Continuing Education Activity
The diaphragm is a dome-shaped musculofibrous structure between the thoracic and abdominal cavities and is crucial for respiration as the primary muscle responsible for breathing. Disorders of the diaphragm, such as diaphragmatic paralysis, result from either muscle weakness or nerve damage. Clinical manifestations vary significantly based on the severity and whether the paralysis is unilateral or bilateral. Some patients may be asymptomatic, while others might depend on ventilators for breathing. Diaphragmatic paralysis can stem from various causes, including direct muscle weakness, phrenic nerve damage, or systemic diseases. Nontraumatic causes include congenital defects, spontaneous ruptures, infections, and conditions such as diaphragmatic hernias and eventration. Traumatic injuries, often due to blunt or penetrating trauma, can lead to significant morbidity and are frequently undiagnosed initially. Compression from tumors or other space-occupying lesions, neuropathic conditions, inflammatory diseases, and idiopathic origins also contribute to diaphragmatic dysfunction.
Evaluation of diaphragm disorders involves imaging studies, pulmonary function tests, and electrophysiological studies. Depending on the persistence and severity of the condition, treatment strategies include noninvasive positive-pressure ventilation, surgical plication, and diaphragmatic pacing. This activity reviews early detection, appropriate evaluation, and available treatment approaches for diaphragmatic disorders. This activity also provides healthcare professionals with the necessary tools and skills to evaluate diaphragmatic disorders, perform the recommended evaluations, and implement an appropriate interprofessional management approach to improve patient outcomes.
Objectives:
Identify signs and symptoms suggestive of diaphragm disorders, such as exertional dyspnea, orthopnea, or paradoxical thoracoabdominal movements.
Implement noninvasive positive-pressure ventilation promptly for symptomatic diaphragmatic paralysis to improve respiratory mechanics.
Select appropriate imaging modalities and electrophysiological studies to confirm the diagnosis and assess the severity of diaphragm disorders.
Collaborate with multidisciplinary healthcare professionals to optimize treatment strategies and enhance outcomes for patients with diaphragmatic dysfunction.
Introduction
The diaphragm is a vital organ in mammals, serving as the primary muscle for respiration. Diaphragmatic paralysis is the loss of muscular power due to muscle weakness or damage to its nerve supply. Depending on the severity of the paralysis and whether it is unilateral or bilateral, patients can exhibit varied clinical manifestations, ranging from asymptomatic to ventilator-dependent presentations.
Anatomical Structure of the Diaphragm
The diaphragm is a dome-shaped musculofibrous structure between the thoracic and abdominal cavities, constituting the thorax floor and the roof of the abdomen. The word "diaphragm" is derived from the Greek words dia, meaning "in between," and phragma, meaning "fence." Although a clear anatomical distinction is not visible, the diaphragm functions as separate units (right and left), each with different vascular and nerve supplies.
The diaphragm's peripheral portion is muscular and comprises 3 distinct muscle groups. The sternal group originates from the xiphoid process, the costal group originates from the inner surface of the lower 6 ribs, and the lumbar group originates from 2 crura and arcuate ligaments, which are, in turn, attached to the lumbar vertebra. The diaphragm's central portion comprises strong aponeurotic tendinous ligaments without bony attachments. The diaphragm is C-shaped and has right lateral, middle, and left lateral leaflets. The anterior sternal attachment of the diaphragm is located more cranially compared to the posterior lumbar attachment.
