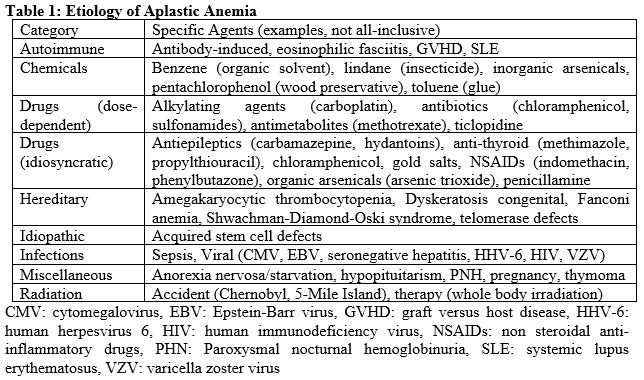
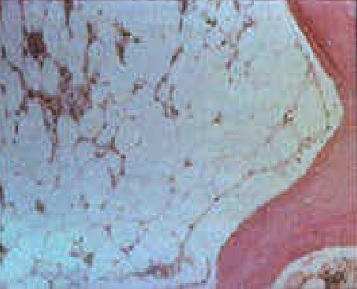
[1]
Ding SX, Chen T, Wang T, Liu CY, Lu WL, Fu R. The Risk of Clonal Evolution of Granulocyte Colony-Stimulating Factor for Acquired Aplastic Anemia: A Systematic Review and Meta-Analysis. Acta haematologica. 2018:140(3):141-145. doi: 10.1159/000491816. Epub 2018 Sep 25
[PubMed PMID: 30253387]
Level 1 (high-level) evidence
[2]
Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood advances. 2018 Aug 14:2(15):2020-2028. doi: 10.1182/bloodadvances.2018021162. Epub
[PubMed PMID: 30108110]
Level 3 (low-level) evidence
[3]
Shallis RM, Ahmad R, Zeidan AM. Aplastic anemia: Etiology, molecular pathogenesis, and emerging concepts. European journal of haematology. 2018 Dec:101(6):711-720. doi: 10.1111/ejh.13153. Epub 2018 Oct 10
[PubMed PMID: 30055055]
[4]
Gadalla SM, Aubert G, Wang T, Haagenson M, Spellman SR, Wang L, Katki HA, Savage SA, Lee SJ. Donor telomere length and causes of death after unrelated hematopoietic cell transplantation in patients with marrow failure. Blood. 2018 May 24:131(21):2393-2398. doi: 10.1182/blood-2017-10-812735. Epub 2018 Apr 9
[PubMed PMID: 29632022]
[5]
Li SS, Hsu YT, Chang C, Lee SC, Yen CC, Cheng CN, Chen JS, Lin SH, Chang KC, Chen TY. Incidence and treatment outcome of aplastic anemia in Taiwan-real-world data from single-institute experience and a nationwide population-based database. Annals of hematology. 2019 Jan:98(1):29-39. doi: 10.1007/s00277-018-3486-3. Epub 2018 Sep 3
[PubMed PMID: 30178191]
[6]
Vaht K, Göransson M, Carlson K, Isaksson C, Lenhoff S, Sandstedt A, Uggla B, Winiarski J, Ljungman P, Brune M, Andersson PO. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000-2011. Haematologica. 2017 Oct:102(10):1683-1690. doi: 10.3324/haematol.2017.169862. Epub 2017 Jul 27
[PubMed PMID: 28751565]
[7]
Schoettler ML, Nathan DG. The Pathophysiology of Acquired Aplastic Anemia: Current Concepts Revisited. Hematology/oncology clinics of North America. 2018 Aug:32(4):581-594. doi: 10.1016/j.hoc.2018.03.001. Epub 2018 May 8
[PubMed PMID: 30047412]
[8]
Yamazaki H. [Acquired aplastic anemia: recent advances in pathophysiology and treatment]. [Rinsho ketsueki] The Japanese journal of clinical hematology. 2018:59(6):711-715. doi: 10.11406/rinketsu.59.711. Epub
[PubMed PMID: 29973449]
Level 3 (low-level) evidence
[9]
Gnanaraj J, Parnes A, Francis CW, Go RS, Takemoto CM, Hashmi SK. Approach to pancytopenia: Diagnostic algorithm for clinical hematologists. Blood reviews. 2018 Sep:32(5):361-367. doi: 10.1016/j.blre.2018.03.001. Epub 2018 Mar 5
[PubMed PMID: 29555368]
[10]
