrofluoric acid exposure reported to Taiwan Poison Control Center, 1991-2010. Human & experimental toxicology. 2014 May:33(5):449-54. doi: 10.1177/0960327113499165. Epub 2013 Jul 25
[PubMed PMID: 23892993]
[2]
Stuke LE, Arnoldo BD, Hunt JL, Purdue GF. Hydrofluoric acid burns: a 15-year experience. Journal of burn care & research : official publication of the American Burn Association. 2008 Nov-Dec:29(6):893-6. doi: 10.1097/BCR.0b013e31818b9de6. Epub
[PubMed PMID: 18849854]
[3]
Kim Y, Shin J, Kang S, Kyung S, Park JW, Lee S, Lee S, Jeong SH. Pulmonary alveolar proteinosis induced by hydrofluoric acid exposure during fire extinguisher testing. Journal of occupational medicine and toxicology (London, England). 2015:10():6. doi: 10.1186/s12995-015-0048-7. Epub 2015 Feb 25
[PubMed PMID: 25737738]
[4]
Bajraktarova-Valjakova E, Korunoska-Stevkovska V, Georgieva S, Ivanovski K, Bajraktarova-Misevska C, Mijoska A, Grozdanov A. Hydrofluoric Acid: Burns and Systemic Toxicity, Protective Measures, Immediate and Hospital Medical Treatment. Open access Macedonian journal of medical sciences. 2018 Nov 25:6(11):2257-2269. doi: 10.3889/oamjms.2018.429. Epub 2018 Nov 20
[PubMed PMID: 30559898]
[5]
Franzblau A, Sahakian N. Asthma following household exposure to hydrofluoric acid. American journal of industrial medicine. 2003 Sep:44(3):321-4
[PubMed PMID: 12929153]
[6]
Smędra-Kaźmirska A, Kędzierski M, Barzdo M, Jurczyk A, Szram S, Berent J. Accidental intoxication with hydrochloric acid and hydrofluoric acid mixture. Archiwum medycyny sadowej i kryminologii. 2014 Jan-Mar:64(1):50-8
[PubMed PMID: 25184427]
[7]
Atley K, Ridyard E. Treatment of hydrofluoric acid exposure to the eye. International journal of ophthalmology. 2015:8(1):157-61. doi: 10.3980/j.issn.2222-3959.2015.01.28. Epub 2015 Feb 18
[PubMed PMID: 25709926]
[8]
Ozsoy G, Kendirli T, Ates U, Perk O, Azapagasi E, Ozcan S, Baran C, Goktug A, Dindar H. Fatal Refractory Ventricular Fibrillation Due to Ingestion of Hydrofluoric Acid. Pediatric emergency care. 2019 Nov:35(11):e201-e202. doi: 10.1097/PEC.0000000000001548. Epub
[PubMed PMID: 30020244]
[9]
Gradinger R, Jung C, Reinhardt D, Mall G, Figulla HR. Toxic myocarditis due to oral ingestion of hydrofluoric acid. Heart, lung & circulation. 2008 Jun:17(3):248-50
[PubMed PMID: 17822953]
[10]
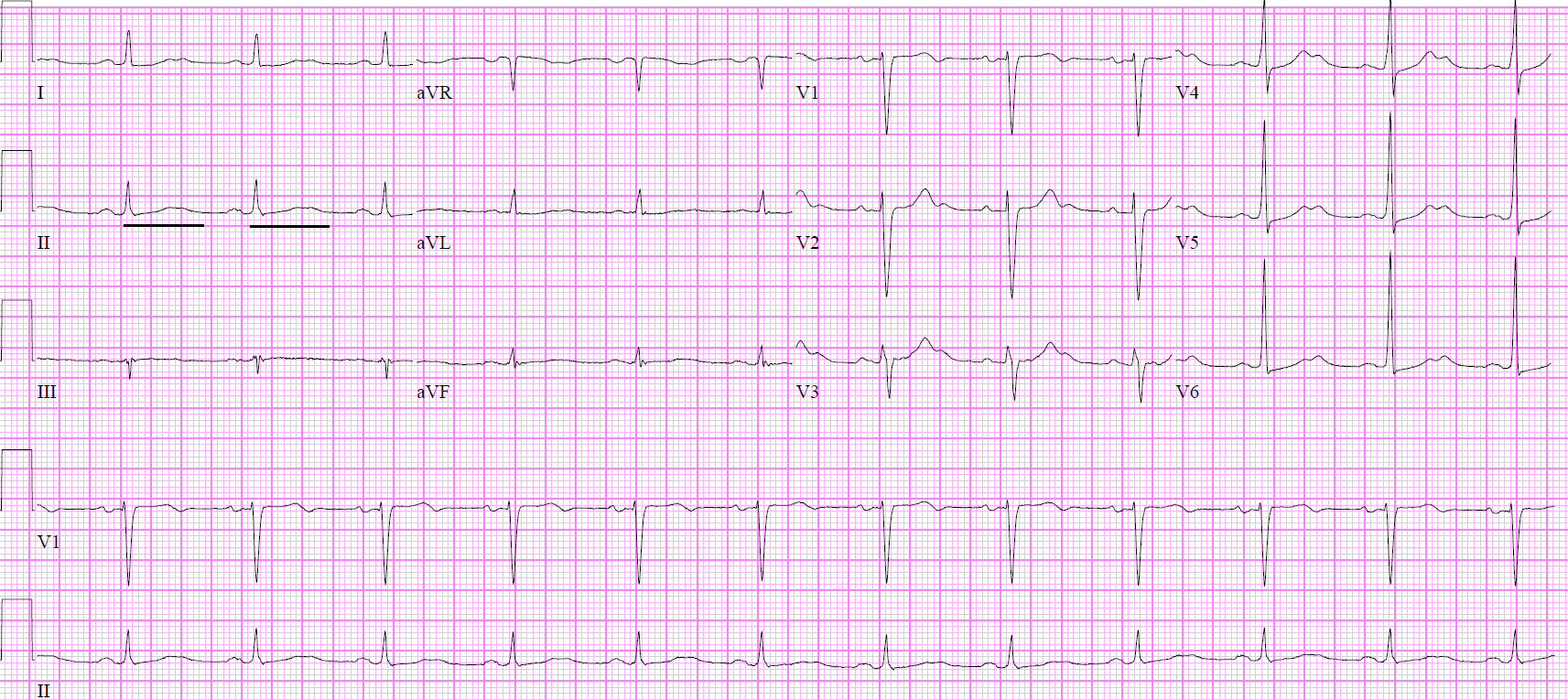
Vohra R, Velez LI, Rivera W, Benitez FL, Delaney KA. Recurrent life-threatening ventricular dysrhythmias associated with acute hydrofluoric acid ingestion: observations in one case and implications for mechanism of toxicity. Clinical toxicology (Philadelphia, Pa.). 2008 Jan:46(1):79-84
[PubMed PMID: 17906993]
Level 3 (low-level) evidence
[11]
