Introduction
The somatic nervous system is a component of the peripheral nervous system associated with the voluntary control of body movements via skeletal muscles. It is responsible for all the functions we know and can consciously influence, including moving our arms, legs, and other body parts. A substantial portion of the peripheral nervous system comprises 43 different segments of nerves (12 pairs of cranial and 31 pairs of spinal nerves), which help us perform daily functions. The somatic nervous system consists of both afferent (sensory) and efferent (motor) nerves [1]. It is also responsible for the reflex arc, which involves using interneurons to perform reflexive actions. Besides these are thousands of other associated nerves in the body (See Image. Somatic Nervous System).
Cranial nerves are responsible for carrying information in and out of the brain. Ten cranial nerves originate from the brain stem and mainly control the voluntary movement and structures of the head, with some exceptions. The olfactory and optic nerve nuclei are located in the forebrain and thalamus and are not considered true cranial nerves. The others originating from the brainstem include oculomotor, trochlear, trigeminal, abducens, facial, vestibulocochlear, glossopharyngeal, vagus, spinal accessory, and hypoglossal. Of note is that the accessory nerve innervates the sternocleidomastoid and trapezius muscles, neither of which control muscles are used exclusively in the head.
Spinal nerves carry somatosensory information and motor instructions out of the spinal cord. They arise from the spinal cord as nerve roots, merge to form a web (plexus) of interconnected nerve roots, and once again branch to form nerve fibers. The formation of nerve plexuses rather than a direct continuation of the nerve roots to peripheral nerves serves as an essential safety measure so that injury at 1 site or body region may not affect the vital functions of our body.
The spinal nerves help to control the function and movement of the rest of the body. The 31 pairs of spinal nerves include 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal. Their names match the adjacent spinal vertebra from which they exit. In the cervical region, the nerve root exits above the corresponding vertebrae (the nerve root between the skull and C1 vertebrae is the C1 spinal nerve). The spinal nerve root originates below the corresponding vertebrae in the thoracic to the coccygeal region. This difference is due to the naming and location of the spinal root between the C7 and T1 vertebrae (C8 spinal nerve root). In the lumbar region, the spinal cord ends at L1 from the conus medullaris region, but the spinal nerve roots travel within the dural sac below the L2 level; this region is known as the cauda equina.