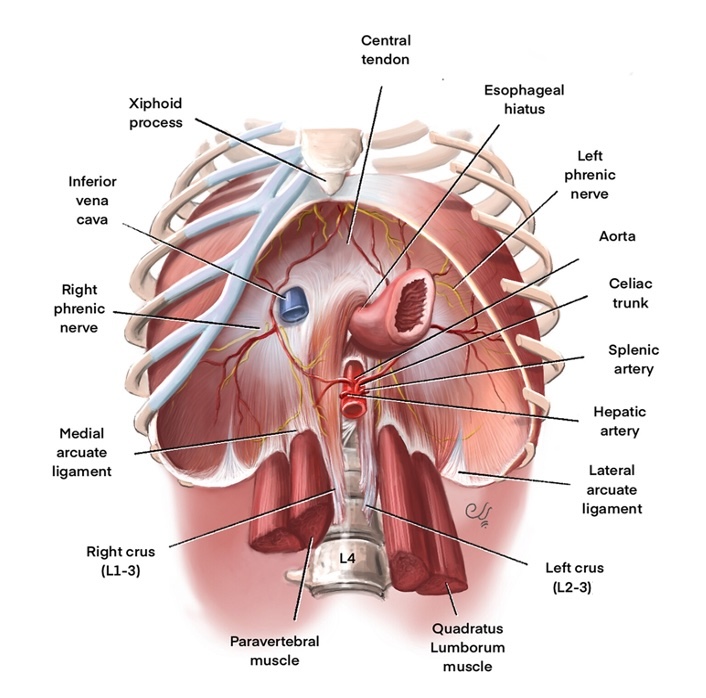
The diaphragm has several openings that allow structures to pass between the thorax and abdomen. At the level of the eighth thoracic vertebra on the right hemidiaphragm, the inferior vena cava enters the thorax from the abdomen through a large opening to join the right atrium. At the level of the tenth thoracic vertebra, a posterior midline opening between the 2 crura of the diaphragm, called the aortic hiatus, allows the descending thoracic aorta to enter the abdomen from the thorax, the thoracic duct to enter the thorax from the abdomen, and the azygos vein to enter the thorax from the abdomen. The esophageal hiatus, located between the fibers of the right crus of the diaphragm, is where the esophagus passes from the thorax to the abdomen (see Image. Anatomy of a Normal Diaphragm).
Nerve and Vascular Supply of the Diaphragm
Diaphragm function is primarily involuntary, with additional voluntary control when needed. The diaphragm is innervated by 2 phrenic nerves originating from cervical nerve roots C3 to C6. The right and left phrenic nerves innervate their respective hemidiaphragms, controlling both sensory and motor functions. The right phrenic nerve courses lateral to the caval hiatus, while the left travels lateral to the pericardium.
Each phrenic nerve divides into 4 trunks—the sternal, anterolateral, posterolateral, and rural. The primary vascular supply for the diaphragm comes from the bilateral phrenic arteries, which are direct branches of the thoracic aorta. Additional blood supply comes from tributaries of the internal mammary and pericardiophrenic arteries. Venous drainage occurs through phrenic veins that empty into the inferior vena cava.
Physiological Function of the Diaphragm
During inhalation, the muscular part of the diaphragm contracts, flattening and expanding the thoracic cavity outward and downward. This diaphragmatic contraction creates negative intrathoracic pressure, facilitating the passive movement of air from the atmosphere into the respiratory system along a pressure gradient. When the diaphragm relaxes, the thoracic cavity constricts, decreasing the subatmospheric pressure and leading to passive air egress from the respiratory system during expiration.[1]
Although external intercostal muscles assist in inspiration, the diaphragm serves as the primary muscle of respiration; thus, diaphragmatic weakness can hinder normal respiratory functions. Paralysis of both hemidiaphragms (ie, bilateral) leads to significant respiratory failure. In contrast, unilateral hemidiaphragm paresis can be asymptomatic due to compensatory function from the opposite side of the diaphragm and recruitment of external intercostal muscles. Voluntary contraction of the diaphragm also increases intraabdominal pressure, supporting essential functions such as vomiting, urination, and defecation while also preventing regurgitation by creating pressure at the lower esophageal sphincter.
Etiology
Diaphragmatic palsy or paresis can result from direct diaphragmatic muscle weakness and atrophy or damage to the phrenic nerves. Unilateral diaphragm weakness is more common than bilateral weakness. Depending on the cause, the weakness can be either temporary or permanent.[2][3][4]
Nontraumatic Etiologies
Nontraumatic diaphragmatic conditions encompass a variety of uncommon congenital and acquired disorders, including diaphragmatic hernias, spontaneous ruptures, endometriosis-related afflictions, eventration, paralysis, infections, and thoracoabdominal fistulas. Diaphragmatic hernias are categorized as either congenital or acquired.[5] Congenital diaphragmatic hernias (CDHs), occurring in about 2.5 out of every 10,000 births, result from an embryologic defect involving the closure of the pleuroperitoneal canal or the fusion of the septum transversum with the anterolateral body wall. This defect allows abdominal organs to herniate into the thoracic cavity due to the negative pressure within the thoracic region.[6] A spontaneous diaphragmatic rupture is a sudden and rare occurrence where the diaphragm tears without trauma, typically due to activities that cause a sudden rise in intra-abdominal pressure (eg, coughing, vaginal delivery, or performing the Valsalva maneuver).[7]
Diaphragmatic endometriosis is a rare condition observed in 0.7% to 1.5% of women of reproductive age who have pelvic endometriosis, although diaphragmatic endometriosis can infrequently occur without concurrent pelvic endometriosis.[8] Diaphragmatic dysfunction can manifest as eventration or weakness and paralysis. Diaphragmatic eventration is the abnormal focal elevation or bulging of part of the hemidiaphragm, often with associated thinning, primarily affecting the anteromedial aspect of the right hemidiaphragm. Diaphragmatic eventration is most often congenital and asymptomatic.
