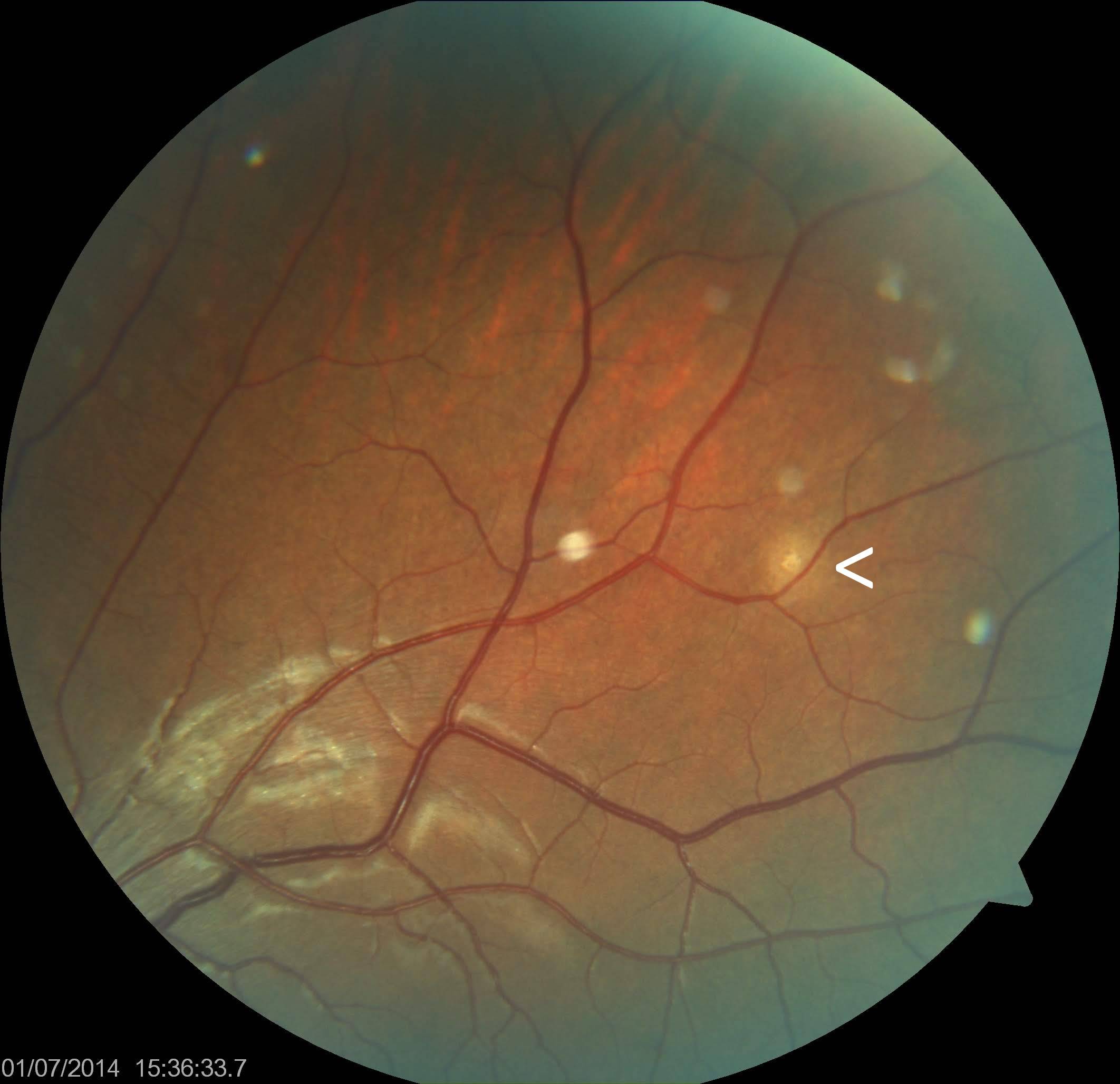
[2]
Helm CJ, Holland GN. Ocular tuberculosis. Survey of ophthalmology. 1993 Nov-Dec:38(3):229-56
[PubMed PMID: 8310395]
Level 3 (low-level) evidence
[3]
EMERY JL, LORBER J. Radiological and pathological correlation of miliary tuberculosis of lungs in children, with special reference to choroidal tubercles. British medical journal. 1950 Sep 23:2(4681):702-4
[PubMed PMID: 14772458]
[4]
Houben RM, Dodd PJ. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS medicine. 2016 Oct:13(10):e1002152. doi: 10.1371/journal.pmed.1002152. Epub 2016 Oct 25
[PubMed PMID: 27780211]
[5]
MacNeil A, Glaziou P, Sismanidis C, Maloney S, Floyd K. Global Epidemiology of Tuberculosis and Progress Toward Achieving Global Targets - 2017. MMWR. Morbidity and mortality weekly report. 2019 Mar 22:68(11):263-266. doi: 10.15585/mmwr.mm6811a3. Epub 2019 Mar 22
[PubMed PMID: 30897077]
[6]
Kon OM, Beare N, Connell D, Damato E, Gorsuch T, Hagan G, Perrin F, Petrushkin H, Potter J, Sethi C, Stanford M. BTS clinical statement for the diagnosis and management of ocular tuberculosis. BMJ open respiratory research. 2022 Mar:9(1):. doi: 10.1136/bmjresp-2022-001225. Epub
[PubMed PMID: 35379660]
[7]
Albert DM, Raven ML. Ocular Tuberculosis. Microbiology spectrum. 2016 Nov:4(6):. doi: 10.1128/microbiolspec.TNMI7-0001-2016. Epub
[PubMed PMID: 27837746]
[9]
Dannenberg AM Jr. Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunology today. 1991 Jul:12(7):228-33
[PubMed PMID: 1822092]
[10]
Jamwal SV, Mehrotra P, Singh A, Siddiqui Z, Basu A, Rao KV. Mycobacterial escape from macrophage phagosomes to the cytoplasm represents an alternate adaptation mechanism. Scientific reports. 2016 Mar 16:6():23089. doi: 10.1038/srep23089. Epub 2016 Mar 16
[PubMed PMID: 26980157]
[11]
Krishnan N, Robertson BD, Thwaites G. The mechanisms and consequences of the extra-pulmonary dissemination of Mycobacterium tuberculosis. Tuberculosis (Edinburgh, Scotland). 2010 Nov:90(6):361-6. doi: 10.1016/j.tube.2010.08.005. Epub 2010 Sep 9
[PubMed PMID: 20829117]
[12]
Drain PK, Bajema KL, Dowdy D, Dheda K, Naidoo K, Schumacher SG, Ma S, Meermeier E, Lewinsohn DM, Sherman DR. Incipient and Subclinical Tuberculosis: a Clinical Review of Early Stages and Progression of Infection. Clinical microbiology reviews. 2018 Oct:31(4):. doi: 10.1128/CMR.00021-18. Epub 2018 Jul 18
[PubMed PMID: 30021818]
[13]
Holmes KK, Bertozzi S, Bloom BR, Jha P, Bloom BR, Atun R, Cohen T, Dye C, Fraser H, Gomez GB, Knight G, Murray M, Nardell E, Rubin E, Salomon J, Vassall A, Volchenkov G, White R, Wilson D, Yadav P. Tuberculosis. Major Infectious Diseases. 2017 Nov 3:():
[PubMed PMID: 30212088]
[14]
Fukunaga R, Glaziou P, Harris JB, Date A, Floyd K, Kasaeva T. Epidemiology of Tuberculosis and Progress Toward Meeting Global Targets - Worldwide, 2019. MMWR. Morbidity and mortality weekly report. 2021 Mar 26:70(12):427-430. doi: 10.15585/mmwr.mm7012a4. Epub 2021 Mar 26
[PubMed PMID: 33764960]
[15]
Alvarez GG, Roth VR, Hodge W. Ocular tuberculosis: diagnostic and treatment challenges. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2009 Jul:13(4):432-5. doi: 10.1016/j.ijid.2008.09.018. Epub 2009 Apr 22
