Continuing Education Activity
Mitral valve repair is a surgical procedure designed to correct mitral stenosis and mitral regurgitation, two conditions that compromise the heart's ability to pump efficiently. The goal of repair is to restore the valve's function while preserving its natural structure, leading to improved long-term outcomes. Techniques such as annuloplasty, leaflet resection, chordal replacement with polytetrafluoroethylene, and advanced transcatheter interventions are employed to address specific issues, such as prolapsing leaflets. Proper patient selection, based on the severity of valve dysfunction, symptoms, and left ventricular changes, is crucial for ensuring successful outcomes. In selected patients, repair is associated with better survival and fewer complications than valve replacement.
In this course, participants gain a deep understanding of mitral valve repair, from the latest surgical advancements to transcatheter options. Learners improve their ability to diagnose mitral valve disease, interpret complex echocardiographic findings, and select the most appropriate treatment strategies. Emphasis is placed on collaborative care, where an interprofessional team of cardiologists, surgeons, and imaging specialists work together to optimize perioperative management. This collaborative approach enhances decision-making, patient safety, and overall clinical outcomes in mitral valve disease management.
Objectives:
Identify the relevant anatomy, indications, and contraindications for mitral valve repair.
Select the equipment, personnel, preparation, and technique regarding mitral valve repair.
Evaluate the potential complications and clinical significance of mitral valve repair.
Coordinate interprofessional team strategies for improving care and communication to advance mitral valve repair and improve outcomes.
Introduction
Although the standard of care for mitral valve (MV) pathology due to degenerative changes is surgical repair, patient outcomes depend on multiple factors, including preoperative status, the severity of mitral regurgitation (MR), the technique of repair, and surgeon and center experience. If MV repair is carried out promptly, the operative risk is low, and life expectancy is close to that of similar sex-aged matched controls. In high-risk patients, the choice among surgical, percutaneous, and conservative approaches can be challenging but should have as its basis patient comorbidities and surgical expertise. Mitral valve repair surgery has advantages over mitral valve replacement, although patient-specific factors must be considered. Of note, close to 50% of patients with severe mitral valve pathology are not candidates for surgical intervention due to age or other comorbidities.[1]
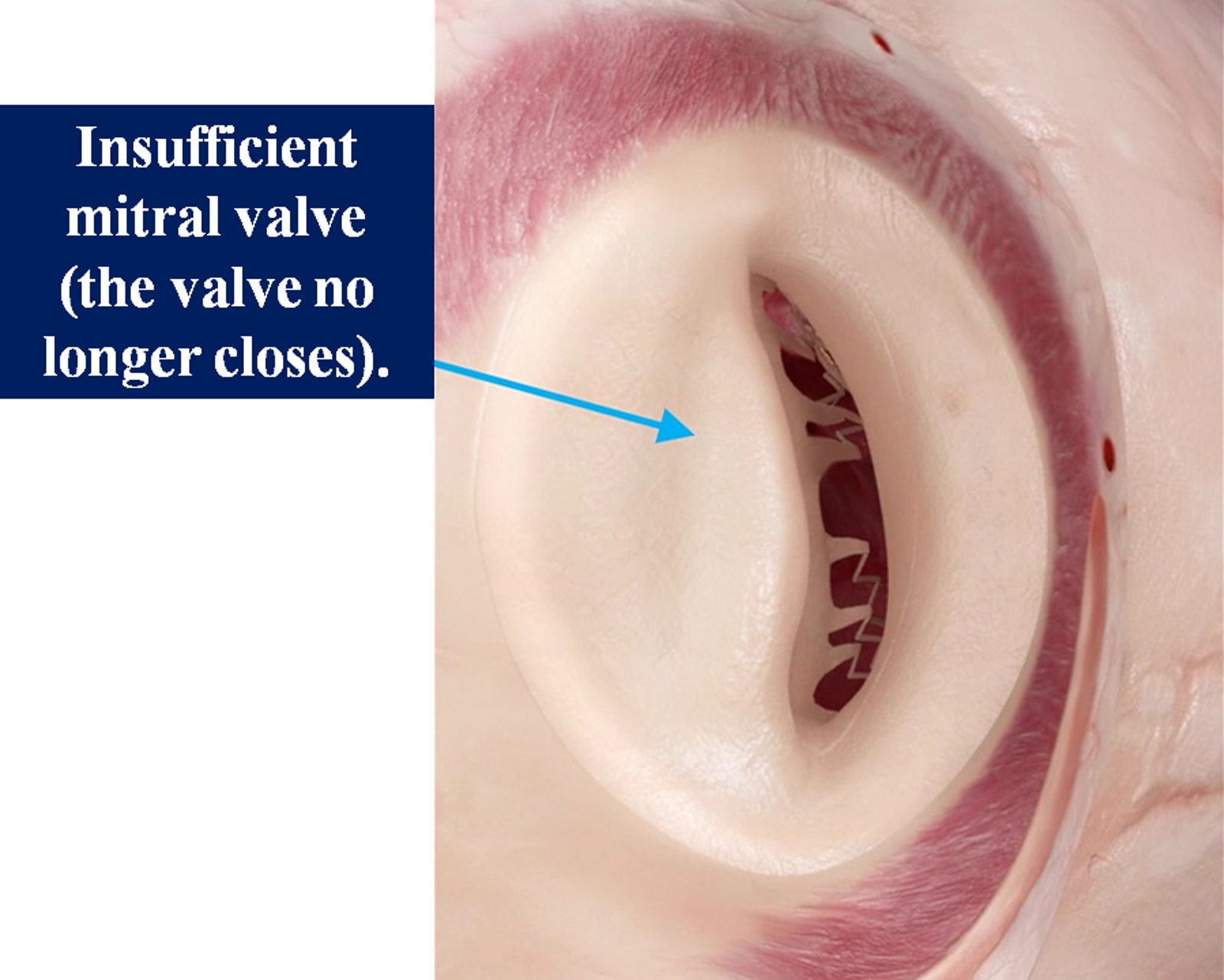
Up to 2% to 3% of adults in the United States have mitral valve disease (see Image. Insufficient Mitral Valve).[2] Patients with degenerative pathology who develop symptoms of MR have a poor prognosis, with annual mortality rates of up to 34%.[3] Mitral stenosis (MS), primarily caused by rheumatic heart disease, is commonly treated by percutaneous mitral balloon commissurotomy (PMBC), also called a percutaneous mitral balloon valvotomy, or mitral valve replacement. Repair is usually not feasible in patients with rheumatic mitral disease.