Additionally, infections can impair the diaphragm.[9] Diaphragmatic dysfunction may occur during or following viral infections such as HIV, herpes zoster, and poliovirus. Diaphragmatic or subphrenic abscesses can develop as postoperative complications or from the spread of adjacent infections (eg, intra-abdominal tuberculosis or intrathoracic mucormycosis). Thoracoabdominal fistulas are abnormal passages between the abdominal and chest cavities through the diaphragm caused by infection, trauma, neoplasms, or iatrogenic factors (eg, surgery). Fistulas can also result from congenital defects. Thoracoabdominal fistulas are classified into several types, including hepatothoracic, pancreaticothoracic, enterothoracic, and nephrothoracic fistulas.[10]
Traumatic Etiologies
Although rare, traumatic injuries to the diaphragm represent a wide range of trauma and can result in significant morbidity and long-term complications. Between 12% and 63% of these injuries are initially undiagnosed, primarily when occurring on the right side. The overall mortality rate for traumatic diaphragmatic injuries ranges from 30% to 41%, frequently due to associated injuries affecting the brain, skeleton, blood vessels, and abdominal organs.[11]
Blunt diaphragmatic injury (BDI) has a prevalence ranging from 0.8% to 8% and occurs as a result of a significant impact. Approximately one-third of all acquired diaphragmatic injuries are BDIs. Compared to penetrating diaphragmatic injuries, BDIs tend to cause more extensive damage due to the large defect size and the presence of concomitant injuries, which increase the risk of herniation. Motor vehicle collisions are the most common cause of BDIs (86%), followed by falls from heights and crushing injuries.[12]
Penetrating diaphragmatic injury accounts for approximately two-thirds of all diaphragmatic injuries and occurs when an external source directly punctures the diaphragm, most frequently due to gunshot wounds, stab wounds, or impalement by foreign objects. Penetrating injuries can also occur without skin penetration if rib fractures or bone fragments breach the diaphragm. Detection sensitivity and specificity for these injuries range from 12.1% to 64.6% and 67.6% to 100%, respectively.[13]
Diaphragmatic Compression
Any space-occupying lesion in the thorax (eg, mediastinal tumor or lung cancer) located close to the phrenic nerve can cause physical compression of the nerve, leading to diaphragm dysfunction. Phrenic nerve involvement is seen in 5% of lung cancer. Other causes of compressive damage include an aortic aneurysm, substernal goiter, and cervical spondylosis.
Neuropathic Etiologies
Many neurological issues can cause phrenic nerve damage, including diabetic neuropathy, inclusion body myositis, dermatomyositis, multiple sclerosis, anterior horn cell disease, chronic demyelinating disease, and neuralgic amyotrophy.[14]
Inflammatory Etiologies
Many systemic diseases can lead to phrenic nerve or diaphragm inflammation, leading to diaphragmatic palsy. Viral infections (eg, HIV, West Nile virus, and poliomyelitis virus), bacterial infections (eg, Lyme disease), and noninfectious causes (eg, sarcoidosis and amyloidosis) have been linked to diaphragmatic weakness.
Idiopathic Etiologies
Nearly 20% of diaphragm disorders are idiopathic, without an apparent cause identified, despite extensive investigations.