[PubMed PMID: 19386531]
[16]
Donahue HC. Ophthalmologic experience in a tuberculosis sanatorium. American journal of ophthalmology. 1967 Oct:64(4):742-8
[PubMed PMID: 6061532]
[17]
Bouza E, Merino P, Muñoz P, Sanchez-Carrillo C, Yáñez J, Cortés C. Ocular tuberculosis. A prospective study in a general hospital. Medicine. 1997 Jan:76(1):53-61
[PubMed PMID: 9064488]
[18]
Singh R, Gupta V, Gupta A. Pattern of uveitis in a referral eye clinic in north India. Indian journal of ophthalmology. 2004 Jun:52(2):121-5
[PubMed PMID: 15283216]
[19]
Morimura Y, Okada AA, Kawahara S, Miyamoto Y, Kawai S, Hirakata A, Hida T. Tuberculin skin testing in uveitis patients and treatment of presumed intraocular tuberculosis in Japan. Ophthalmology. 2002 May:109(5):851-7
[PubMed PMID: 11986087]
[20]
Pillai S, Malone TJ, Abad JC. Orbital tuberculosis. Ophthalmic plastic and reconstructive surgery. 1995 Mar:11(1):27-31
[PubMed PMID: 7748819]
[21]
Heiden D, Saranchuk P, Keenan JD, Ford N, Lowinger A, Yen M, McCune J, Rao NA. Eye examination for early diagnosis of disseminated tuberculosis in patients with AIDS. The Lancet. Infectious diseases. 2016 Apr:16(4):493-9. doi: 10.1016/S1473-3099(15)00269-8. Epub 2016 Feb 18
[PubMed PMID: 26907735]
[22]
Ludi Z, Sule AA, Samy RP, Putera I, Schrijver B, Hutchinson PE, Gunaratne J, Verma I, Singhal A, Nora RD, van Hagen PM, Dik WA, Gupta V, Agrawal R. Diagnosis and biomarkers for ocular tuberculosis: From the present into the future. Theranostics. 2023:13(7):2088-2113. doi: 10.7150/thno.81488. Epub 2023 Apr 1
[PubMed PMID: 37153734]
[23]
Gupta V, Gupta A, Rao NA. Intraocular tuberculosis--an update. Survey of ophthalmology. 2007 Nov-Dec:52(6):561-87
[PubMed PMID: 18029267]
Level 3 (low-level) evidence
[24]
Chawla R, Venkatesh P, Tripathy K, Chaudhary S, Sharma SK. Successful Management of Proliferative Diabetic Retinopathy and Multiple Choroidal Tubercles in a Patient with Miliary Tuberculosis. Journal of ophthalmic & vision research. 2018 Apr-Jun:13(2):210-211. doi: 10.4103/jovr.jovr_203_16. Epub
[PubMed PMID: 29719654]
[25]
Tripathy K, Chawla R, Sharma YR. Intravitreal Bevacizumab for Choroidal Neovascular Membrane at the Edge of a Healed Choroidal Tuberculoma. Ocular immunology and inflammation. 2018:26(2):239-241. doi: 10.1080/09273948.2016.1206205. Epub 2016 Aug 19
[PubMed PMID: 27541084]
[26]
Tripathy K, Chawla R. Choroidal tuberculoma. The National medical journal of India. 2016 Mar-Apr:29(2):106
[PubMed PMID: 27586221]
[28]
Biswas J, Therese L, Madhavan HN. Use of polymerase chain reaction in detection of Mycobacterium tuberculosis complex DNA from vitreous sample of Eales' disease. The British journal of ophthalmology. 1999 Aug:83(8):994
[PubMed PMID: 10413707]
[29]
Madhavan HN, Therese KL, Gunisha P, Jayanthi U, Biswas J. Polymerase chain reaction for detection of Mycobacterium tuberculosis in epiretinal membrane in Eales' disease. Investigative ophthalmology & visual science. 2000 Mar:41(3):822-5
[PubMed PMID: 10711699]
[31]
Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. American family physician. 2005 Nov 1:72(9):1761-8
