Introduction
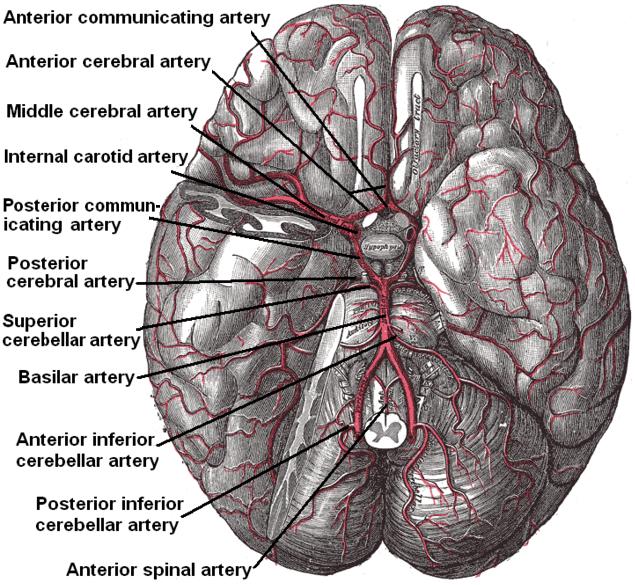
The brain receives vascular supply from a network of arteries that anastomose to form the circle of Willis. Because the brain has a constant high metabolic demand and no energy supply of its own, it requires a significant blood supply, consuming 15% of total cardiac output; any blockage of blood flow leads to severe damage and a host of neurological pathologies (see Figure. Diagram of the Brain Blood Circulation).[1]
The brain is supplied by the internal carotid arteries (ICAs), which branch from the common carotid arteries, and the vertebral arteries, which branch from the subclavian. The ICA gives rise to the anterior cerebral artery (ACA) and the middle cerebral artery (MCA). The 2 vertebral arteries unite to form the basilar artery, terminating in the 2 posterior cerebral arteries (PCA). The circle of Willis is the combination of these anterior and posterior divisions (see Image. Outer Surface of the Cerebral Hemisphere).[2]
Structure and Function
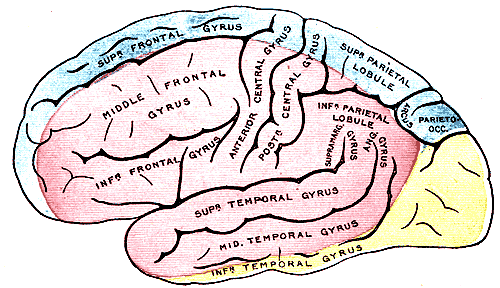
The internal carotid arteries penetrate the temporal bone through the carotid canal before giving off branches supplying the eyes via the ophthalmic artery and its daughter, the central retinal artery.[3] It then gives off branches supplying the hypothalamus regions via the posterior communicating arteries, the areas surrounding the globus pallidus, and the amygdala via the anterior choroidal arteries, and the medial surfaces of the anterior segment of the brain via the anterior cerebral artery.[4][5] The 2 anterior cerebral arteries then connect by the anterior communicating artery (ACoA), the junction with which is a frequent location of berry aneurysms.[6] Finally, the internal carotid arteries end in the middle cerebral arteries, which, together with the anterior cerebral arteries, represent its terminal branches and supply a wide territory of the lateral aspects of the cerebral hemispheres (see Image. Arterial Circulation of the Brain). These broad territories include the centers controlling speech production: Broca and Wernicke areas.[7] The lenticulostriate arteries, which branch from the proximal part of the middle cerebral arteries, further serve the deeper structures of this region.