Whiteley PM, Aks SE. Case files of the Toxikon Consortium in Chicago: survival after intentional ingestion of hydrofluoric acid. Journal of medical toxicology : official journal of the American College of Medical Toxicology. 2010 Sep:6(3):349-54. doi: 10.1007/s13181-010-0088-4. Epub
[PubMed PMID: 20661686]
Level 3 (low-level) evidence
[12]
McKee D, Thoma A, Bailey K, Fish J. A review of hydrofluoric acid burn management. Plastic surgery (Oakville, Ont.). 2014 Summer:22(2):95-8
[PubMed PMID: 25114621]
[14]
Ye C, Wang X, Zhang Y, Ni L, Jiang R, Liu L, Han C. Ten-year epidemiology of chemical burns in western Zhejiang Province, China. Burns : journal of the International Society for Burn Injuries. 2016 May:42(3):668-74. doi: 10.1016/j.burns.2015.12.004. Epub 2016 Jan 20
[PubMed PMID: 26803372]
[15]
Zhang Y, Zhang J, Jiang X, Ni L, Ye C, Han C, Sharma K, Wang X. Hydrofluoric acid burns in the western Zhejiang Province of China: a 10-year epidemiological study. Journal of occupational medicine and toxicology (London, England). 2016:11():55
[PubMed PMID: 27980604]
Level 2 (mid-level) evidence
[16]
Pu Q, Qian J, Tao W, Yang A, Wu J, Wang Y. Extracorporeal membrane oxygenation combined with continuous renal replacement therapy in cutaneous burn and inhalation injury caused by hydrofluoric acid and nitric acid. Medicine. 2017 Dec:96(48):e8972. doi: 10.1097/MD.0000000000008972. Epub
[PubMed PMID: 29310404]
Level 2 (mid-level) evidence
[17]
Dennerlein K, Kiesewetter F, Kilo S, Jäger T, Göen T, Korinth G, Drexler H. Dermal absorption and skin damage following hydrofluoric acid exposure in an ex vivo human skin model. Toxicology letters. 2016 Apr 25:248():25-33. doi: 10.1016/j.toxlet.2016.02.015. Epub 2016 Feb 27
[PubMed PMID: 26930472]
Level 3 (low-level) evidence
[18]
Hultén P, Höjer J, Ludwigs U, Janson A. Hexafluorine vs. standard decontamination to reduce systemic toxicity after dermal exposure to hydrofluoric acid. Journal of toxicology. Clinical toxicology. 2004:42(4):355-61
[PubMed PMID: 15461243]
[19]
Dibbell DG, Iverson RE, Jones W, Laub DR, Madison MS. Hydrofluoric acid burns of the hand. The Journal of bone and joint surgery. American volume. 1970 Jul:52(5):931-6
[PubMed PMID: 5479482]
[20]
Edelman P. Hydrofluoric acid burns. Occupational medicine (Philadelphia, Pa.). 1986 Jan-Mar:1(1):89-103
[PubMed PMID: 3299779]
[21]
Robinson EP, Chhabra AB. Hand chemical burns. The Journal of hand surgery. 2015 Mar:40(3):605-12; quiz 613. doi: 10.1016/j.jhsa.2014.07.056. Epub 2015 Feb 1
[PubMed PMID: 25653184]
[22]
Ohtani M, Nishida N, Chiba T, Muto H, Yoshioka N. Pathological demonstration of rapid involvement into the subcutaneous tissue in a case of fatal hydrofluoric acid burns. Forensic science international. 2007 Mar 22:167(1):49-52
[PubMed PMID: 16426786]
Level 3 (low-level) evidence