Epidemiology
CDH is a severe congenital disability characterized by a malformation of the diaphragm that allows abdominal organs to protrude into the thoracic cavity. Globally, CDH affects approximately 2.3 in every 10,000 live births. Respiratory failure, often due to pulmonary hypertension and underdeveloped lungs (pulmonary hypoplasia), is the primary cause of mortality associated with CDH. Approximately 64% of CDH cases are isolated, while 36% involve additional anomalies. Infants born with CDH experience significant morbidity and mortality, with mortality rates ranging from 30% to 60%, and even up to 89% in cases with additional chromosomal or structural anomalies. In recent years, notable advancements have been made in the prenatal and postnatal diagnosis, clinical management, and treatment of CDH. Despite these improvements, overall mortality rates have remained high over the past 3 decades.[15]
BDI has a prevalence ranging from 0.8% to 8% and occurs as a result of a significant impact. Approximately one-third of all acquired diaphragmatic injuries are BDIs. Compared to penetrating diaphragmatic injuries, BDIs cause a greater degree of damage due to concomitant injuries and the large size of the defect, which predisposes to herniation.[16] Penetrating diaphragmatic injury refers to trauma where an external source directly breaches the diaphragm and constitutes approximately two-thirds of all diaphragmatic injuries. These injuries are typically caused by gunshot wounds, stab wounds, or penetration by foreign bodies. Penetrating injuries can also occur without breaking the skin when rib fractures or fragments directly puncture the diaphragm.[16]
Diaphragmatic paralysis refers to abnormal weakness and elevation of one or both hemidiaphragms, depending on the underlying cause. Paralysis is typically acquired and can be symptomatic. Unilateral diaphragm weakness may be asymptomatic and often incidentally discovered during imaging. However, severe or bilateral weakness usually leads to symptoms such as respiratory distress, necessitating significant use of accessory muscles or resulting in respiratory failure.[9]
Pathophysiology
Acute diaphragmatic abnormalities encompass a diverse range of relatively rare pathological conditions that disrupt the normal structure and function of the diaphragm. Structural compromise can result in tearing and rupture of the diaphragm, leading to herniation of organs and the potential spread of disease. Additionally, impaired diaphragmatic function can contribute to cardiopulmonary distress and exacerbate associated injuries. Herniated organs are vulnerable to complications such as incarceration, strangulation, ischemia, and perforation. Early and precise identification of these conditions, often through various imaging techniques, is crucial for stabilizing and treating patients effectively.[17][18]