Mitral regurgitation is classified as primary or secondary, depending on whether the lesion is located at the valvular apparatus or due to left ventricular changes, respectively. While severe primary MR still receives treatment with surgical intervention, percutaneous techniques for repair and replacement are also gaining traction. Mitral valve replacement may be considered in patients with MR caused by papillary muscle rupture, degenerative and ischemic MR, or in patients with a failed repair undergoing reoperation.[4]
Anatomy and Physiology
Anatomy of the Mitral Valve
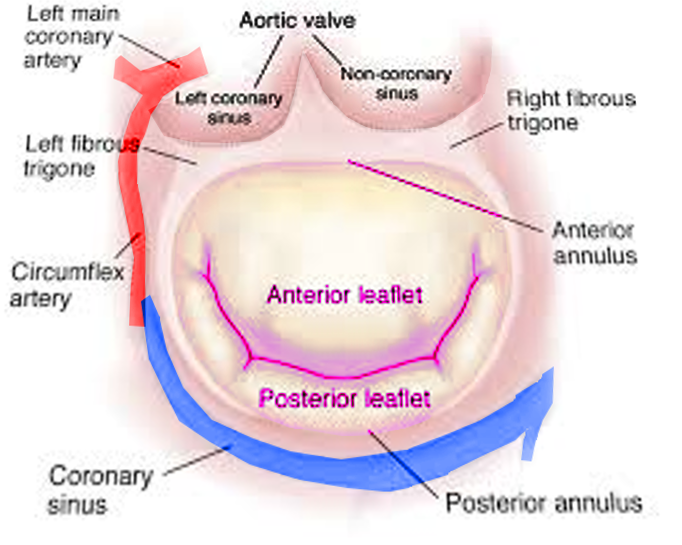
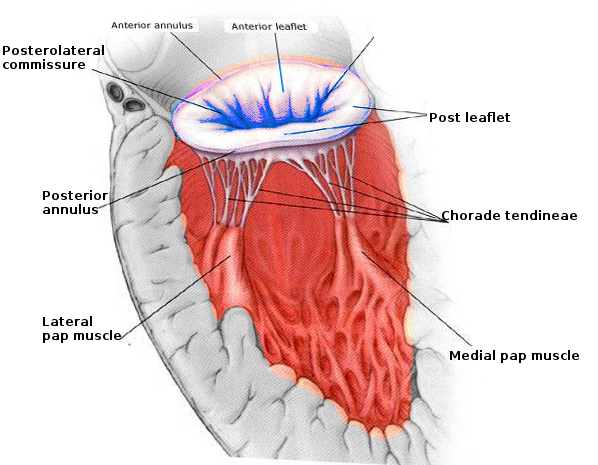
The mitral valve apparatus has a complex anatomy, including an annulus, 2 leaflets, 3 types of chordae tendinae, and 2 papillary muscles (see Image. Mitral Valve Anatomy). The mitral annulus, shaped like a "D," is at the junction of the left atrium (LA), left ventricle (LV), and mitral leaflets.[5] The left circumflex artery runs laterally around the mitral annulus, while the coronary sinus lies posteriorly, both playing a role in supplying blood to the mitral leaflets (see Image. Mitral Valve, Transverse View).[6]
The mitral valve annulus is a saddle-shaped structure consistent with the aortic valve. The annulus is most vulnerable to dilatation at its insertion on the posterior leaflet because it is the thinnest at this junction. There are 2 mitral valve leaflets: the anterior is tall and narrow, and the posterior is shorter and broader. These 2 leaflets meet at their respective commissures, known as the anterolateral and posteromedial commissures. Each leaflet is divided into 3 scallops, 1 to 3, from lateral to medial, and they are designated A1, A2, A3 (anterior) and P1, P2, and P3 (posterior).[7] The anterior leaflet occupies two-thirds of the valvular area and one-third of the annular area, whereas the posterior leaflet comprises two-thirds of the annular circumference. The leaflets have a normal line of coaptation from 7 to 9 mm, allowing for a range of normal physiologic pressures and volumes. The posterior, or mural leaflet, is more vulnerable to prolapse because it is attached to the ventricular free wall, exposing it to the recurrent stress of ventricular contraction.[8]
Chordae tendinae, fibrous structures that connect the mitral leaflets to the papillary muscles, come in 3 types based on their insertion: primary (attached to the leaflet's free margin to prevent prolapse), secondary (inserted on the rough surface of the leaflet), and tertiary (attached to the basal portion). The 2 papillary muscles are anterolateral and posteromedial, with the posteromedial muscle receiving blood from the posterior descending artery and the anterolateral muscle receiving dual supply from the left anterior descending artery and the left circumflex artery.[7] These valvular components work in concert to maintain proper blood flow from the LA to the LV. A normal mitral valve area measures 4 to 6 cm². In cases of MR, the LV often becomes thin, dilated, and dysfunctional, with an increased risk of arrhythmias.
Anomalies of the mitral valve apparatus are increasingly recognized as a phenotypic expression of hypertrophic cardiomyopathy (HCM).[5] These anomalies include:
- Anterior displacement of the mitral valve
- Elongated mitral leaflets: The anterior mitral leaflet (AML) is often longer than 30 mm, with some cases exceeding 40 mm, compared to an average of 25 mm in controls. The posterior mitral leaflet (PML) is also elongated, typically measuring more than 17 mm. The elongation of the AML shifts the coaptation plane toward its body rather than near the free edge, causing the distal portion of the AML to bend into the LV cavity during systole. This results in a sharp angulation of the distal AML toward the septum in midsystole. A residual distal AML and abnormal leaflet coaptation are necessary to develop systolic anterior motion (SAM) and LV outflow tract (LVOT) obstruction.
- Results from a cardiac magnetic resonance imaging (MRI) study found that AML length in those with HCM averaged 26 ± 5 mm (range 17-41 mm), significantly longer than in controls, which averaged 19 ± 5 mm (range 8-29 mm). The PML length in HCM patients was 14 ± 4 mm (range 6-28 mm), also significantly exceeding that of controls at 10 ± 3 mm (range 2-17 mm). A ratio of AML length to transverse LVOT diameter greater than 2.0 was more common in patients with LVOT gradients exceeding 30 mm Hg at rest.[9]
- Excessive AML area [9]
- Papillary muscle anomalies: Over 50% of patients with HCM exhibit anomalies in the papillary muscles, which may include:
- Hypertrophy of papillary muscle heads with or without accompanying septal or posterior wall hypertrophy, potentially leading to mid-cavity obstruction
- Increased number of papillary muscles; greater than 50% of those with HCM have 3 to 4 papillary muscle heads.[10]
- Anterior and apical displacement of the papillary muscles, which shifts the mitral leaflets toward the LVOT, contributes to chordal and leaflet laxity.[10]
- Direct insertion of papillary muscle onto the ventricular aspect of the AML; observed in approximately 13% of patients with HCM.