[PubMed PMID: 16300038]
Level 3 (low-level) evidence
[32]
Khan FY. Review of literature on disseminated tuberculosis with emphasis on the focused diagnostic workup. Journal of family & community medicine. 2019 May-Aug:26(2):83-91. doi: 10.4103/jfcm.JFCM_106_18. Epub
[PubMed PMID: 31143078]
[33]
Chawla R, Garg S, Venkatesh P, Kashyap S, Tewari HK. Case report of tuberculous panophthalmitis. Medical science monitor : international medical journal of experimental and clinical research. 2004 Oct:10(10):CS57-9
[PubMed PMID: 15448600]
Level 3 (low-level) evidence
[34]
Thompson MJ, Albert DM. Ocular tuberculosis. Archives of ophthalmology (Chicago, Ill. : 1960). 2005 Jun:123(6):844-9
[PubMed PMID: 15955987]
[36]
Dhakal S, Neupane S, Arjyal Kafle P, Badhu BP, Upadhyaya Kafle S. Primary Orbital Tuberculosis with Cold Abscess: A Case Report. JNMA; journal of the Nepal Medical Association. 2024 Feb 24:62(270):148-151. doi: 10.31729/jnma.8441. Epub 2024 Feb 24
[PubMed PMID: 38409977]
Level 3 (low-level) evidence
[37]
Dalvin LA, Smith WM. Orbital and external ocular manifestations of Mycobacterium tuberculosis: A review of the literature. Journal of clinical tuberculosis and other mycobacterial diseases. 2016 Aug:4():50-57. doi: 10.1016/j.jctube.2015.11.001. Epub 2015 Nov 27
[PubMed PMID: 31723688]
[38]
Thomas S, Suhas S, Pai KM, Raghu AR. Lupus vulgaris--report of a case with facial involvement. British dental journal. 2005 Feb 12:198(3):135-7
[PubMed PMID: 15706374]
Level 3 (low-level) evidence
[39]
Madhukar K, Bhide M, Prasad CE, Venkatramayya. Tuberculosis of the lacrimal gland. The Journal of tropical medicine and hygiene. 1991 Jun:94(3):150-1
[PubMed PMID: 2051518]
[40]
Gupta A, Gupta V, Arora S, Dogra MR, Bambery P. PCR-positive tubercular retinal vasculitis: clinical characteristics and management. Retina (Philadelphia, Pa.). 2001:21(5):435-44
[PubMed PMID: 11642371]
[41]
Goyal JL, Jain P, Arora R, Dokania P. Ocular manifestations of tuberculosis. The Indian journal of tuberculosis. 2015 Apr:62(2):66-73. doi: 10.1016/j.ijtb.2015.04.004. Epub 2015 Jun 16
[PubMed PMID: 26117474]
[42]
Latiff N, Lakshmipathy M, Janani MK, Dutta Majumder P. Tuberculous corneal ulcer with hypopyon: A case report. Indian journal of ophthalmology. 2020 May:68(5):922-924. doi: 10.4103/ijo.IJO_1368_19. Epub
[PubMed PMID: 32317491]
Level 3 (low-level) evidence
[43]
Nanda M, Pflugfelder SC, Holland S. Mycobacterium tuberculosis scleritis. American journal of ophthalmology. 1989 Dec 15:108(6):736-7
[PubMed PMID: 2512813]
[44]
Tabbara KF. Ocular tuberculosis: anterior segment. International ophthalmology clinics. 2005 Spring:45(2):57-69
[PubMed PMID: 15791158]
[48]
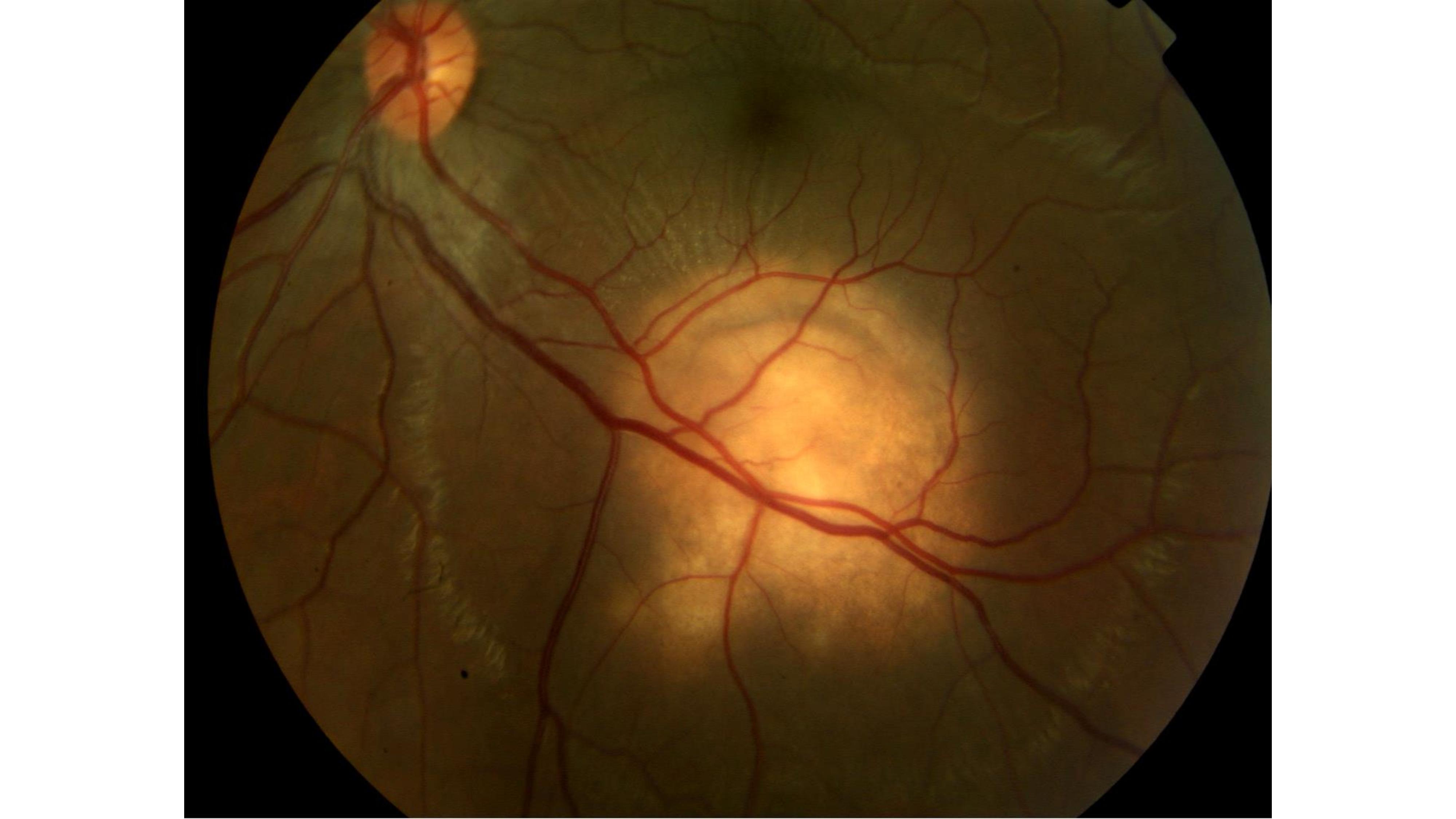
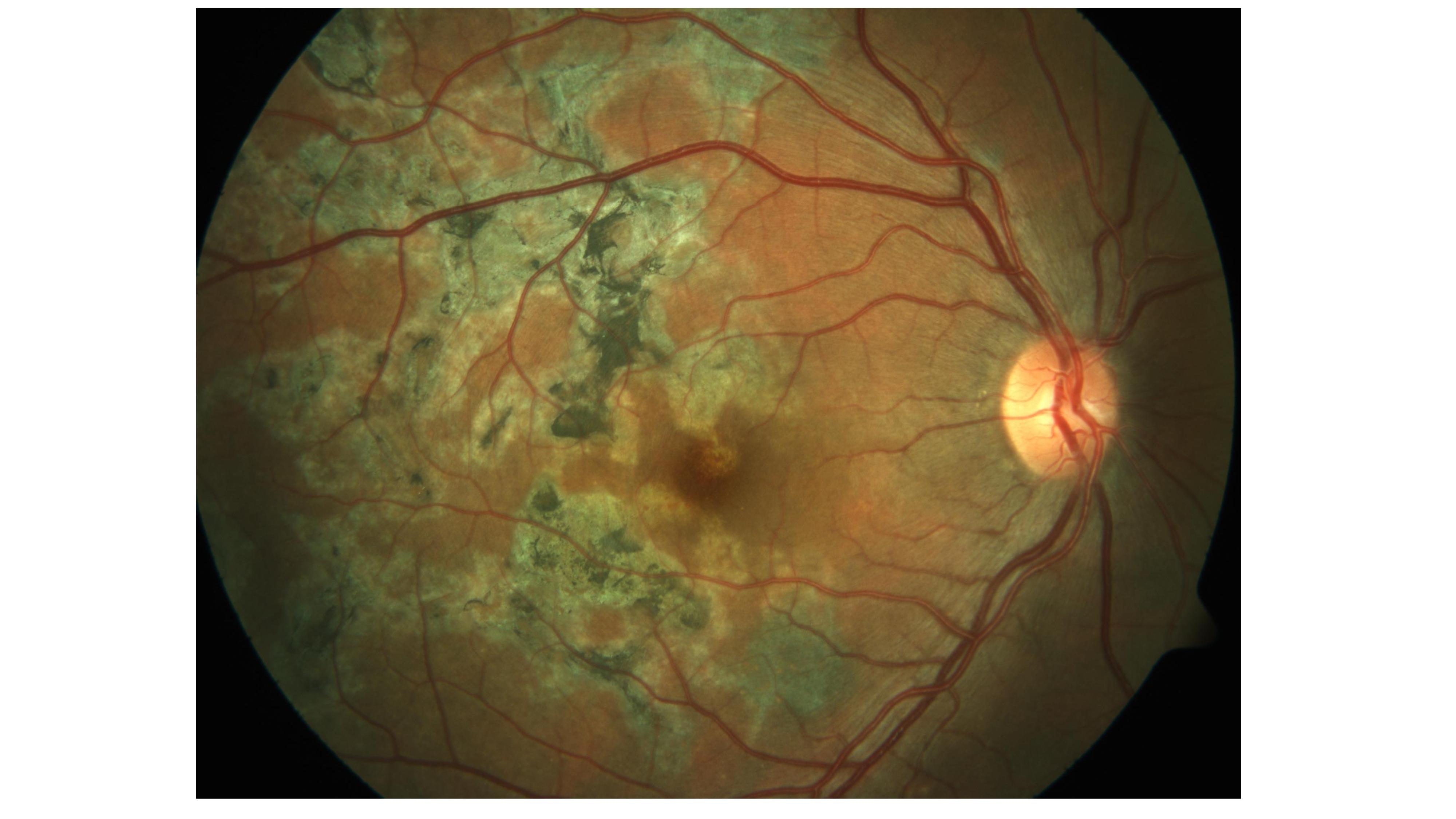
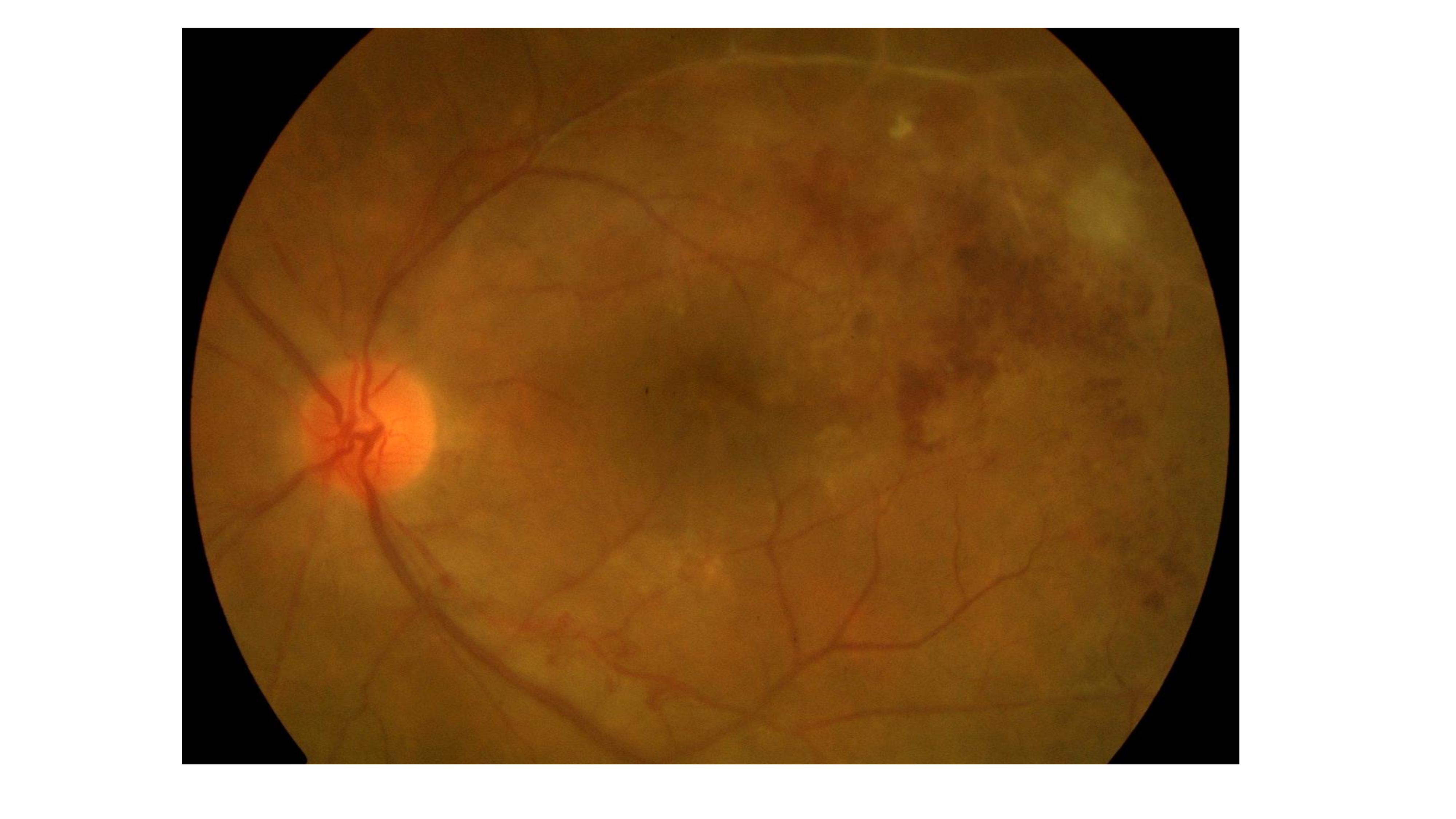
Bansal R, Gupta A, Gupta V, Dogra MR, Sharma A, Bambery P. Tubercular serpiginous-like choroiditis presenting as multifocal serpiginoid choroiditis. Ophthalmology. 2012 Nov:119(11):2334-42. doi: 10.1016/j.ophtha.2012.05.034. Epub 2012 Aug 11
[PubMed PMID: 22892153]
[49]
