Continuing Education Activity
Tubo-ovarian abscess (TOA) is a severe complication of pelvic inflammatory disease (PID) that predominantly affects sexually active women of reproductive age, with a significant proportion being nulliparous. This condition is characterized by an inflammatory mass in the fallopian tubes and ovaries, often filled with pus, typically resulting from an ascending infection of the upper genital tract. Common symptoms of TOA include fever, abdominal pain, adnexal mass, and foul-smelling vaginal discharge, although the presentation can vary widely. TOAs pose significant risks, including infertility, chronic pelvic pain, and ectopic pregnancy, with rupture potentially leading to life-threatening sepsis.
Diagnosis is supported by imaging, such as ultrasound, computed tomography, or magnetic resonance imaging, which often reveals fluid-filled, inflamed tubes or abscesses. Initial treatment typically involves broad-spectrum antibiotics, which are effective in many cases. However, larger abscesses or cases unresponsive to antibiotics within 72 hours may require surgical intervention or image-guided drainage. Laboratory tests and imaging help differentiate TOA from other conditions. The decision to use antibiotics, drainage, or surgery to reduce the need for radical surgery depends on factors such as infection severity and the patient’s overall health. Despite these treatment improvements, delayed diagnosis or inadequate management can still lead to serious long-term complications. This activity reviews the etiology, diagnosis, evaluation, and management of TOAs, highlighting the importance of early recognition and timely intervention. This activity also provides healthcare professionals with the knowledge and skills needed to perform accurate evaluations and implement effective interprofessional management strategies to improve patient outcomes.
Objectives:
Identify the clinical signs and symptoms of tubo-ovarian abscesses, including fever, abdominal pain, adnexal mass, and abnormal discharge, in sexually active women of reproductive age.
Implement evidence-based guidelines for the management of tubo-ovarian abscesses, including the use of broad-spectrum antibiotics and appropriate surgical interventions when necessary.
Apply appropriate diagnostic imaging techniques, such as ultrasound, CT, and MRI, to confirm the presence of tubo-ovarian abscess and evaluate its size and complexity.
Collaborate with interprofessional healthcare providers, such as gynecologists, radiologists, and other specialists, to ensure timely and accurate diagnosis and comprehensive management of tubo-ovarian abscesses.
Introduction
Tubo-ovarian abscess (TOA) is a severe complication of pelvic inflammatory disease (PID) that primarily affects sexually active women of reproductive age, although it may occur without other PID sequelae. This condition is characterized by adnexal inflammation, typically involving pus-filled fallopian tubes and ovaries, caused by an ascending infection from the upper genital tract. TOA can lead to systemic infections and impact nearby organs, such as the bowel or bladder, with mortality rates reaching up to 12% before the advent of antibiotic treatment.[1] Common symptoms include fever, abdominal pain, and foul-smelling vaginal discharge, with diagnosis supported by imaging showing fluid-filled, inflamed tubes or abscesses. However, the clinical presentation can vary widely.
TOA presents serious risks, including infertility, chronic pelvic pain, and a heightened risk of ectopic pregnancy. A ruptured abscess can result in life-threatening sepsis, underscoring the need for prompt evaluation and treatment at the first clinical suspicion.[2][3][4] Initial management typically involves broad-spectrum antibiotics, which are effective in many cases. However, larger abscesses or those unresponsive to antibiotics within 72 hours may require surgical intervention or image-guided drainage.[5] Surgical approaches vary and include laparoscopy or laparotomy, with considerations based on factors such as abscess size, patient age, fertility considerations, and surgical history.