- Degenerative, myxomatous, and restrictive valves: A study of 851 patients with HCM who underwent surgery at the Cleveland Clinic revealed that 115 required a concomitant mitral valve procedure. The most common abnormalities were degenerative changes (31%), myxomatous degeneration (20%), papillary muscle anomalies (20%), chordal restriction (19%), leaflet restriction (70%), and abnormally long leaflets (56%).[11]
Physiology of Mitral Stenosis and Mitral Regurgitation
The physiology of MS and MR centers around the disruption of normal MV function, leading to significant hemodynamic changes. In MS, restricted valve opening impairs blood flow from the LA to the LV, while in MR, valve incompetence causes retrograde blood flow during systole, contributing to progressive cardiac dysfunction.
Mitral stenosis
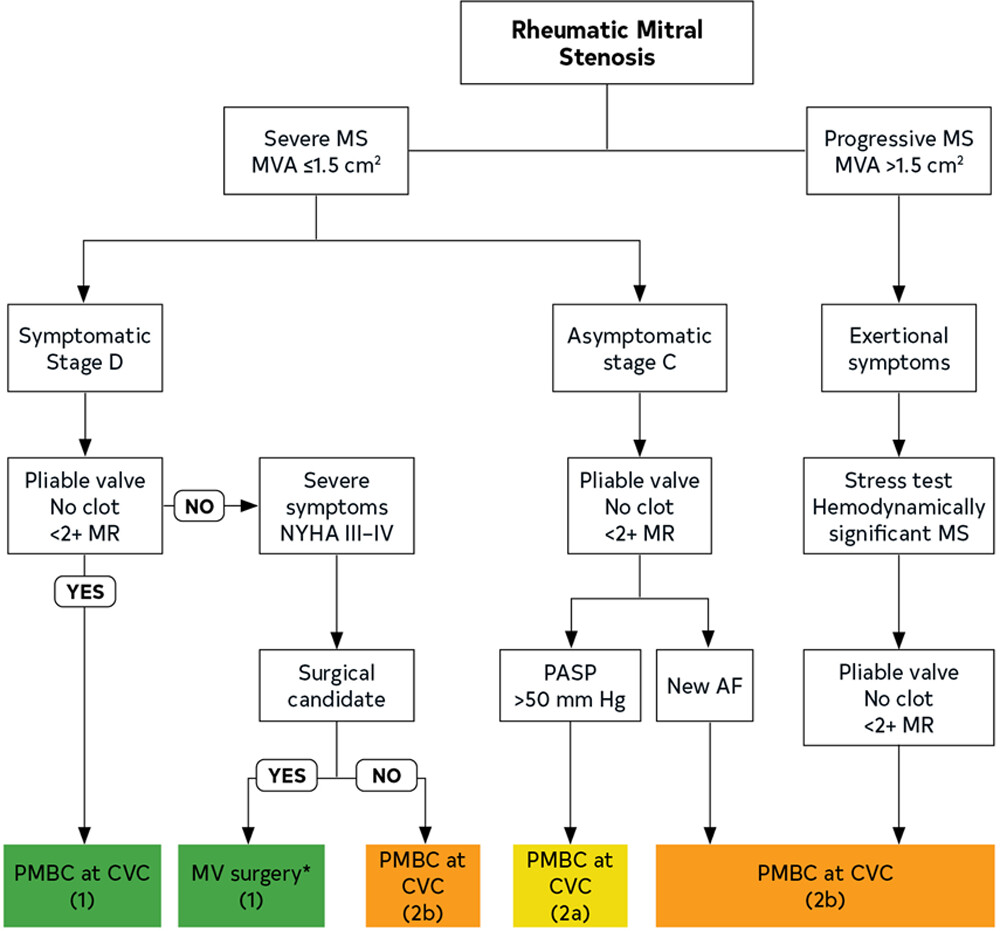
Rheumatic heart disease is a common cause of MS, often linked to an exaggerated immune response due to cross-reactivity between streptococcal antigens and valvular tissue.[12] This condition leads to characteristic changes such as a "fish-mouth" appearance of the mitral valve orifice, commissural fusion, leaflet thickening, and fusion of chordae tendinae, culminating in a "hockey-stick" appearance of the anterior leaflet on echocardiography (see Image. Treatment Flowchart for Rheumatic Mitral Valve Stenosis).
Other causes of MS include radiation-induced valvulitis, systemic inflammatory disorders, obstructive lesions, infectious vegetations, congenital valvular abnormalities, and mitral annular calcification, prevalent in older patients or those with advanced renal disease.[13] Progressive MS, especially with a valve area less than 2 cm², leads to a diastolic pressure gradient between the LA and LV, resulting in increased LA pressures and potentially reduced forward flow. Tachycardia exacerbates this condition by decreasing diastolic filling time and increasing the transmitral gradient. Elevated LA pressures can cause atrial enlargement, increasing the risk of thromboembolism and arrhythmias, particularly atrial fibrillation. Additionally, elevated pulmonary pressures can lead to pulmonary edema, hypertension, right ventricular (RV) failure, and tricuspid regurgitation, while decreased forward flow results in poor LV filling and reduced cardiac output.
To quantify MS, clinicians use echocardiography to assess the mean diastolic transmitral pressure gradient, valvular area, and sizes and pressures of the LA and right-sided chambers.[14] The mean diastolic pressure gradient is measured with continuous-wave Doppler from an apical 4-chamber view, with velocity measurements converted to pressure using the Bernoulli equation. Severe MS is indicated by a mean gradient of 10 mm Hg or more, moderate MS by 5 to 10 mm Hg, and mild MS by a gradient below 5 mm Hg. The 2020 American College of Cardiology (ACC) and the American Heart Association (AHA) guidelines classify MS as very severe if the MV area is ≤1 cm² and severe if it is ≤1.5 cm².[15]
Mitral regurgitation
MR can be categorized as primary or secondary, depending on whether the abnormality originates from the valvular apparatus or the left ventricle. In low-resource countries, mitral valve prolapse (MVP) is the most common cause of MR requiring surgical repair, whereas degenerative disease is more prevalent in the United States.[16] MVP is characterized by systolic displacement of the leaflet(s) more than 2 mm above the mitral annulus plane on a long-axis view. Barlow disease, marked by mucopolysaccharide accumulation and fibroelastic deficiency, compromises the mechanical integrity of the mitral valve. Primary MR may also result from infective endocarditis, mitral annular calcification, rheumatic heart disease, connective tissue disorders, congenital anomalies, and drug use. Secondary MR, or functional MR, is associated with LV remodeling, mitral annular dilation, and impaired LV contractility. Secondary MR can be further divided into ischemic and nonischemic categories. Ischemic mitral regurgitation due to LV dysfunction from coronary artery disease (CAD) often signifies a poor prognosis. Nonischemic MR arises from various cardiomyopathies or atrial fibrillation with subsequent annular dilation.
