Continuing Education Activity
Arthrocentesis is a procedure that is performed to obtain synovial fluid from within a joint capsule, both for diagnostic and for therapeutic purposes. It is used in multiple disease processes, including arthritis, gout, and infectious processes such as septic arthritis. This activity outlines the use of arthrocentesis in clinical settings and highlights the role of the interprofessional team in evaluating and treating patients who undergo this procedure.
Objectives:
Identify the indications and contraindications of arthrocentesis.
Describe the equipment, personnel, preparation, and technique required for arthrocentesis.
Review the clinical significance of synovial fluid analysis findings after arthrocentesis.
Outline interprofessional team strategies for improving care coordination and efficiency to advance arthrocentesis and improve outcomes.
Introduction
Arthrocentesis is a procedure performed to collect synovial fluid from joint spaces for the identification of a disease process or the relief of painful or bothersome symptoms. There are numerous indications for joint fluid aspiration, the most important of which includes the evaluation of synovial fluid for evidence of infection or inflammation. While the procedure specifics vary depending on the joint being aspirated, the general technique and steps remain consistent. The procedure tends to be very safe overall with few complications if performed correctly, and only a small number of contraindications exist.[1][2][3][4]
Anatomy and Physiology
Anatomy and relevant landmarks depend on which joint is being accessed. Please refer to specific articles of elbow arthrocentesis, knee arthrocentesis, and shoulder arthrocentesis for the relevant anatomy of the joints and surrounding structures.[5][6][7]
Indications
Synovial fluid aspiration is indicated for the following:
- Evaluation for an intra-articular infectious process
- Diagnosis of inflammatory disease (e.g., crystalline arthropathy, spondyloarthropathies)
- Administration of medications for acute or chronic arthritis
- Symptom relief in a swollen, painful joint or inflammatory conditions (e.g., rheumatoid arthritis)
- Evacuation of possible hemarthrosis in a traumatic effusion
- Identifying communication between the joint space and a laceration
Contraindications
The only absolute contraindication to arthrocentesis is a peri-articular infection such as cellulitis, as this may introduce overlying bacteria into the joint space. There are a number of relative contraindications to this procedure. One is bacteremia, as it is theorized that the procedure can introduce bacteria into the joint space. Coagulopathy is a debated contraindication. The possibility of traumatic hemarthrosis is a concern, but a number of studies have shown little evidence of harm.[8][9]
Personnel
This procedure can be performed without an assistant; however, having a nurse or other team member at the patient's bedside may help with alleviating patient anxiety, unforeseen equipment failure, and documentation of the procedure.
Preparation
Appropriate preparation is essential to a successful procedure. Double-check to make sure all of the required equipment is at the bedside. Position the patient for the highest likelihood of success (depending on the joint being aspirated). Provide the patient with analgesia and anxiolytics if required.
Technique or Treatment
This article will go over the general steps of arthrocentesis. The specific techniques of arthrocentesis of various joints are not to be discussed here. For those specifics, see the articles titled "Elbow Arthrocentesis," "Knee Arthrocentesis," and "Shoulder Arthrocentesis Technique."[5][6][7]
- Define the joint anatomy by palpating the surrounding bony landmarks. Ultrasound may be helpful in locating effusions.
- Select a puncture site and an approach to the joint based on the appropriate anatomy. Be sure to avoid tendons, major blood vessels, and major nerves.
- Apply the antiseptic solution to the area of needle insertion and surrounding skin. Allow the skin to dry and then put down the sterile drape surrounding the area of needle insertion.
- Using a 25 gauge to 27 gauge needle, first create a wheal of local anesthetic at the point of insertion. After the skin is anesthetized, infiltrate the skin down to the area of the joint capsule. For extremely painful joints, a regional nerve block can be used.
- Attach a larger needle of appropriate length to an appropriately sized syringe. Insert the needle into the joint space along the anesthetized track.
- For draining larger effusions, a three-way stopcock can be placed between the needle and the syringe.
- To change the syringe during the procedure, grasp the hub of the needle with a sterile hemostat and hold it tightly while removing the syringe.
- An attempt should be made to remove as much fluid or blood as possible. If fluid stops flowing, the joint is either drained completely, the needle tip is dislodged, or debris is obstructing the needle. Slightly advance or retract the tip, rotate the bevel, or aspirate less forcefully.
- Place fluid into appropriate tubes and send the synovial fluid for studies as indicated by the clinical scenario.