Several small branches of the ACA branch at or near its junction with the ACoA. These include the recurrent artery of Heubner (RAH), the orbitofrontal artery, and the frontopolar artery. Identification of these arteries is essential, particularly the RAH, as their location places them at risk of injury in surgery for aneurysms and tumors.[8] The RAH is the largest medial lenticulostriate arteries, which also branch from the ACA and serve the basal ganglia with lateral branches from the MCA. In addition to those lenticulostriate arteries, the MCA also gives off the polar and anterior temporal arteries and the uncal artery, followed by numerous cortical branches that come off the distal part of the MCA.[9]
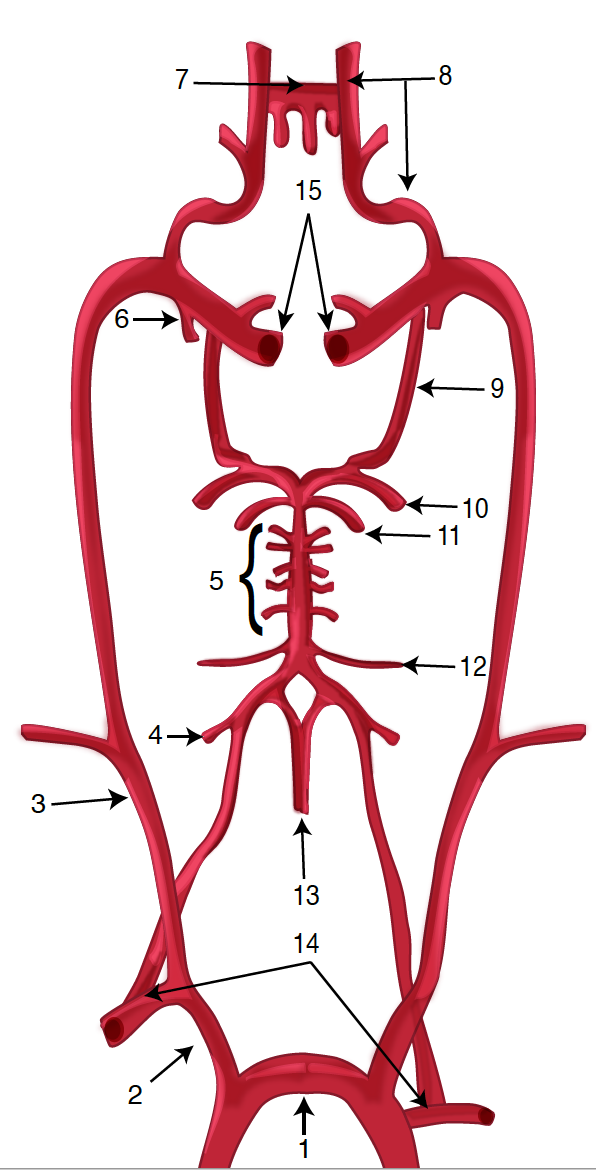
The vertebral arteries arise from the corresponding subclavian arteries and course via the transverse foramina of the cervical spine into the foramen magnum.[10] Before joining to form the basilar artery, they give off the posterior inferior cerebellar arteries and the anterior spinal arteries. Following the joining, the basilar artery gives off the paramedian pontine arteries, labyrinthine arteries, the long circumferential anterior inferior cerebellar arteries, and superior cerebellar arteries. Cranial nerve III passes between the superior cerebellar and posterior cerebral arteries as it exits the midbrain.[11] The anterior inferior cerebellar arteries supply various brainstem territories and pontine cranial nerve nuclei. Finally, the basilar artery bifurcates into its 2 terminal branches, the posterior cerebral arteries, which supply the midbrain and much of the posterior brain, including the visual cortex and related centers in the occipital and temporal lobes (see Figure. Diagram of the Posterior Cerebral Artery and its Branches). They also give off the medial and lateral posterior choroidal arteries supplying deeper structures and cortical branches, including the splenial artery.[12]
Occlusion of each arterial territory causes damage to different functional areas of the brain. As a result, each branch has a unique constellation of symptoms that may present when affected.
Embryology
The proximal ICA originates from the third aortic arch, and the distal portions come from the dorsal aorta. At roughly 28 to 30 days, the ICA has fully divided into cranial and caudal segments. The cranial segment includes the primitive olfactory artery (POA) that eventually forms the anterior choroidal and MCA. Together with the median artery of the corpus callosum (MACC), the POA is involved as part of the normal development of the ACA. Failure of the MACC to regress can lead to a variant called azygos ACA, described below. The MCA develops later, beginning around 32 to 40 days, and develops together with the cerebral hemispheres. Initially, numerous anastomoses exist between the carotid and vertebrobasilar systems, most of which regress. Occasionally, the hypoglossal artery or the trigeminal artery may persist into adulthood. The PCA develops from the caudal segments of the ICA; it is initially a continuation of the posterior communicating artery, which regresses in most individuals.[13]
Blood Supply and Lymphatics
Vasa vasorum, the microscopic vessel networks supplying large blood vessels, are rarely found in the brain and, when present, are usually related to intracranial cardiovascular pathologies such as atherosclerosis. Rather than through vasa vasorum, intracranial vessels likely get their blood supplied by simple diffusion with the cerebrospinal fluid (CSF).[14]
The brain does not have a lymphatic system as peripheral tissues do; instead, it has a similar system by which CSF enters the brain parenchyma, known as the glymphatic system. CSF initially travels through the pial arteries into the perivascular Virchow-Robin space, entering brain cells via aquaporin channels found on astrocytes. Eventually, it exits into lymphatics running along the spinal and cranial nerves and through the arachnoid granulations.[15]
Physiologic Variants