Ischemic MR is a severe complication of CAD, contributing to increased mortality and morbidity. While optimal medical therapy and surgical revascularization are effective for moderate-to-severe ischemic MR, the best surgical approach remains debated. Mitral valve repair demonstrates clear advantages for primary MR, but these benefits are less consistent for functional regurgitation due to the distinct pathophysiology of ischemic MR. Unlike degenerative MR, ischemic MR results not from direct damage to the valve leaflets but from dysfunction of the subvalvular apparatus and the LV wall due to acute or chronic ischemia. Echocardiographic study results suggest that LV remodeling, leading to papillary muscle displacement, increased leaflet tethering, and impaired coaptation, is the primary mechanism behind ischemic MR. Neither mitral valve repair nor replacement directly addresses these underlying issues, and results from early randomized trials have suggested that replacement may offer a more durable solution. However, advancements in subvalvular procedures enhance the stability of repair techniques, and the debate over the best surgical approach is far from resolved.[17]
Depending on whether the MR is acute or chronic and the magnitude of the regurgitant volume, MR leads to LA and LV volume overload. Acute MR leads to a sudden increase in preload and LV filling pressures that can cause pulmonary edema. Cardiac output is reduced since blood flow is directed to the LA, which can precipitate cardiogenic shock; this is a joint presentation in patients with infective endocarditis, chordae tendinae rupture, or papillary muscle rupture following myocardial infarction.
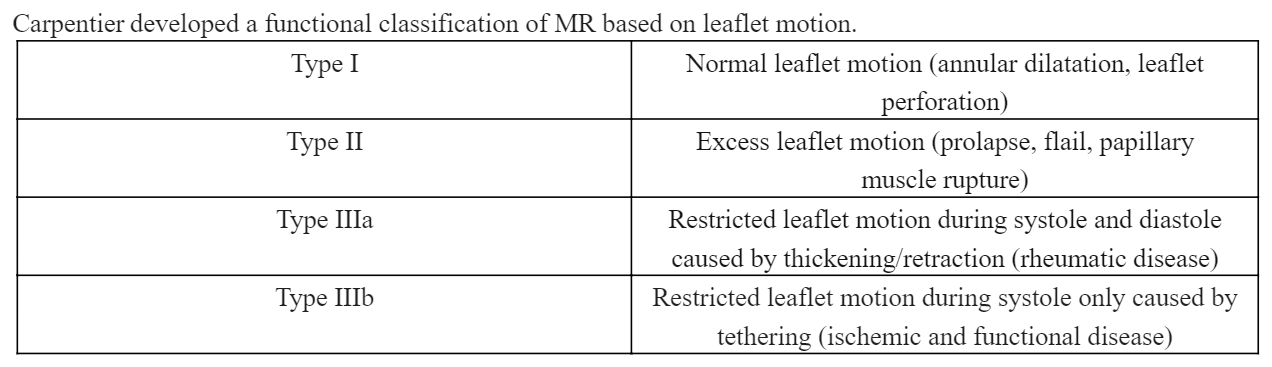
Chronic MR is divided into 3 stages: a compensated stage, where most patients are asymptomatic; a transitional stage with LV remodeling; and a decompensated stage marked by insidious symptom development. Chronic MR also leads to LA enlargement, which increases the risk for atrial arrhythmias and thromboembolic episodes. Carpentier developed a functional classification of MR based on leaflet motion (see Image. Carpentier Functional Classification of Mitral Regurgitation). Results from the Olmsted County community study showed that 54% of patients with moderate-to-severe MR also had atrial fibrillation.[18]
Echocardiography is the modality of choice for the categorization of MR. Color Doppler is useful in assessing the jet area and its ratio to the LA area. A jet over 40% of the LA area suggests severe MR. In severe MR, measured peak mitral inflow velocity is usually more than 120 cm/sec and diastolic pulmonary venous flow reversal is present.[19] Worsening MR leads to systolic flow reversal and a blunting of the systolic component. Prompt surgical intervention is paramount in patients with severe MR and preserved ejection fraction to prevent adverse LV remodeling. Beta natriuretic peptide, global longitudinal strain, exercise capacity, and RV systolic pressure are important prognostic indicators in such patients.[20]
Indications
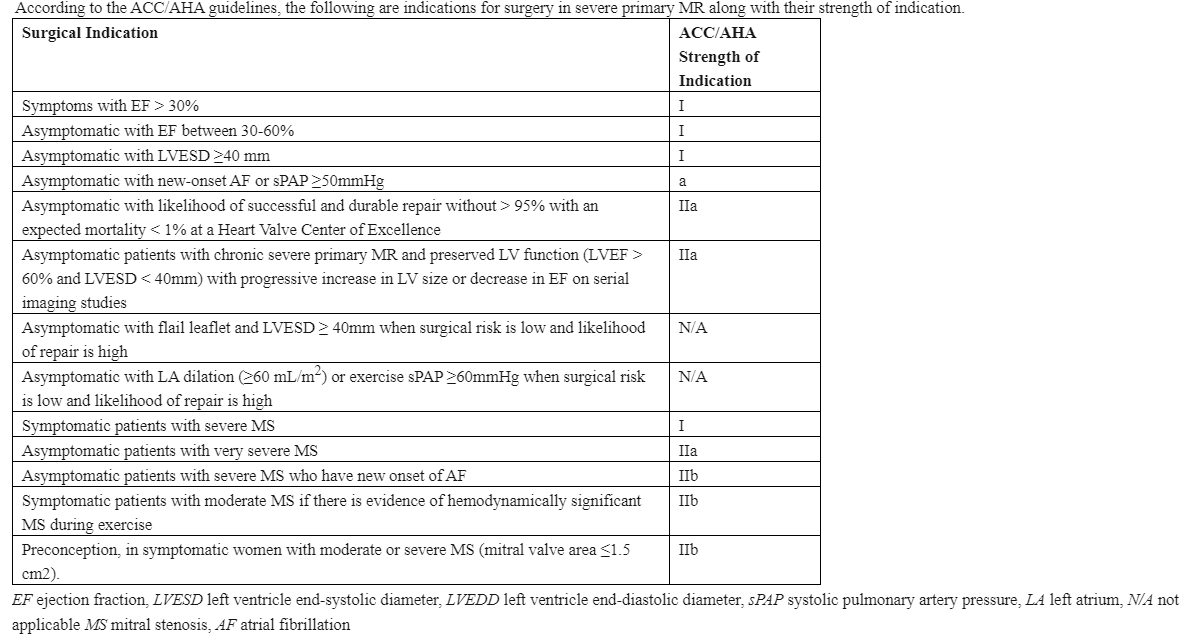
The American College of Cardiology (ACC)/American Heart Association (AHA) guidelines provide specific criteria for managing MS and MR, particularly regarding mitral valve repair (see Image. Indications for Intervention in Severe Primary Mitral Regurgitation).[21] The relevant guidelines for each condition are summarized here.