Information in electrical impulses is relayed to and from the CNS (brain and spinal cord) to the neuromuscular junction (NMJ), which converts electrical signals into chemical signals for muscle contraction. Information from the periphery is detected by sensory receptors and coveted as electrical signals back to the central nervous system.
Structure and Function
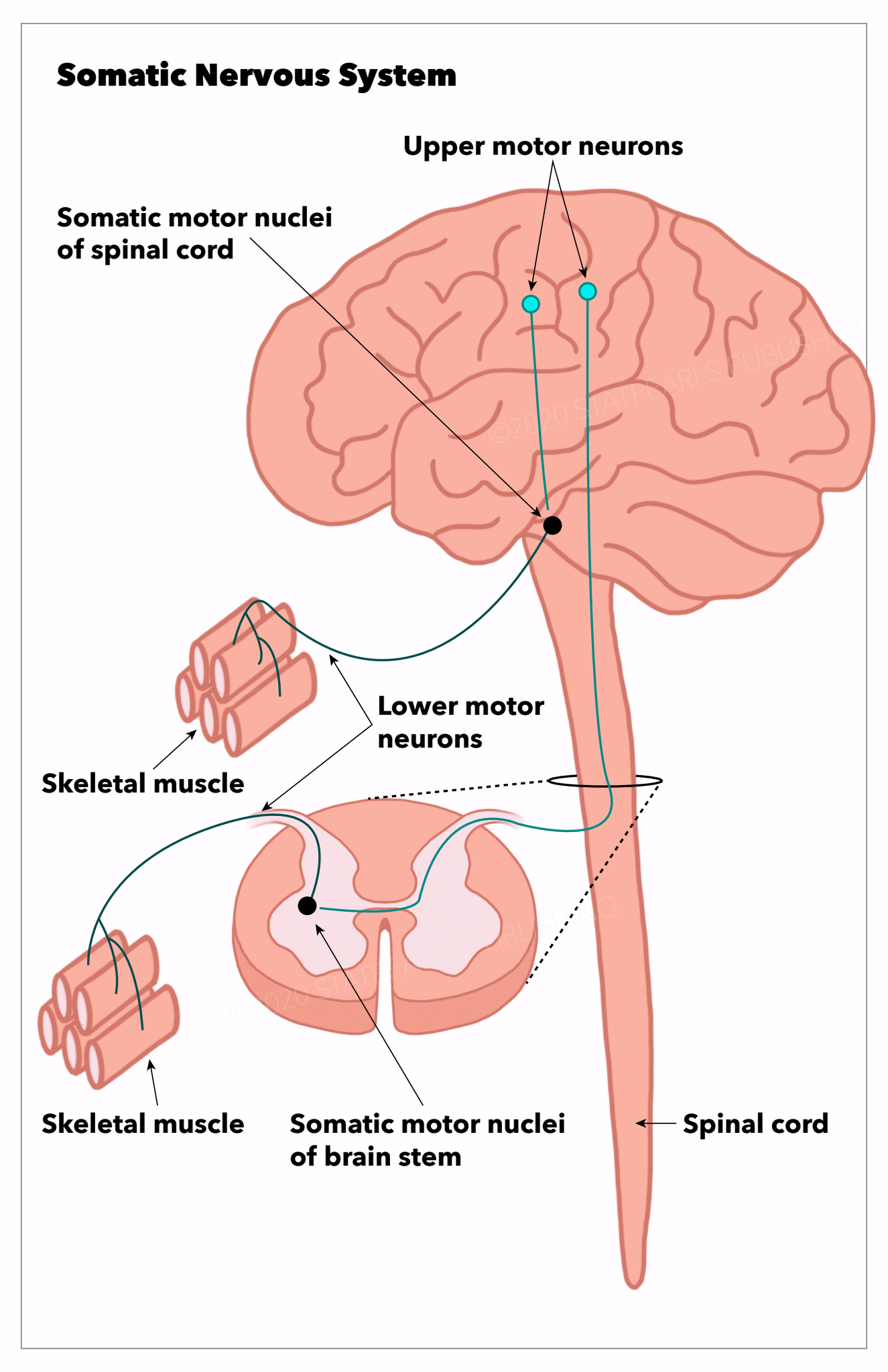
The somatic nervous system contains afferent nerves traveling from the periphery toward the CNS, and efferent nerves are responsible for sending signals from the CNS to the periphery. The brain and spinal cord are responsible for processing and integrating the various sources of information to allow us to develop a response. Therefore, the main function of the somatic nervous system is to connect the CNS with organs and striated muscles to perform our daily functions. The basic motor pathway involves the upper motor neurons in the precentral gyrus (primary motor cortex), which sends signals through the corticospinal tract via axons in the spinal cord to the lower motor neurons. These signals travel through the ventral horn of the spinal cord synapse with the lower motor neurons and send their signals through peripheral axons to the NMJ of skeletal muscle. The upper motor neuron releases neurotransmitters acetylcholine, which binds to nicotinic acetylcholine receptors of the alpha-motor neurons, creating a stimulus that propagates towards the NMJ, which innervates muscles.
The somatic nervous system's peripheral(outside of the CNS) processes form the somatic peripheral nerves, structurally and functionally different from the autonomic nervous system. The somatic peripheral nervous system is a single-neuron system with the motor neurons in the brainstem or spinal cord and the sensory neurons in the dorsal root ganglia. The autonomic peripheral nervous system is a 2-neuron system with a neuron lying outside the CNS in the autonomic ganglia. The nerve fibers have different sizes with different conduction velocities. The size depends on the actual size of the axons and, more importantly, the degree of myelination, which provides electrical insulation and fast conduction seed through the so-called "saltatory conduction." The fastest conducting fibers are, therefore, the most heavily myelinated. The motor and sensory fibers from the motor neurons serve discriminative touch, joint position, and vibratory sensations. The small fibers are mainly sensory fibers serving pain and temperature sensations.