The ACA is divided into 5 segments, denoted A1 through A5. Its distal part runs along the corpus callosum, sometimes called the pericallosal artery. The largest branch of this pericallosal segment is the callosomarginal artery, which can be present in more than half of individuals. Most branches of the ACA serve the cortex branch either from the callosomarginal artery or, if it is not present, directly from the pericallosal artery. There are numerous additional variants, of which significant examples are the azygos ACA, in which the A2 segments of the 2 ACAs fuse before dividing again; bihemispheric, in which the A2 segment of 1 ACA diminishes and the other divides to feed both hemispheres; and medial ACA, in which a third ACA appears and feeds the distal territories.[16] Also, many individuals have fenestration of the anterior communicating artery, which can mimic an aneurysm.[13]
The MCA is divided into 4 parts: M1, from the ICA bifurcation through M4 to the lateral cerebral cortex. Corresponding with their relative locations, they are alternatively called the sphenoidal, insular, opercular, and cortical segments, respectively.[9] There may be a duplicate or accessory MCA in rare cases.[13]
The PCA is divided into 4 sections: P1, P2A (anterior), P (posterior), P3, and P4. The anastomosis with the posterior communicating artery separates P1 and P2.[17] As mentioned above, the PCA derives from the caudal segments of the ICA that form the posterior communicating artery. In many patients, the posterior communicating artery does not regress to a normal degree and remains a significant contributor to the PCA; the term for this is a "fetal" PCA.[13]
Surgical Considerations
As mentioned above, knowing cerebral branches and variants during surgery for intracranial aneurysms and neoplasms is vital. For example, avoiding occlusion of the fetal PCA is important, as it provides much of the posterior circulation during treating aneurysms of the posterior communicating artery.[13]
Clinical Significance
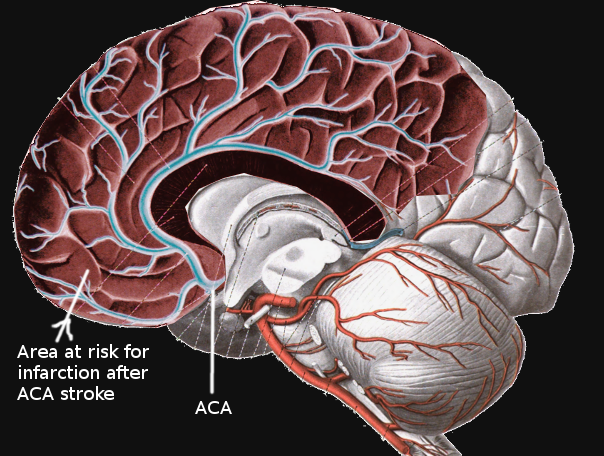
Occlusion of the cerebral arteries can lead to stroke, leading to significant morbidity and mortality (see Image. Anterior Cerebral Artery Stroke). Understanding cerebral arterial anatomy is also significant in diagnosing and managing intracranial aneurysms and tumors.
Ischemic occlusion of branches of the ICA results in anterior circulation strokes. Occlusion of cortical (pial) branches of the anterior circulation results in cortical stroke syndromes with clinical findings of aphasia, apraxia, cortical sensory impairment such as neglect or agraphesthesia, and contralateral hemiparesis. Occlusion of deep perforating branches, most commonly the lenticulostriate arteries, results in lacunar infarcts with the most common type of pure motor hemiparesis. Cortical infarction of the posterior cerebral artery results in homonymous hemianopia. Occlusion of the perforators from the PCA (thalamic perforators) may result in the lacunar syndrome of pure sensory hemianesthesia.
The ophthalmic artery arises from the ICA. A transient ischemic attack presenting with transient monocular blindness indicates a transient ischemic attack (TIA) in the ipsilateral ICA territory. The imaging modality of choice is a carotid duplex ultrasound or computed tomogram (CT) angiogram.
The anterior circulation supplies 80% of the brain, and the posterior circulation 20% of the brain. Logically, 80% of strokes occur in the anterior and 20% in the posterior circulation.
Moyamoya disease is an occlusive condition resulting from bilateral blockage of the terminal ICA and the development of abnormal collateral vessels in the basal ganglia and internal capsule region. These tiny collaterals give rise to the radiological appearance of a "puff of smoke," which is the literal meaning of Moyamoya in Japanese. It is a major cause of both ischemic and hemorrhagic strokes in childhood and early adulthood and is often treated with surgical bypass to revascularize the MCA distribution.[18]