- Place a dressing or bandage over the puncture site, and apply pressure to achieve hemostasis.
Complications
Complications with arthrocentesis are rare.[1][2] They include the following:
- Infection. If the procedure is not done with a completely aseptic technique, or if there is an overlying infection, skin bacteria may be introduced into the joint space. This can be limited by maintaining strict sterile technique and avoiding insertion through infected skin or subcutaneous tissue.
- Bleeding. Hemarthrosis after arthrocentesis is rare but more common in patients with a bleeding diathesis. When inserting the needle during the procedure, do so in a linear fashion without side-to-side movements to avoid shearing blood vessels and other structures.
- Allergic reaction. Patients with allergy to local anesthetic should not receive that class of medications.
Clinical Significance
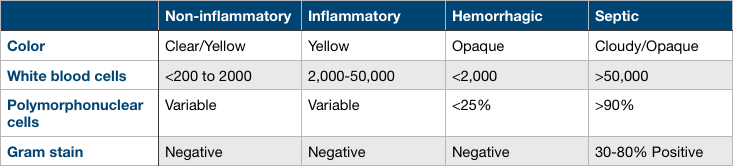
Analysis of synovial fluid is important in determining the cause of the effusion. Synovial fluid can be categorized into non-inflammatory, inflammatory, hemorrhagic, or septic. Please see the attached table for fluid characteristics in each category.
Non-inflammatory synovial fluid may be caused by osteoarthritis.
Inflammatory causes of joint effusion include gout and other crystalline arthropathies, rheumatoid arthritis, and seronegative spondyloarthropathies.
Hemorrhagic effusions may be seen in patients with a bleeding diathesis, either spontaneously or after trauma. These effusions may also be seen in any patient after significant trauma.
Septic arthritis is a severe diagnosis with significant morbidity and mortality if not identified and treated promptly. Septic arthritis can be categorized into nongonococcal arthritis and gonococcal arthritis. More than 80% of septic arthritis is caused by nongonococcal pathogens. Gram-positive staphylococci and streptococci are the most common causes of nongonococcal septic arthritis. Risk factors include intravenous drug abuse, endocarditis, osteomyelitis, abscesses, and other skin wounds. Gonococcal arthritis is more common in young, sexually active patients. This infection can have other clinical symptoms, including migratory arthritis, tenosynovial inflammation, and genitourinary complaints.
General Appearance
Visual inspection of synovial fluid can provide diagnostic clues. The clarity of synovial fluid is determined by the leukocyte count. Normal synovial fluid is clear or straw-colored. The inflammatory fluid is more opaque. Hemarthrosis may be seen spontaneously in patients with hemophilia or effusions seen after trauma. Additionally, the presence of lipohemarthrosis may indicate an occult fracture.
Fluid Analysis
Common laboratory analyses include cell count, gram stain, crystal analysis, and glucose and protein levels.
Normal synovial fluid will have the following characteristics: Clear or straw-colored, less than 200 leukocytes per microL, variable neutrophil count, and negative gram stain.
Inflammatory synovial fluid will have the following characteristics: Yellow color, 2,000 to 50,000 leukocytes per microL, variable neutrophil count, and negative gram stain.
Hemorrhagic synovial fluid will have the following characteristics: Opaque appearance, less than 2,000 leukocytes per microL, less than 25% neutrophils, negative gram stain.
Septic synovial fluid will have the following characteristics: Cloudy/opaque appearance, greater than 50,000 leukocytes per microL, greater than 90% neutrophils, and a positive gram stain between 30% to 80% of the time.
Crystal Analysis
Microscopy can be performed to assess synovial fluid for crystalline pathology. Monosodium urate crystals present in gout, are needle-shaped and negatively birefringent. Calcium pyrophosphate crystals present in pseudogout are rhomboid and positively birefringent.
Enhancing Healthcare Team Outcomes
All procedures done in clinical practice should be performed with an interprofessional team-based approach. Most commonly, arthrocentesis is performed by the physician in the emergency department, hospital, or outpatient setting, but other members of the interprofessional team play an integral role. This procedure does not commonly require procedural sedation, but in the extremely anxious patient, a provider may wish to perform sedation before the procedure is performed. In that situation, other members of the interprofessional team, including a nurse and a respiratory therapist, have important roles in monitoring the patient and documentation of the procedure. Other roles that nurses play regarding arthrocentesis include educating the patient and family about the procedure, preparing the patient and room, and assisting the physician during the procedure as needed to ensure optimal outcomes.[10]