Somatic Reflex Arc
These neural pathways are responsible for the automatic response between a sensory and motor neuron. The sensory input generates a specific motor output. The simplest spinal reflex is mediated by a single synaptic process called the monosynaptic reflex. It contains only 1 synapse between the 2 neurons involved in the arc (sensory and motor). An example to illustrate this is the patellar reflex- Striking the patellar ligament just below the patella with a reflex hammer leads to automatic contraction of the quadriceps, which results in knee extension. The next order of a simple reflex involves 2 or more synapses, termed the polysynaptic reflex (1 or more interneurons). For example, the sensory neuron becomes stimulated, which activates the interneuron, which then directly stimulates the motor neuron, causing a movement.
Embryology
The motor nerves originate from the ventral neural tube. Ventrilization is induced by morphogenetic molecules like the sonic hedgehog and other growth factors, as well as myelinating proteins.[2][1] On the other hand, the sensory nerves derive from a specific population of cells in the roof of the neural tube called neural crest cells.[1] The neural crest cells are divided axially into cranial, vagal, truncal, and lumbosacral. These truncal cells are responsible for forming the dorsal root ganglia, the autonomic ganglia, and the paraganglia, which include the neurons of the adrenals.[3]
Surgical Considerations
Dorsal root ganglion (DRG) targeted delivery of drugs is a targeted tool in pain research, and research currently focuses on capitalizing on new knowledge of the peripheral sensory nerve function in painful conditions.[4] New techniques for sustained infusion at a single DRG level and gene delivery using viral vectors are probable new avenues for therapeutic approaches to control pain at the level of the single DRG.[4]
Severe trauma to the upper extremities can result in mixed transection of both motor and sensory somatic peripheral nerves, which can lead to functional losses without surgical management. This condition can seriously affect the patient's overall quality of life. To regain function, these nerves can be transected and reconstructed to permit axonal regeneration into distal target muscles and terminal receptors in the skin. Some techniques used involved nerve autografts, which sacrifice healthy nerves in cases where a bridging material is needed if surgical management with tension is not appropriate.[5][6] Other alternatives include commercially processed nerve allografts donated to human peripheral nerve tissue.[5]
Clinical Significance
Diseases affecting the peripheral nerve fibers of the somatic nervous system are called peripheral neuropathy. They can be classified according to their causes, including congenital and acquired diseases. They can also be classified according to the primary pathology of the axons (axonal neuropathy) or the myelin sheath (demyelinating neuropathy). Axonal peripheral neuropathy consists of a big group of causes, mostly toxic-metabolic, such as diabetes mellitus and B-group vitamin deficiency. They present mainly with length-dependent sensory impairment and weakness, the so-called glove and stocking distribution. Pain and temperature sensations are more selectively affected. The demyelinating neuropathies are not length-dependent. They are often immune-mediated, resulting in more diffuse sensorimotor involvement and early loss of deep tendon reflexes. The sensory involvement is more selective with joint position and vibratory sensory loss.
Numerous congenital sensory and motor control diseases arise from CNS, peripheral nervous system, or muscle defects. Due to the extensive range covered by the somatic nervous system, these conditions can either localize to a region of the body or can be widespread and generalized.
Inherited Peripheral Neuropathy
These conditions include:
- Charcot-Marie-Tooth disease
- Fabry disease
- Refsum disease
- Porphyrias
Acquired Peripheral Neuropathy
These conditions include:
- Diabetes mellitus
- Trauma: Cauda equine syndrome, conus medullaris, herniated disk, brachial and lumbar plexus injury
- Spinal stenosis: Radiculopathy
- Familial/Sporadic: Amyotrophic lateral sclerosis (ALS)
- Autoimmune: Guillain Barre syndrome, Lambert-Eaton syndrome, amyloid neuropathy
- Infectious: Lepromatous form of leprosy, diphtheria [3]