Gupta V, Gupta A, Arora S, Bambery P, Dogra MR, Agarwal A. Presumed tubercular serpiginouslike choroiditis: clinical presentations and management. Ophthalmology. 2003 Sep:110(9):1744-9
[PubMed PMID: 13129872]
[50]
Shakarchi FI. Ocular tuberculosis: current perspectives. Clinical ophthalmology (Auckland, N.Z.). 2015:9():2223-7. doi: 10.2147/OPTH.S65254. Epub 2015 Nov 26
[PubMed PMID: 26648690]
Level 3 (low-level) evidence
[51]
Tripathy K. Choroidal neovascular membrane in intraocular tuberculosis. GMS ophthalmology cases. 2017:7():Doc24. doi: 10.3205/oc000075. Epub 2017 Sep 1
[PubMed PMID: 28944155]
Level 3 (low-level) evidence
[52]
Gupta A, Bansal R, Gupta V, Sharma A, Bambery P. Ocular signs predictive of tubercular uveitis. American journal of ophthalmology. 2010 Apr:149(4):562-70. doi: 10.1016/j.ajo.2009.11.020. Epub 2010 Feb 10
[PubMed PMID: 20149341]
[53]
Chawla R, Tripathy K, Sharma YR, Venkatesh P, Vohra R. Periarterial Plaques (Kyrieleis' Arteriolitis) in a Case of Bilateral Acute Retinal Necrosis. Seminars in ophthalmology. 2017:32(2):251-252. doi: 10.3109/08820538.2015.1045153. Epub 2015 Jul 10
[PubMed PMID: 26161821]
Level 3 (low-level) evidence
[54]
MINTON J. Ocular manifestations of tuberculous meningitis: a pictorial survey. Transactions. Ophthalmological Society of the United Kingdom. 1956:76():561-79
[PubMed PMID: 13409572]
Level 3 (low-level) evidence
[55]
Sharma P, Sharma R. Toxic optic neuropathy. Indian journal of ophthalmology. 2011 Mar-Apr:59(2):137-41. doi: 10.4103/0301-4738.77035. Epub
[PubMed PMID: 21350283]
[56]
Zuhaimy H, Leow SN, Vasudevan SK. Optic disc swelling in a patient with tuberculous meningitis: a diagnostic challenge. BMJ case reports. 2017 Aug 9:2017():. pii: bcr-2017-221170. doi: 10.1136/bcr-2017-221170. Epub 2017 Aug 9
[PubMed PMID: 28794092]
Level 3 (low-level) evidence
[57]
Gupta A, Sharma A, Bansal R, Sharma K. Classification of intraocular tuberculosis. Ocular immunology and inflammation. 2015 Feb:23(1):7-13. doi: 10.3109/09273948.2014.967358. Epub 2014 Oct 14
[PubMed PMID: 25314361]
[58]
Gupta A, Gupta V. Tubercular posterior uveitis. International ophthalmology clinics. 2005 Spring:45(2):71-88
[PubMed PMID: 15791159]
[61]
Hoang QV, Cunningham ET Jr, Sorenson JA, Freund KB. The "pitchfork sign" a distinctive optical coherence tomography finding in inflammatory choroidal neovascularization. Retina (Philadelphia, Pa.). 2013 May:33(5):1049-55. doi: 10.1097/IAE.0b013e31827e25b8. Epub
[PubMed PMID: 23514797]
[62]
Socci da Costa D, Gomes E Silva A, Melichar A, Neves DB, Correa PA, Moraes RT. Bacillary layer detachment in serpiginous-like choroiditis of presumed intraocular tuberculosis: Report of two cases. American journal of ophthalmology case reports. 2022 Sep:27():101653. doi: 10.1016/j.ajoc.2022.101653. Epub 2022 Jul 7
[PubMed PMID: 35845750]
Level 3 (low-level) evidence
[63]
Salman A, Parmar P, Rajamohan M, Vanila CG, Thomas PA, Jesudasan CA. Optical coherence tomography in choroidal tuberculosis. American journal of ophthalmology. 2006 Jul:142(1):170-2
[PubMed PMID: 16815274]
[64]
Lee CS, Lee AY, Forooghian F, Bergstrom CS, Yan J, Yeh S. Fundus autofluorescence features in the inflammatory maculopathies. Clinical ophthalmology (Auckland, N.Z.). 2014:8():2001-12. doi: 10.2147/OPTH.S68446. Epub 2014 Sep 29