History and Physical
Symptoms of diaphragmatic weakness vary depending on the cause and duration. Many patients with unilateral diaphragmatic weakness are asymptomatic and are identified incidentally, although one-third of patients report exertional breathlessness. Patients with coexisting debilitating cardiopulmonary conditions can have dyspnea at rest. Most patients with unilateral diaphragmatic weakness demonstrate some exercise capacity limitations and lower resting oxygen saturation levels. Patients with bilateral weakness often complain of varying degrees of dyspnea, ranging from mild exertional breathlessness to dyspnea at rest. Patients report orthopnea as diaphragm function becomes further compromised in the supine position. Progressive hypoventilation can lead to hypercapnia and right heart failure. Hypoxemia and hypercapnia are commonly worse during sleep.
Evaluation
Because diaphragm disorders encompass various pathological conditions, clinicians must evaluate patients for a range of differential diagnoses and etiologies of diaphragmatic dysfunction, along with associated complications involving surrounding anatomic structures. Timely identification of these conditions is crucial for stabilizing and effectively treating patients. Evaluation of diaphragm disorders typically includes imaging studies, pulmonary function tests, and electrophysiological studies.[19]
Diagnostic Imaging Studies
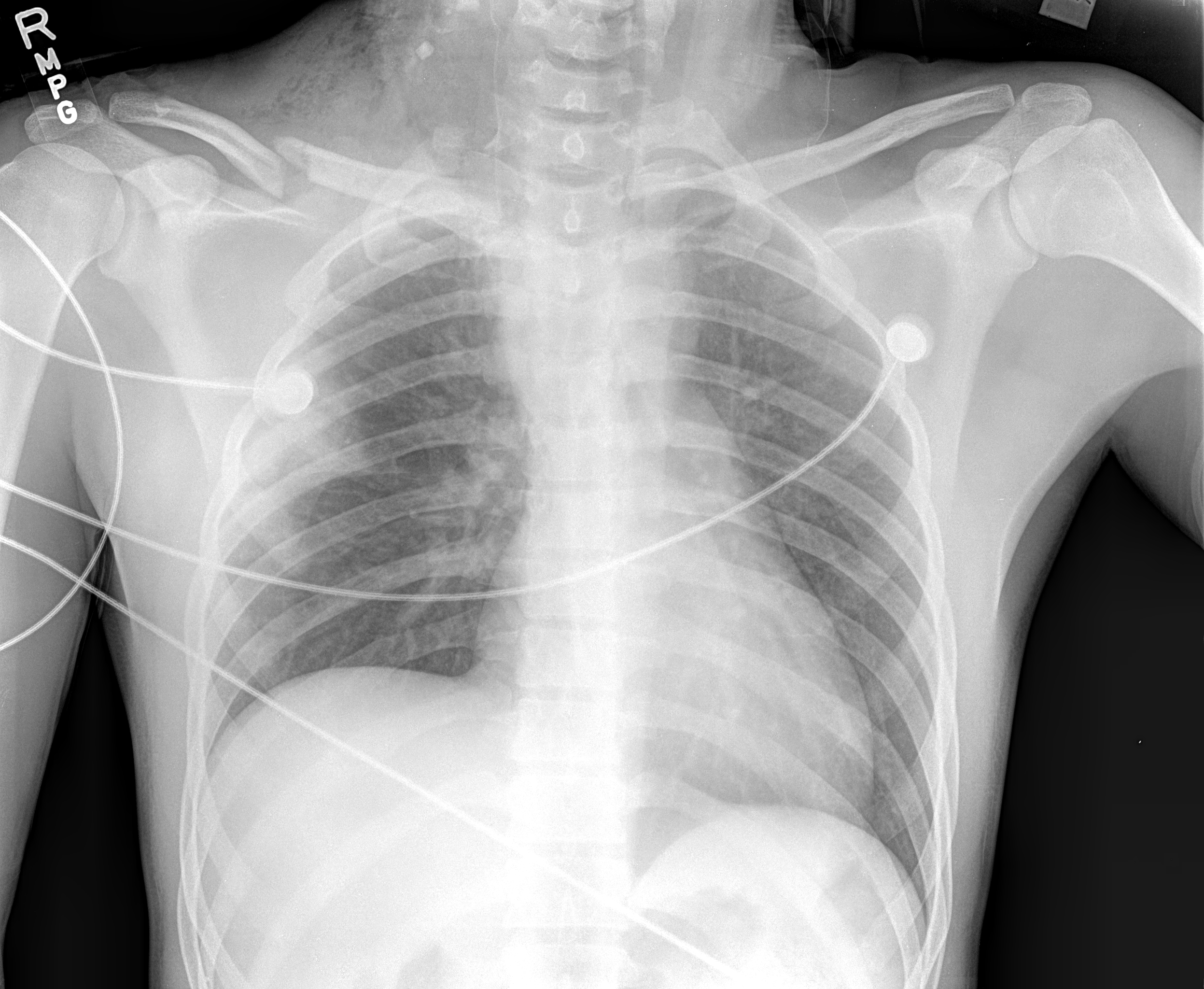
As the diaphragm is positioned obliquely, the dome of the right diaphragm is at the level of the fifth rib anteriorly and the level of the tenth rib posteriorly. Usually, the right hemidiaphragm is located slightly higher than the left hemidiaphragm by around 1 intercostal space. If a side of the diaphragm is weak, negative intrathoracic pressure will pull the diaphragm cranially into the thoracic cavity, making the paralyzed diaphragm appear higher (see Image. Elevated Right Hemidiaphragm). When the right side is paralyzed, the distance between the right and left diaphragms will be more than 2 intercostal spaces. If the left side is paralyzed, both hemidiaphragms will appear at the same level. Up to 90% of unilateral diaphragmatic palsy can be diagnosed based on chest x-rays or radiographs alone (see Image. Chest Radiograph Showing Diaphragm Disorder). In bilateral weakness, both hemidiaphragms will be higher and might be missed on routine chest radiograms. The costophrenic and craniovertebral angles will be more acute as the diaphragms curve. An upward-located diaphragm can also cause secondary atelectasis of the lower lung segments.