Bluteau O, Sebert M, Leblanc T, Peffault de Latour R, Quentin S, Lainey E, Hernandez L, Dalle JH, Sicre de Fontbrune F, Lengline E, Itzykson R, Clappier E, Boissel N, Vasquez N, Da Costa M, Masliah-Planchon J, Cuccuini W, Raimbault A, De Jaegere L, Adès L, Fenaux P, Maury S, Schmitt C, Muller M, Domenech C, Blin N, Bruno B, Pellier I, Hunault M, Blanche S, Petit A, Leverger G, Michel G, Bertrand Y, Baruchel A, Socié G, Soulier J. A landscape of germ line mutations in a cohort of inherited bone marrow failure patients. Blood. 2018 Feb 15:131(7):717-732. doi: 10.1182/blood-2017-09-806489. Epub 2017 Nov 16
[PubMed PMID: 29146883]
[11]
Yoshida N, Kojima S. Updated Guidelines for the Treatment of Acquired Aplastic Anemia in Children. Current oncology reports. 2018 Jun 30:20(9):67. doi: 10.1007/s11912-018-0716-8. Epub 2018 Jun 30
[PubMed PMID: 29961134]
[12]
Samarasinghe S, Veys P, Vora A, Wynn R. Paediatric amendment to adult BSH Guidelines for aplastic anaemia. British journal of haematology. 2018 Jan:180(2):201-205. doi: 10.1111/bjh.15066. Epub 2017 Dec 28
[PubMed PMID: 29285764]
[13]
Zakaria Z, Kaliaperumal C, Crimmins D, Caird J. Neurosurgical management in children with bleeding diathesis: auditing neurological outcome. Journal of neurosurgery. Pediatrics. 2018 Jan:21(1):38-43. doi: 10.3171/2017.6.PEDS16574. Epub 2017 Nov 10
[PubMed PMID: 29125443]
[14]
Dietz AC, Mehta PA, Vlachos A, Savage SA, Bresters D, Tolar J, Boulad F, Dalle JH, Bonfim C, de la Fuente J, Duncan CN, Baker KS, Pulsipher MA, Lipton JM, Wagner JE, Alter BP. Current Knowledge and Priorities for Future Research in Late Effects after Hematopoietic Cell Transplantation for Inherited Bone Marrow Failure Syndromes: Consensus Statement from the Second Pediatric Blood and Marrow Transplant Consortium International Conference on Late Effects after Pediatric Hematopoietic Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2017 May:23(5):726-735. doi: 10.1016/j.bbmt.2017.01.075. Epub 2017 Jan 20
[PubMed PMID: 28115275]
Level 3 (low-level) evidence
[15]
Rice C, Eikema DJ, Marsh JCW, Knol C, Hebert K, Putter H, Peterson E, Deeg HJ, Halkes S, Pidala J, Anderlini P, Tischer J, Kroger N, McDonald A, Antin JH, Schaap NP, Hallek M, Einsele H, Mathews V, Kapoor N, Boelens JJ, Mufti GJ, Potter V, Pefault de la Tour R, Eapen M, Dufour C. Allogeneic Hematopoietic Cell Transplantation in Patients Aged 50Years or Older with Severe Aplastic Anemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2019 Mar:25(3):488-495. doi: 10.1016/j.bbmt.2018.08.029. Epub 2018 Sep 5
[PubMed PMID: 30194027]
[16]
Scheinberg P. Recent Advances and Long-Term Results of Medical Treatment of Acquired Aplastic Anemia: Are Patients Cured? Hematology/oncology clinics of North America. 2018 Aug:32(4):609-618. doi: 10.1016/j.hoc.2018.03.003. Epub 2018 May 18
[PubMed PMID: 30047414]
Level 3 (low-level) evidence