[PubMed PMID: 25302012]
[65]
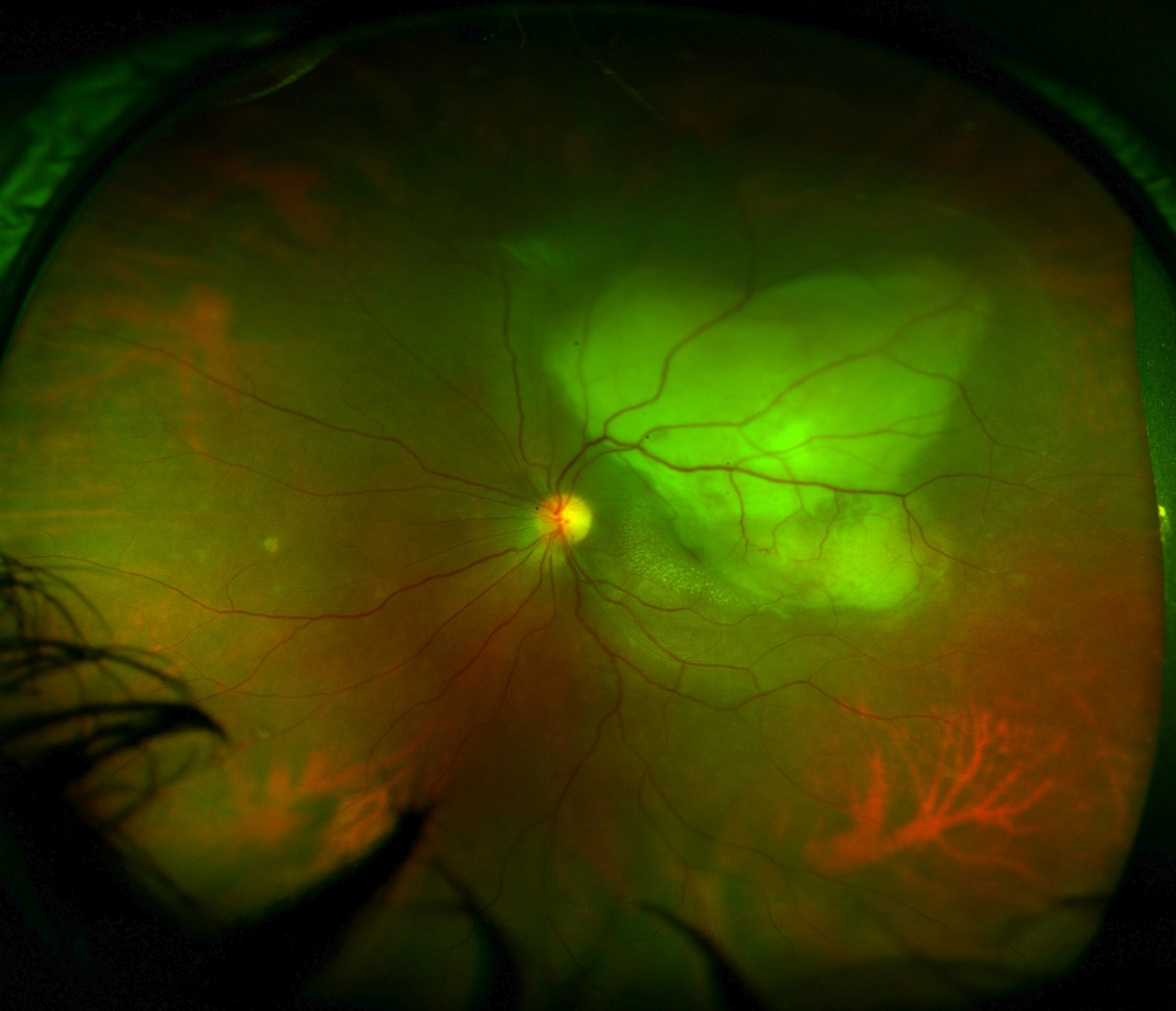
Gupta A, Bansal R, Gupta V, Sharma A. Fundus autofluorescence in serpiginouslike choroiditis. Retina (Philadelphia, Pa.). 2012 Apr:32(4):814-25. doi: 10.1097/IAE.0b013e3182278c41. Epub
[PubMed PMID: 22080913]
[66]
Raveendran R, Wattal C. Utility of multiplex real-time PCR in the diagnosis of extrapulmonary tuberculosis. The Brazilian journal of infectious diseases : an official publication of the Brazilian Society of Infectious Diseases. 2016 May-Jun:20(3):235-41. doi: 10.1016/j.bjid.2016.01.006. Epub 2016 Mar 26
[PubMed PMID: 27020707]
[67]
Sehgal V, Sharma M, Lnu P, Sharma K, Sharma A, Sharma N, Modi M. Comparison of Protein B Polymerase Chain Reaction (PCR) With IS6110 PCR for Diagnosis of Tuberculous Meningitis Patients. Cureus. 2023 Jan:15(1):e33783. doi: 10.7759/cureus.33783. Epub 2023 Jan 15
[PubMed PMID: 36798623]
[68]
Ortega-Larrocea G, Bobadilla-del-Valle M, Ponce-de-León A, Sifuentes-Osornio J. Nested polymerase chain reaction for Mycobacterium tuberculosis DNA detection in aqueous and vitreous of patients with uveitis. Archives of medical research. 2003 Mar-Apr:34(2):116-9
[PubMed PMID: 12700006]
[69]
Bajgai P, Sharma K, Bansal R, Gupta N, Sharma A, Gupta A. Detection of Mycobacterium tuberculosis Genome in Subretinal Fluid of Patients with Latent Tuberculosis Infection. Ocular immunology and inflammation. 2016 Dec:24(6):615-620
[PubMed PMID: 26645647]
[70]
Vasconcelos-Santos DV, Zierhut M, Rao NA. Strengths and weaknesses of diagnostic tools for tuberculous uveitis. Ocular immunology and inflammation. 2009 Sep-Oct:17(5):351-5. doi: 10.3109/09273940903168688. Epub
[PubMed PMID: 19831571]
[72]
Tripathy K. Comment on Trad et al.'s "Update on Immunological Test (Quantiferon-TB Gold) Contribution in the Management of Tuberculosis-Related Ocular Inflammation". Ocular immunology and inflammation. 2019:27(1):138-139. doi: 10.1080/09273948.2017.1371766. Epub 2017 Oct 11
[PubMed PMID: 29020492]
Level 3 (low-level) evidence
[73]
. Use of Tuberculosis Interferon-Gamma Release Assays (IGRAs) in Low- and Middle- Income Countries: Policy Statement. 2011:():
[PubMed PMID: 26269875]
[74]
M A, El-Asrar A, Abouammoh M, Al-Mezaine HS. Tuberculous uveitis. Middle East African journal of ophthalmology. 2009 Oct:16(4):188-201. doi: 10.4103/0974-9233.58421. Epub
[PubMed PMID: 20404986]
[75]
Cimino L, Herbort CP, Aldigeri R, Salvarani C, Boiardi L. Tuberculous uveitis, a resurgent and underdiagnosed disease. International ophthalmology. 2009 Apr:29(2):67-74
[PubMed PMID: 17486298]
[76]
Ganesh SK, Roopleen, Biswas J, Veena N. Role of high-resolution computerized tomography (HRCT) of the chest in granulomatous uveitis: a tertiary uveitis clinic experience from India. Ocular immunology and inflammation. 2011 Feb:19(1):51-7. doi: 10.3109/09273948.2010.525680. Epub
[PubMed PMID: 21250925]
[77]
Balkan A, Balci E, Yüksekol I, Ozkan M, Taşan Y, Papuşcu Y, Ekiz K, Bilgiç H, Demirci N, Seber O. [The role of high resolution computerized tomography (HRCT) in the diagnosis and treatment of pulmonary tuberculosis]. Tuberkuloz ve toraks. 2004:52(1):38-46