Mitral Stenosis
Mitral valve repair for MS is typically less common than for MR, and the primary intervention for MS often involves PMBC. However, valve repair or surgical commissurotomy is considered in specific situations where PMBC is not feasible. The ACC/AHA guidelines include:
Symptomatic patients with severe MS (class I): Patients with severe symptomatic MS (valve area ≤1.5 cm²) who are not candidates for PMBC due to unfavorable valve anatomy or those with contraindications such as left atrial thrombus should be considered for mitral valve repair or surgical commissurotomy.
Severe MS with new-onset atrial fibrillation (class IIa): For patients with severe MS who develop new-onset atrial fibrillation or have recurrent systemic embolization, mitral valve repair or replacement should be considered if PMBC is not an option.
Pulmonary hypertension in severe MS (class IIa): In patients with asymptomatic severe MS but who develop pulmonary hypertension, defined as a pulmonary artery systolic pressure >50 mm Hg, surgical intervention, including mitral valve repair, is recommended when PMBC is not appropriate.
Symptomatic moderate MS (class IIb): Surgical mitral valve repair may be considered in patients with moderate MS (MV area >1.5 cm² but <2 cm²) who are symptomatic and have other cardiac indications for surgery, such as concomitant coronary artery bypass grafting (CABG).
Mitral Regurgitation
Mitral valve repair is often preferred over replacement for MR, especially in primary MR. The ACC/AHA guidelines emphasize early intervention in certain patient populations to prevent long-term complications. The key recommendations include:
Symptomatic severe primary MR (class I): Surgical mitral valve repair is recommended for symptomatic individuals with severe primary MR and preserved LV function (ejection fraction [EF] >30%).
Asymptomatic severe primary MR with LV dysfunction (class I): In patients with severe primary MR who are asymptomatic but have evidence of LV dysfunction, defined as LVEF ≤60% or LV end-systolic diameter ≥40 mm, early surgical intervention with mitral valve repair is strongly recommended to prevent further LV remodeling and heart failure.
Severe primary MR with atrial fibrillation or pulmonary hypertension (class IIa): Asymptomatic individuals with severe primary MR and preserved LV function who develop new-onset atrial fibrillation or resting pulmonary hypertension defined as above should be considered for mitral valve repair.
Asymptomatic severe primary MR with normal LV function and high likelihood of durable repair (class IIa): Early mitral valve repair may be considered in patients with asymptomatic severe primary MR who have normal LV function and a high likelihood of successful and durable repair. This is especially relevant if the surgical team has a high success rate with repair procedures.
Severe secondary MR (class IIb): For patients with severe secondary MR and heart failure, mitral valve repair may be considered if the patient is undergoing another cardiac surgery, such as CABG or aortic valve surgery. However, depending on the underlying cause and LV function, mitral valve replacement may sometimes be more appropriate, especially in patients with ischemic MR.
Moderate secondary MR in patients undergoing other cardiac surgery (class IIa): In patients with moderate secondary MR undergoing surgery for another cardiac condition, such as CABG or aortic valve replacement, mitral valve replacement should be considered to address MR and improve long-term outcomes.
Contraindications
A cardiac surgeon with experience in mitral valve surgery must evaluate patients with severe MR. The standard preoperative evaluation must be undertaken to determine surgical candidacy, including assessment of coronary arteries, medical comorbidities, and prior surgical history. The Society of Thoracic Surgeons (STS) risk calculation can be performed to aid with the determination of surgical risk, including that of mortality and significant morbidity.
Patients with aortic calcification, RV dysfunction, or severe mitral annular calcification are considered to have relative contraindications to mitral valve repair. Severe LV dysfunction is also a relative contraindication as repair of the mitral valve produces a competent valve, thereby increasing afterload on the LV. Thus, the preoperative LV function and EF usually overestimate the true LV function in cases of severe MR since the regurgitant valve acts as a "pop-off" valve. Severe emphysema, restrictive lung disease, and pulmonary hypertension are also commonly seen comorbidities with severe longstanding MR and pose relative contraindications to surgery.[22]
Equipment
Mitral valve repair requires cardiopulmonary bypass and, in the vast majority of cases, full cardiac arrest. Standard equipment will include instruments necessary for open cardiac operations. Transesophageal echocardiography is mandatory for intraoperative repair and ventricular function evaluation.
Personnel
Mitral valve repair requires standard open cardiac surgical staff, which includes, at minimum, a cardiac surgeon experienced in mitral valve surgery, a cardiac anesthesiologist, a perfusionist, a surgical assistant, a surgical nurse or technician, and a circulating or operating room nurse. Postoperatively, these patients require care from a critical care intensivist, cardiologist, physical therapist, social worker, and various levels of nursing staff.
Preparation
The preoperative assessment of patients undergoing cardiac surgery requires an appraisal of the patient and procedural risks, including a thorough medical history, focused physical examination, a review of diagnostic studies, and pertinent consultant preoperative evaluations. Significant cardiovascular risk factors include myocardial ischemia, ventricular dysfunction with heart failure, and atherosclerotic disease of the carotid arteries or proximal aorta. The etiology of ventricular dysfunction is essential to establish optimal perioperative hemodynamic parameters.