All patients with suspected diaphragmatic weakness should undergo a fluoroscopic test to diagnose the functional weakness. During tidal breathing in normal individuals, diaphragmatic contraction leads to the descent of both hemidiaphragms by at least one intercostal space. During deep breathing or sniffing, the descent of the hemidiaphragm is more rapid and distal. In cases of unilateral weakening of the hemidiaphragm, diaphragmatic excursion will be reduced or absent. Occasionally, fluoroscopic imaging can show paradoxical diaphragm movement, where the affected hemidiaphragm moves into the thoracic cavity during inspiration, while the unaffected hemidiaphragm moves downward toward the abdominal cavity. The intercostal muscles and the functioning hemidiaphragm generate negative intrathoracic pressure, pulling the paralyzed diaphragm into the thorax. With bilateral weakness, the diaphragm either does not move or moves paradoxically, depending on the severity of the weakness.
Ultrasound examination for thoracic disease is increasingly used. In B mode, the diaphragm appears as a thick echogenic line. In M mode, a paralyzed hemidiaphragm shows no cranial or paradoxical caudal movements, similar to a sniff test. Computed tomography (CT) of the chest should be performed in all patients with diaphragm weakness to exclude thoracic etiologies. Unfortunately, diaphragmatic weakness is not easily recognized and cannot be detected in static CT images.
Diagnostic Pulmonary Function Studies
As the diaphragm accounts for 80% of the muscular respiration power, weakness can be measured by pulmonary function tests. With unilateral diaphragmatic weakness, forced vital capacity typically declines by 50% of predicted volume and up to 75% in bilateral weakness. Usually, lung function declines by <15% in the supine position due to the restriction of thoracic expansion and positive pressure from abdominal structures. With diaphragmatic weakness, this decrease is accentuated, and on repeating the spirometry in a supine position, forced vital capacity will further decrease by 15% to 20% in unilateral weakness and 20% to 30% in bilateral weakness. Maximum inspiratory and expiratory pressure will also be reduced. Total lung capacity, functional residual capacity, residual volume, and diffusion factor remain unchanged in unilateral weakness but can be reduced in bilateral weakness.
Electrophysiological Studies
In diaphragmatic electromyography, the phrenic nerve is electrically stimulated, and the diaphragm's mechanical response is detected along with transdiaphragmatic pressure. In phrenic nerve pathology, prolonged latency and reduced transdiaphragmatic pressure are observed. However, in primary diaphragmatic pathology, the phrenic nerve conduction is normal, but the compound action potential is reduced.
Treatment / Management
The 3 primary management approaches for diaphragmatic palsy, if the weakness persists, are mentioned below.
Noninvasive Positive-Pressure Ventilation
Positive-pressure and bilevel positive-pressure ventilation show success in diaphragmatic weakness. This type of noninvasive pressure ventilation creates dynamic pressure splints in the airways, preventing paradoxical diaphragmatic movements. Positive-pressure ventilation also augments minute ventilation by working in coordination with intercostal muscles. This therapy typically starts at night when respiration is compromised in the supine position. In severe cases, intermittent daytime therapy can also be used.
A tracheostomy is an option for more severe cases requiring high-pressure settings and for patients unable to tolerate oral or nasal masks. Advancements in noninvasive technology have enabled noninvasive therapy with a tracheostomy interface. A mechanical ventilator can be used if the patient cannot tolerate a high-pressure setting. Diaphragmatic paralysis following surgeries is usually temporary and can be treated with noninvasive positive-pressure ventilation until the diaphragm or phrenic nerve regains function.
Surgical Plication of Paralyzed Diaphragm
This complicated, invasive procedure, performed either through open thoracotomy or video-assisted thoracic surgery, effectively relieves symptoms and improves lung function. Surgical plication prevents the paralyzed diaphragm from causing a mechanical disadvantage in the thoracic cavity.
Diaphragmatic Pacing
This approach can be used in unilateral phrenic nerve palsy. In diaphragmatic pacing, the phrenic nerve is electrically stimulated to generate mechanical power in the diaphragm. The electric pacemaker can be placed in the neck or near the diaphragm. The location of the generator depends on the cause of the site of phrenic nerve damage.[20][21][22]
Differential Diagnosis
Differential diagnoses to consider when evaluating diaphragm disorders include:
- Alveolar hypoventilation
- Anterior horn cell or neuromuscular junction disease
- Cerebral haemorrhage
- Cervical fracture
- Cervical spine fracture
- Decreased pulmonary compliance
- Guillain-Barré syndrome
- Myasthenia gravis
- Peripheral neuropathies
- Pleural adhesions
Prognosis
Phrenic nerve injury leads to paralysis of the diaphragm muscle, which is crucial in generating inspiratory effort and stabilizing posture and spinal alignment. Unilateral deficits typically manifest with exertional dyspnea, orthopnea, and sleep-disordered breathing, while bilateral paralysis can necessitate oxygen supplementation or ventilator support. Common causes of phrenic nerve injuries include cervical trauma, iatrogenic injury during neck or chest procedures, and neuralgic amyotrophy. Some cases are idiopathic, with no identifiable cause.