[PubMed PMID: 15143371]
[78]
Skoura E, Zumla A, Bomanji J. Imaging in tuberculosis. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2015 Mar:32():87-93. doi: 10.1016/j.ijid.2014.12.007. Epub
[PubMed PMID: 25809762]
[79]
Malherbe ST, Chen RY, Dupont P, Kant I, Kriel M, Loxton AG, Smith B, Beltran CGG, van Zyl S, McAnda S, Abrahams C, Maasdorp E, Doruyter A, Via LE, Barry CE 3rd, Alland D, Richards SG, Ellman A, Peppard T, Belisle J, Tromp G, Ronacher K, Warwick JM, Winter J, Walzl G. Quantitative 18F-FDG PET-CT scan characteristics correlate with tuberculosis treatment response. EJNMMI research. 2020 Feb 10:10(1):8. doi: 10.1186/s13550-020-0591-9. Epub 2020 Feb 10
[PubMed PMID: 32040770]
[80]
Khatri GD, Krishnan V, Antil N, Saigal G. Magnetic resonance imaging spectrum of intracranial tubercular lesions: one disease, many faces. Polish journal of radiology. 2018:83():e524-e535. doi: 10.5114/pjr.2018.81408. Epub 2018 Dec 29
[PubMed PMID: 30800191]
[82]
Figueira L, Fonseca S, Ladeira I, Duarte R. Ocular tuberculosis: Position paper on diagnosis and treatment management. Revista portuguesa de pneumologia. 2017 Jan-Feb:23(1):31-38. doi: 10.1016/j.rppnen.2016.10.004. Epub 2016 Dec 14
[PubMed PMID: 27988134]
[83]
Sanghvi C, Bell C, Woodhead M, Hardy C, Jones N. Presumed tuberculous uveitis: diagnosis, management, and outcome. Eye (London, England). 2011 Apr:25(4):475-80. doi: 10.1038/eye.2010.235. Epub 2011 Feb 4
[PubMed PMID: 21293496]
[84]
Khan FY, Aladab AH. Role of Fiberoptic Bronchoscopy in the Rapid Diagnosis of Sputum Smear-negative Disseminated Tuberculosis with Pulmonary Miliary Infiltrates. Oman medical journal. 2020 Jan:35(1):e87. doi: 10.5001/omj.2020.05. Epub 2020 Jan 15
[PubMed PMID: 31993225]
[85]
Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine. 1984 Jan:63(1):25-55
[PubMed PMID: 6419006]
[86]
Saxena R, Singh D, Phuljhele S, Kalaiselvan V, Karna S, Gandhi R, Prakash A, Lodha R, Mohan A, Menon V, Garg R. Ethambutol toxicity: Expert panel consensus for the primary prevention, diagnosis and management of ethambutol-induced optic neuropathy. Indian journal of ophthalmology. 2021 Dec:69(12):3734-3739. doi: 10.4103/ijo.IJO_3746_20. Epub
[PubMed PMID: 34827033]
Level 3 (low-level) evidence
[87]
Kee AR, Gonzalez-Lopez JJ, Al-Hity A, Gupta B, Lee CS, Gunasekeran DV, Jayabalan N, Grant R, Kon OM, Gupta V, Westcott M, Pavesio C, Agrawal R. Anti-tubercular therapy for intraocular tuberculosis: A systematic review and meta-analysis. Survey of ophthalmology. 2016 Sep-Oct:61(5):628-53. doi: 10.1016/j.survophthal.2016.03.001. Epub 2016 Mar 10
[PubMed PMID: 26970263]
Level 1 (high-level) evidence
[88]
Ang M, Hedayatfar A, Wong W, Chee SP. Duration of anti-tubercular therapy in uveitis associated with latent tuberculosis: a case-control study. The British journal of ophthalmology. 2012 Mar:96(3):332-6. doi: 10.1136/bjophthalmol-2011-300209. Epub 2011 Jun 30
[PubMed PMID: 21719564]
Level 2 (mid-level) evidence
[89]
Bansal R, Gupta A, Gupta V, Dogra MR, Bambery P, Arora SK. Role of anti-tubercular therapy in uveitis with latent/manifest tuberculosis. American journal of ophthalmology. 2008 Nov:146(5):772-9. doi: 10.1016/j.ajo.2008.06.011. Epub 2008 Aug 16