Depending on their presentation, patients can be stratified into low-, intermediate-, or high-risk. Low-risk patients present with angina but no preoperative myocardial infarction (MI) and are scheduled for elective surgery. Intermediate-risk patients may have an acute MI but remain hemodynamically stable, often requiring hospitalization with heparin and antiplatelet therapy. High-risk patients are hemodynamically unstable after an acute MI and face a heightened risk of morbidity and mortality.[23] For those with heart failure, preoperative assessment should include evaluation of left and right ventricular dysfunction. Anesthetic planning must consider the need for intraoperative monitoring, appropriate anesthetic agents, vasoactive support, and possible mechanical assistance. Pulmonary hypertension, defined by a mean pulmonary artery pressure greater than 25 mm Hg at rest, significantly raises perioperative morbidity and mortality risks.[23]
Patients with severe atherosclerosis of the proximal aorta or carotid arteries are at an increased risk for perioperative stroke. Additional comorbidities that must be evaluated include diabetes, hypertension, prior cerebrovascular events, peripheral vascular disease, and smoking-related pulmonary pathology. Stroke risk can be mitigated by preoperatively treating patients with aspirin, beta-blockers, and statins. Those with extremely high stroke risk may require consultation with a vascular surgeon before surgery. During cardiac procedures, aortic plaques can dislodge due to clamping, unclamping, or turbulent blood flow, increasing the risk of embolic events such as stroke.[24]
Noncardiac risk factors that cannot be modified include female sex, advanced age, New York Heart Association intravenous functional status, preexisting renal insufficiency, prior transient ischemic attack, anemia, and a history of tobacco use. Preexisting renal insufficiency can lead to acute kidney injury in 1% to 2% of patients, which significantly increases mortality.[25] These patients should avoid exposure to nephrotoxic agents and have their volume status optimized. Reduced cardiac output or hypotension needs to be promptly treated. Anemia is a common perioperative finding in cardiac surgical individuals and is independently associated with increased transfusion risk.[25] In patients with diabetes, hypoglycemia, and marked hyperglycemia should be strictly avoided to prevent perioperative complications. Patients with hypertension should have chronically administered oral antihypertensive agents until surgery, excluding angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers.
Patients with chronic obstructive pulmonary disease should be at their optimal baseline level of pulmonary function before the intervention. Patients who smoke or use tobacco receive counsel about preoperative cessation.[26] Ensuring optimal preoperative nutrition and postoperative rehabilitation programs have improved functional capacity in older patients. For elective surgical procedures, prolonged aortic cross-clamp time and total cardiopulmonary bypass time confer a higher risk. The mortality risk also directly correlates with the procedural volume or experience of the surgeon and institution.[27] Medical management of mitral valve pathology includes diuretics and rate-controlling agents, such as beta-blockers, for symptomatic relief. Patients with atrial fibrillation should receive anticoagulation with warfarin. Vasodilators are frequently used to reduce arterial resistance and improve cardiac output, which increases the mitral valve closing force and reduces backflow. Although acute MR reduction is effective with vasodilators, sustained improvement can be challenging.
Technique or Treatment
Mitral valve repair aims to restore proper valve function and improve hemodynamics in patients with MS or MR. Advances in surgical and percutaneous approaches have refined repair strategies, prioritizing valve preservation whenever possible. This section outlines the key procedural steps, decision-making criteria, and therapeutic interventions that guide MV repair for optimal patient outcomes.
Standard Mitral Valve Repair
Standard mitral valve repair is performed utilizing full cardiopulmonary bypass and ischemic arrest. Numerous possible surgical approaches exist, including median sternotomy, right thoracotomy, and robot-assisted. Regardless of the incisional approach, the core principles of mitral valve repair remain the same: creating a competent valve with good coaptation depth, ring annuloplasty, and avoiding systolic anterior motion. The surgical technique for mitral valve repair via median sternotomy is described here; this approach is essential for patients needing additional concomitant procedures such as CABG, ascending aortic intervention, or extra/multiple valve intervention:
- Cannulation for CPB is achieved via the ascending aorta and bicaval venous cannulation. The cardioplegic arrest is achieved via the antegrade and retrograde routes. After aortic cross-clamping and adequate diastolic cardioplegic arrest, the interatrial groove (Søndergaard or Waterston) is exposed. Left atriotomy is created away from the pulmonary veins. Alternatively, a transseptal approach can be performed via right atriotomy and incising the septum by the fossa ovalis. Appropriate retractors are placed for exposure. The valve is inspected systematically using saline injection and visual inspection of each segment. The repair technique will depend on the findings of valve pathology. Isolated P2 prolapse, for instance, can be repaired by triangular or quadrangular resection and ring annuloplasty or by creating neochordae to the appropriate papillary muscle. We recommend ring annuloplasty for all repairs. Annuloplasty sutures are placed around the annulus, and the anterior leaflet height is sized. The ring can be true-sized or undersized depending on MR pathology (primary or secondary, ischemic or nonischemic). Testing the valve for coaptation depth is essential; the ideal depth approximates 1 cm. Importantly, excessive undersizing of the ring should be avoided as this may cause systolic anterior motion.
Carpentier and Lawrie Techniques
Alain Carpentier’s technique for correction of MR, developed through autopsy and pathology studies of the mitral valve, includes leaflet repair with quadrangular resection and rigid annuloplasty to correct annular dilatation.[28] Lawrie et al described a functional correction of MR, which spares valve leaflets and chordae during the repair.[27] A flexible annuloplasty ring corrects annular dilatation, and proprietary artificial fabric chordae are used to repair prolapse and realign leaflets.[29] In patients with degenerative MR, the mitral valve annulus can double in size, leading to flattening of the valvular orifice and reduced leaflet edge apposition. With inadequate leaflet coaptation, ventricular systole stresses the leaflet bodies and marginal chordae, leading to further dysfunction and failure.
According to reports, when employing the Lawrie technique, avoidance of reoperation and significant recurrent MR as assessed by echocardiography at 1 year is 90.1% and 93.9%, respectively. Also, if the ventricular filling is optimized through adequate preload and afterload, atrioventricular dyssynchrony is avoided, hypercontractility is limited, and there is almost no postoperative systolic anterior movement.[30]
Braunberger et al reported long-term results of valve repair in nonrheumatic MR.[29] In patients with isolated posterior leaflet prolapse, 10- and 20-year freedom from reoperation was 98.5% and 96.9%, respectively. In patients with isolated anterior prolapse, it was 86.2% and 82.2%, respectively. In patients with bileaflet prolapse, freedom from reoperation was 88.1% and 82.6%, respectively. These data confirm the stability of the Carpentier repair over an extended period.