Diagnostic evaluation involves radiographic imaging, pulmonary function tests, and electrodiagnostic assessments to quantify nerve deficits and assess denervation atrophy. Treatment options for symptomatic diaphragm paralysis have historically been limited. Medical therapies and nocturnal positive airway pressure can offer symptomatic relief. Surgical repair, such as phrenic nerve reconstruction, has emerged as a safe and effective alternative to static repositioning of the diaphragm (diaphragm plication) in appropriately selected patients. Phrenic nerve reconstruction is increasingly considered a standard surgical approach for diaphragm paralysis resulting from phrenic nerve injury. Achieving optimal long-term outcomes often requires a multidisciplinary approach at specialized referral centers, encompassing diagnostic evaluation, surgical intervention, and rehabilitation.[23]
Complications
Diaphragm dysfunction is characterized by diminished contractile function of the diaphragm muscle, presenting as muscular weakness, partial loss of contractile ability, or complete paralysis affecting one or both hemidiaphragms. This condition can result in respiratory pump failure, leading to insufficient airflow due to decreased respiratory effort against heightened resistance. Alterations in the ventilation-perfusion ratio may elevate physiological dead space, representing the portion of air volume not participating in gas exchange.[24]
Various factors can lead to diaphragm dysfunction, including surgical procedures, mechanical ventilation, metabolic disorders, inflammatory conditions, neuromuscular disorders, and mediastinal or abdominal masses causing lung hyperinflation. Patients with unilateral diaphragmatic paralysis may not show symptoms at rest but can experience exertional dyspnea, while those with bilateral dysfunction are more likely to experience symptoms even at rest. Conditions such as underlying cardiac disease, obesity, or respiratory conditions such as chronic obstructive pulmonary disease can worsen dyspnea, especially when in the supine position.
Severe bilateral diaphragmatic dysfunction can present with resting tachypnea and abnormal activation of accessory respiratory muscles. A common sign of diaphragm paralysis is the "abdominal paradox" or "thoracoabdominal asynchrony," characterized by inward abdomen movement during inspiration.[25] The prognosis of diaphragm dysfunction varies depending on its underlying cause. In degenerative neuromuscular diseases, the condition usually progresses, whereas after cardiac surgery, only a small percentage of patients (2%) show persistent diaphragm paralysis on chest radiography after a 6-month follow-up.[26]
Deterrence and Patient Education
Diaphragmatic dysfunction can lead to significant clinical consequences. A thorough evaluation is necessary to identify its cause, address symptoms, and mitigate its impact on sleep quality and exercise capacity. Patients should be reassured that most diaphragm disorders, whether muscular or neurological, are treatable.
Pearls and Other Issues
The prognosis of diaphragmatic paralysis depends on its etiology. Temporary weakness after cardiac bypass typically improves over time, whereas progressive neuromuscular diseases can lead to respiratory failure. With supportive treatment, many patients demonstrate stable or improved lung function within 1 to 2 years.
Enhancing Healthcare Team Outcomes
Enhancing patient-centered care and outcomes, patient safety, and team performance in the management of diaphragmatic dysfunction requires coordinated efforts from a comprehensive interprofessional healthcare team. This team should include internists, surgeons, intensivists, neurologists, pulmonologists, nurse practitioners, and other healthcare professionals. Physicians and advanced practitioners must conduct thorough assessments, including complete lung function tests in both sitting and supine positions, and utilize bedside thoracic ultrasound for diagnosing diaphragmatic palsy without radiation risks. Nurses are critical in monitoring patient status and providing bedside care, ensuring continuous observation and prompt intervention. Pharmacists contribute by managing medications that support respiratory function or address underlying conditions effectively.
Effective interprofessional communication and care coordination are essential for developing and implementing individualized treatment plans, which may involve both surgical and nonsurgical interventions. By fostering collaboration and shared responsibilities, the healthcare team can ensure optimal patient outcomes, enhance safety, and improve overall team performance in managing diaphragmatic disorders.