[PubMed PMID: 18708180]
[90]
Seung KJ,Keshavjee S,Rich ML, Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold Spring Harbor perspectives in medicine. 2015 Apr 27;
[PubMed PMID: 25918181]
Level 3 (low-level) evidence
[91]
Lyon CE, Grimson BS, Peiffer RL Jr, Merritt JC. Clinicopathological correlation of a solitary choroidal tuberculoma. Ophthalmology. 1985 Jun:92(6):845-50
[PubMed PMID: 3897938]
[92]
Young L, Yakin M, Sen HN. Choroidal granuloma resolution with tuberculosis treatment. American journal of ophthalmology case reports. 2020 Dec:20():100969. doi: 10.1016/j.ajoc.2020.100969. Epub 2020 Oct 16
[PubMed PMID: 33117917]
Level 3 (low-level) evidence
[93]
Basu S, Elkington P, Rao NA. Pathogenesis of ocular tuberculosis: New observations and future directions. Tuberculosis (Edinburgh, Scotland). 2020 Sep:124():101961. doi: 10.1016/j.tube.2020.101961. Epub 2020 Jul 24
[PubMed PMID: 33010848]
Level 3 (low-level) evidence
[94]
Rao NA, Saraswathy S, Smith RE. Tuberculous uveitis: distribution of Mycobacterium tuberculosis in the retinal pigment epithelium. Archives of ophthalmology (Chicago, Ill. : 1960). 2006 Dec:124(12):1777-9
[PubMed PMID: 17159041]
[95]
Sharma A, Thapa B, Lavaju P. Ocular tuberculosis: an update. Nepalese journal of ophthalmology : a biannual peer-reviewed academic journal of the Nepal Ophthalmic Society : NEPJOPH. 2011 Jan-Jun:3(1):52-67. doi: 10.3126/nepjoph.v3i1.4280. Epub
[PubMed PMID: 21587325]
[96]
Schutz C, Davis AG, Sossen B, Lai RP, Ntsekhe M, Harley YX, Wilkinson RJ. Corticosteroids as an adjunct to tuberculosis therapy. Expert review of respiratory medicine. 2018 Oct:12(10):881-891. doi: 10.1080/17476348.2018.1515628. Epub 2018 Sep 6
[PubMed PMID: 30138039]
[97]
Ganesh SK, Abraham S, Sudharshan S. Paradoxical reactions in ocular tuberculosis. Journal of ophthalmic inflammation and infection. 2019 Sep 6:9(1):19. doi: 10.1186/s12348-019-0183-x. Epub 2019 Sep 6
[PubMed PMID: 31493128]
[98]
Agarwal A, Marchese A, Rabiolo A, Agrawal R, Bansal R, Gupta V. Clinical and Imaging Factors Associated With the Outcomes of Tubercular Serpiginous-like Choroiditis. American journal of ophthalmology. 2020 Dec:220():160-169. doi: 10.1016/j.ajo.2020.07.024. Epub 2020 Jul 23
[PubMed PMID: 32710829]
[99]
Jain S, Agarwal A, Gupta V. Resolution of Large Choroidal Tuberculoma following Monotherapy with Intravitreal Ranibizumab. Ocular immunology and inflammation. 2020 Apr 2:28(3):494-497. doi: 10.1080/09273948.2019.1582786. Epub 2019 Apr 15
[PubMed PMID: 30986122]
[100]
Agrawal R, Ludi Z, Betzler BK, Testi I, Mahajan S, Rousellot A, Kempen JH, Smith JR, McCluskey P, Nguyen QD, Pavesio C, Gupta V. The Collaborative Ocular Tuberculosis Study (COTS) calculator-a consensus-based decision tool for initiating antitubercular therapy in ocular tuberculosis. Eye (London, England). 2023 May:37(7):1416-1423. doi: 10.1038/s41433-022-02147-7. Epub 2022 Jun 28
[PubMed PMID: 35764876]
Level 3 (low-level) evidence
[101]