Percutaneous Mitral Balloon Commissurotomy
Randomized trials have demonstrated that PMBC is safe and effective compared to surgical closed or open commissurotomy in patients with favorable valve morphology characterized by less than 2+ MR and no left atrial thrombus. During PMBC, 1 or more balloon catheters are advanced across the MV and inflated to split the commissures. Ideal candidates for PMBC have valve leaflets that are mobile and relatively thin, free of calcium, and without significant subvalvular fusion. An anatomic mitral morphology score can help determine a patient’s suitability for PMBC by assessing the commissures’ appearance and the extent of calcification. Clinical factors such as age, New York Heart Association class, and presence or absence of atrial fibrillation also predict outcomes.[31]
Commissurotomy
Mitral valve surgery is a well-established treatment for rheumatic MS, with commissurotomy being the preferred method when the valvular anatomy is suitable. This procedure can be performed as a closed commissurotomy, where the valve is opened without direct visualization through the LA or LV, or as an open commissurotomy, allowing for more extensive surgery under direct observation. However, mitral valve replacement may be the most appropriate option for significant valvular thickening and subvalvular fibrosis with associated leaflet tethering. For patients with suboptimal valve anatomy, those who have experienced a failed PMBC, and those with moderate or severe tricuspid regurgitation, a surgical approach that includes tricuspid valve repair may yield better outcomes. Additional procedures, such as atrial fibrillation ablation and tricuspid valve repair, are strongly recommended (class 1) in conjunction with mitral valve replacement.[31]
Minimally Invasive Mitral Valve Surgery
Minimally invasive mitral valve surgery (MIMVS) can be divided into 2 groups: partial sternotomy and right thoracotomy, including the open and video-assisted methods, with or without robotic assistance.[28][31] MIMVS has been shown to reduce surgical trauma by avoidance of a full sternotomy incision. MIMVS approaches will require different cannulation techniques, generally involving the femoral vessels. Sündermann et al showed equivalent short- and mid-term outcomes with MIMVS, as compared to conventional surgery, regarding the incidence of stroke, mortality, and durability of repair.[30] Patients who undergo MIMVS have reduced blood loss, need for blood transfusion, mechanical ventilation time, intensive care unit stay, and a quicker resumption of regular activity.[32] Iribarne et al reported that MIMVS is associated with lower hospital costs.[33] Due to these highly optimistic results, there has been an increase in the proportion of MIMVS from 10% in 2004 to 20% in 2008. The least invasive approach for MIMVS is robotically-assisted, eliminating the need for thoracotomy or significant rib spreading. However, this method has higher operative costs. The robotically-assisted approach allows for excellent 3-dimensional visualization of the valvular and subvalvular apparatus, thanks to EndoWrist® technology, which enables complex surgical maneuvers.
Transapical Beating-Heart Mitral Valve Repair
An innovative approach for treating degenerative mitral valve disease due to posterior leaflet prolapse is transapical beating-heart mitral valve repair with polytetrafluoroethylene (PTFE) chordae implantation. This procedure avoids CPB by accessing the cardiac apex through a small left anterolateral thoracotomy incision at the fourth or fifth intercostal space. The neochordae device lets the surgeon grasp and pierce the prolapsing leaflet, pulling the PTFE chord through the segment. The neochordae are then externalized at the cardiac apex and adjusted under echocardiographic guidance to ensure maximal coaptation and resolution of MR. Early follow-up has shown most patients to be stable. This technique is utilized to treat MR due to prolapsing lesions early in the history of the disease when annular dilatation is minimal, and LV remodeling is limited.[33] Transcatheter techniques targeting both the leaflets and annulus are increasingly employed to achieve durable results in appropriate patients. However, the omission of ring annuloplasty is associated with poor long-term outcomes, underscoring the importance of careful patient selection.
Complications
Recurrent MR is the most common complication following primary mitral valve repair. The pulmonary capillary wedge pressure (PCWP) and LA pressures are equivalent. The a wave and v wave are key components of heart pressures, and variations in their size can provide critical diagnostic insights. The significance of the v wave is linked to any pathological condition that impacts atrial filling during systole when the atrioventricular valves are closed. The size of the v wave is partially influenced by the volume of blood entering the atrium, with MR being the most common cause of large v waves. In cases of acute MR, such as with a ruptured chordae tendineae, giant v waves can occur. V waves more than twice the mean PCWP indicate severe MR. As an example, in severe mitral paravalvular leak, elevated mean LA pressure and prominent v waves can lead to pulmonary hypertension and right heart failure. Closing the paravalvular leak typically results in a rapid reduction of v waves and mean LA pressure, improvement in the echocardiographic grade of regurgitation, and immediate positive effects on the lungs and right heart.[34]
Intraoperative assessment via TEE of the repaired valve is crucial, as it can aid in the immediate valve assessment. If persistent mild or greater MR exists, the valve must be reexplored to be either re-repaired or replaced. This decision is critical in cases of impaired LV function, as these patients may not tolerate a long repeat ischemic/arrest period.