Agrawal R, Testi I, Bodaghi B, Barisani-Asenbauer T, McCluskey P, Agarwal A, Kempen JH, Gupta A, Smith JR, de Smet MD, Yuen YS, Mahajan S, Kon OM, Nguyen QD, Pavesio C, Gupta V, Collaborative Ocular Tuberculosis Study Consensus Group. Collaborative Ocular Tuberculosis Study Consensus Guidelines on the Management of Tubercular Uveitis-Report 2: Guidelines for Initiating Antitubercular Therapy in Anterior Uveitis, Intermediate Uveitis, Panuveitis, and Retinal Vasculitis. Ophthalmology. 2021 Feb:128(2):277-287. doi: 10.1016/j.ophtha.2020.06.052. Epub 2020 Jun 27
[PubMed PMID: 32603726]
Level 3 (low-level) evidence
[102]
Shukla D. Re: Agrawal et al.: Collaborative Ocular Tuberculosis Study consensus guidelines on the management of tubercular uveitis - Report 2: guidelines for initiating antitubercular therapy in anterior uveitis, intermediate uveitis, panuveitis, and retinal vasculitis (Ophthalmology. 2021;128:277-287). Ophthalmology. 2021 Jul:128(7):e34-e35. doi: 10.1016/j.ophtha.2021.03.024. Epub 2021 Apr 15
[PubMed PMID: 33865623]
Level 3 (low-level) evidence
[103]
Chawla R, Venkatesh P, Kumar V, Azad S. Re: Agrawal et al.: Collaborative Ocular Tuberculosis Study Consensus Guidelines on the Management of Tubercular Uveitis-Report 1 (Ophthalmology. 2021;128:266-276). Ophthalmology. 2021 Dec:128(12):e217. doi: 10.1016/j.ophtha.2021.08.008. Epub 2021 Sep 6
[PubMed PMID: 34503846]
Level 3 (low-level) evidence
[104]
Agrawal R, Testi I, Mahajan S, Yuen YS, Agarwal A, Kon OM, Barisani-Asenbauer T, Kempen JH, Gupta A, Jabs DA, Smith JR, Nguyen QD, Pavesio C, Gupta V, Collaborative Ocular Tuberculosis Study Consensus Group. Collaborative Ocular Tuberculosis Study Consensus Guidelines on the Management of Tubercular Uveitis-Report 1: Guidelines for Initiating Antitubercular Therapy in Tubercular Choroiditis. Ophthalmology. 2021 Feb:128(2):266-276. doi: 10.1016/j.ophtha.2020.01.008. Epub 2020 Jan 11
[PubMed PMID: 32115264]
Level 3 (low-level) evidence
[105]
Gülbay BE, Gürkan OU, Yildiz OA, Onen ZP, Erkekol FO, Baççioğlu A, Acican T. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respiratory medicine. 2006 Oct:100(10):1834-42
[PubMed PMID: 16517138]
[106]
Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. American journal of respiratory and critical care medicine. 2007 Feb 15:175(4):367-416
[PubMed PMID: 17277290]
[107]
Madhavan HN, Therese KL, Doraiswamy K. Further investigations on the association of Mycobacterium tuberculosis with Eales' disease. Indian journal of ophthalmology. 2002 Mar:50(1):35-9
[PubMed PMID: 12090085]
[108]
Abu El-Asrar AM, Aljazairy AH. Acute posterior multifocal placoid pigment epitheliopathy with retinal vasculitis and papillitis. Eye (London, England). 2002 Sep:16(5):642-4
[PubMed PMID: 12194084]
[109]
Cozubas R, Ungureanu E, Instrate SL, Alexandrescu C, Nanu RV, Carstocea L, Voinea LM, Ciuluvica R. Similarities and differences between three different types of white dot syndrome and the therapeutic possibilities. Romanian journal of ophthalmology. 2018 Jul-Sep:62(3):183-187
[PubMed PMID: 30505986]
[110]
Barron GJ, Tepper L, Iovine G. Ocular toxicity from ethambutol. American journal of ophthalmology. 1974 Feb:77(2):256-60
[PubMed PMID: 4204592]