The long-term durability of a mitral valve repair is more difficult to predict. Flameng et al reported that only 50% of patients remain free from mitral valve incompetence 7 years following repair.[35] Results from a study by Chikwe et al demonstrated that mitral valve repair did not confer a long-term survival benefit in patients over 60 who required concomitant CABG surgery.[36] Fifty-nine octogenarians undergoing mitral valve surgery for nonrheumatic disease demonstrated similar outcomes between repair and replacement.[37] A national Society of Thoracic Surgeons (STS) database analysis included 8523 mitral valve repairs and 3520 replacements, concluding that there was operative mortality reduction in the repair cohort compared to replacement with and without chordal preservation.[38]
Following successful mitral valve repair, patients with pulmonary hypertension and atrial fibrillation have worse long-term survival and event-free survival, as well as increased compromise of the repair.[39] Based on findings by the Mayo Clinic group, postoperative atrial fibrillation occurs in up to 24% of patients previously in sinus rhythm, particularly those with LA enlargement, and is associated with increased mortality.[40] There has been a recent trend to perform surgical ablation of atrial fibrillation during mitral valve repair to aid with this complication. Up to 32.2% of patients presenting for repair have atrial fibrillation, with concomitant atrial fibrillation ablation occurring in 61.5% of patients, according to the STS database.[41] Gillinov et al reported that adding atrial fibrillation ablation to mitral valve surgery increased the freedom from atrial fibrillation at 1 year (63.2% vs. 39.4%) with similar mortality in both groups. Still, pacemaker implantation increased in the ablation group.[42]
Systolic anterior motion (SAM) of the mitral valve can result if there is a discrepancy between the annular area and leaflet tissue following repair. Systolic anterior motion results from anterior MVP into the LVOT during systole and most commonly occurs with an undersized annuloplasty ring or excess leaflet tissue.[43] SAM can lead to residual MR and LVOT obstruction, both observable on intraoperative TEE. If SAM is seen intraoperatively following the repair, the ventricular filling should immediately be optimized, AV pacing should be implemented to improve AV synchrony, and ventricular hypercontractility should be lessened. Postoperative beta blockers are helpful in this regard. David and colleagues report that freedom from MR ranges from 65% to 80% at 12 years postoperatively, depending on which mitral valve leaflet suffered prolapse.[44] Jouan et al describe a 9.8% recurrence rate of moderate or severe MR in patients with Barlow disease.[45]
Iatrogenic injury to the circumflex artery following mitral valve surgery can occur due to the artery's proximity to the anterolateral commissure of the mitral annulus, particularly in the mid-to-distal segment. This risk is heightened in cases of Barlow disease, where annular dilatation and hypermobility of the valvular leaflets make it challenging to predict the precise location of the circumflex artery, increasing the potential for coronary artery injury. The limited data available on the incidence of this complication, reported to be between 0.3% and 1.8%, complicates the development of effective strategies for its early detection and management. The likelihood of iatrogenic MI due to arterial injury is slightly higher following mitral valve repair (2.2%) compared to isolated mitral valve replacement (1.7%), with no significant decrease in incidence during minimally invasive mitral valve repair.[18]
While the urgent restoration of coronary blood flow is crucial, no universally established treatment protocol exists. The choice between percutaneous coronary intervention (PCI) and surgical myocardial revascularization typically depends on the specific situation. If the left circumflex artery injury is identified during surgery, immediate surgical myocardial revascularization is generally advised. However, PCI is often preferred if the injury is detected postoperatively due to its quicker execution and the advantage of avoiding a repeat sternotomy. Nonetheless, surgical intervention should be considered if technical challenges arise during PCI, such as difficulty navigating the lesion, incomplete balloon expansion, or failure to restore blood flow.[46][47]
AV groove disruption is a severe complication that can occur due to surgical techniques or stretch injury resulting from removing the posterior leaflet and the subsequent untethering of the LV. This complication occurs in approximately 1.2% of mitral valve replacements and carries a mortality rate of up to 75%. Repairing this disruption is challenging, with both external and internal repair approaches documented. Although internal approaches are generally preferred, achieving reliable exposure for the repair can be difficult.[48] Age is a recognized risk factor for AV groove rupture due to the higher prevalence of annular calcification and fragile tissues in older patients. Preserving the posterior leaflet and basal chordae can reduce the risk of AV groove rupture, though this is not always feasible. While certain types of AV dissociation might be managed through atriotomy, complete perforation and rupture present significant risks if not adequately exposed. This challenge arises in part from the complexity of the surgical approach and the proximity of the circumflex artery.[49]
Clinical Significance
With advanced age, degenerative mitral valve disease is the most common cause of MR. Mitral valve repair is the gold standard for degenerative MR and is the recommendation of the current guidelines for managing valvular heart disease. American and European guidelines set the same criteria for defining LV dysfunction in patients with primary MR, using a threshold of LVEF less than 60% or LV end-systolic diameter greater than 40 mm. There is strong consensus between the guidelines regarding mitral valve surgery for symptomatic patients with severe primary MR, with both recommending it as a class I intervention, regardless of LV function. Surgery is also advised in both sets of guidelines (class I) for asymptomatic patients who exhibit LV dysfunction as defined by these criteria. Additionally, both guidelines prefer mitral valve repair over replacement.[50] The ACC/AHA guidelines recommend that surgery take place before LV dysfunction (class IIa) in experienced centers. MR imposes significant volume overload on the LV, leading to permanent structural and functional deterioration of the myocardium and subsequent heart failure. Timely correction of MR is paramount to the preservation of cardiac function. MIMVS and transcatheter mitral valve repair technologies have been instrumental in allowing the repair of pathology in high-risk patients who are not appropriate for open-heart surgery.
Enhancing Healthcare Team Outcomes
Mitral regurgitation is the second most common valvular heart disease after aortic stenosis in the general population and requires an interprofessional healthcare team for management. In industrialized countries, the most common etiology is degenerative mitral valve disease, leading to prolapse caused by either myxomatous degeneration or fibroelastic deficiency. Mitral valve repair is preferred over replacement if complete and durable repair can be achieved. While the natural history of this valvular pathology is poor, leading to eventual LV decompensation, an appropriate and timely repair can be lifesaving and prolong life expectancy to that of the healthy age-matched population. Mortality is increased in patients with symptoms of heart failure with reduced EF. Transcatheter techniques may be challenging in patients with certain anatomical limitations, including calcified leaflets and advanced disease. Repair is not always practical, and patient selection is imperative to prevent the recurrence of MR.
The success of repair depends partially on the center and the surgeon’s level of experience. Intraoperative collaboration with cardiac anesthesia and the use of TEE is critical. Current guidelines propose that these procedures should occur at “Heart Valve Centers of Excellence” (HVCE), which offer comprehensive options for diagnosing and managing valvular disease.[51] An HVCE should deliver superior quality of care due to a larger volume of repair procedures, advanced imaging techniques, and greater transparency regarding patient outcomes.[52] An annual surgical volume of fewer than 25 cases per year correlates with a lower repair rate, increased 1-year mortality, and a higher incidence of subsequent reoperation.[53]
An interprofessional team dedicated to these patients should include cardiologists, anesthesiologists, nurse anesthetists, and intensivists. Cardiology specialty nursing should be available to assist at every step, both perioperatively and during the procedures, working in collaboration with the clinicians and specialists to provide monitoring and patient and family education. Perioperative evaluation should consist of high-quality TEE with 3-dimensional technology. Goals for repair should include below 1% mortality for isolated repair, a near 100% repair rate, and less than 5% repair failure at a 5-year follow-up. Centers should be involved in research and innovation of techniques for procedural improvement. A successful HVCE should adhere to international guidelines, engage in the appropriate and timely referral of patients, evaluate and enhance patient outcomes, and participate in regional or national outcome registries. With an interprofessional team approach, including specialists, clinicians, nurses, and pharmacists, patient results can improve, and adverse events can be kept to a minimum, resulting in a better quality of life and better outcomes.
Nursing, Allied Health, and Interprofessional Team Interventions
The nurse is crucial to the care of the postoperative patient. Their duties include:
- Educating the patient on the course of the illness
- Encouraging incentive spirometry
- Helping the patient to ambulate
- Offering and encouraging a healthy diet
- Ensuring medication compliance
- Monitoring vital signs and neurological exam
- Monitoring fluid balance
- Checking daily labs and radiographs
- Monitoring output from the mediastinal tubes
- Wound care