Image-guided drainage, such as transvaginal aspiration, is a minimally invasive treatment option with high success rates, especially for smaller abscesses.[6] However, larger or more complex abscesses may require catheter drainage or surgical intervention. The decision between antibiotics, drainage, and surgery depends on factors such as the severity of the infection and the patient’s overall health.[7] Despite advancements in treatment, delayed diagnosis or improper management can lead to long-term complications, including the need for more radical surgical procedures such as salpingo-oophorectomy or, in severe cases, hysterectomy.[8]
Etiology
TOA often results from untreated PID, which typically begins with a lower genital tract infection that ascends into the fallopian tubes and ovaries. As a result, infectious pathogens are the most common cause of TOAs. However, the specific organisms identified in TOA differ from the typical pathogens associated with PID. In premenopausal women, sexually transmitted pathogens such as Chlamydia trachomatis and Neisseria gonorrhoeae are commonly reported.[9] However, Escherichia coli, Bacteroides fragilis, and Peptostreptococcus species are more frequently found in TOA, especially in postmenopausal women.[9][10]
TOA infections are generally polymicrobial, involving a combination of enteric, respiratory, and anaerobic bacteria, with less frequent involvement of traditional sexually transmitted pathogens. Emerging pathogens such as Mycoplasma genitalium have been identified as contributors to PID, but standard antibiotic regimens may not fully target this organism. Rarely, organisms such as Mycobacterium and Actinomyces can also lead to TOA in specific cases.[1][11] Additionally, TOAs can also result from the spread of infection from an adjacent organ, most commonly the appendix. Less frequently, they may arise from hematogenous dissemination from a distant infection site or be associated with pelvic organ malignancies.[12][13]
Tubo-Ovarian Abscess Risk Factors
The primary risk factors for PID, and consequently TOA, include:
- Age 25 and younger
- Multiple or new sexual partners
- Placement or removal of an intrauterine contraceptive device
- Endometrial biopsy
- In vitro fertilization
- Unprotected intercourse
- Sexual activity beginning before age 15
- History of sexually transmitted infections (STIs), such as C trachomatis and N gonorrhoeae [6][14]
Epidemiology
The prevalence of PID in females in the United States is estimated to exceed 2 million cases.[15] PID occurs most frequently among non-Hispanic Black women residing in Southern states.[15] Sexually transmitted and commensal vaginal pathogens are implicated in over 85% of PID cases, while respiratory and intestinal colonizing organisms account for the remaining incidences of PID. Notably, TOAs are predominantly polymicrobial in nature.[1]
Approximately 15% to 35% of patients hospitalized for PID develop TOAs.[14][8] TOAs are less prevalent in postmenopausal women, who account for 6% to 18% of cases.[11] Mortality rates associated with TOAs have significantly declined with the advent of effective antibiotic regimens, now estimated at approximately 1 in 740 cases.[1]
Pathophysiology
Organisms from the lower genital tract typically ascend following inflammatory epithelial damage, forming an inflammatory mass that involves the fallopian tube, ovary, and sometimes adjacent pelvic organs. Infections, including tubercular organisms, can also reach the upper genital tract via lymphatic or hematogenous pathways.[1][6] Please refer to the Epidemiology section for more information.
History and Physical
Due to the shared underlying etiologies of TOA and PID, and the fact that PID often leads to TOAs, the recommended diagnostic evaluation is similar, with additional procedures used in patients suspected of having TOAs or presenting with nonspecific or atypical findings. However, diagnosing both TOA and PID can be challenging due to the wide variability in clinical presentations. Many women with PID exhibit subtle or nonspecific symptoms, making early detection difficult.[6] Delayed diagnosis can result in severe complications, including infertility, ectopic pregnancy, and chronic pelvic pain.[5][13]
Clinical History
The clinical diagnosis of PID is primarily based on symptom assessment. A classical presentation of a TOA includes abdominal pain, a pelvic mass on examination, fever, and leukocytosis. Studies suggest that the positive predictive value of a clinical diagnosis of PID ranges from 65% to 90% when compared to laparoscopy.[5][13] The accuracy of this diagnosis is influenced by epidemiological factors, such as the prevalence of STIs in specific populations, particularly sexually active young women, adolescents, and high-risk communities. Due to the limitations of clinical diagnosis, it is essential for clinicians to consider risk factors such as age, sexual activity, and a history of STIs. In populations with a high prevalence of STIs, the positive predictive value of a clinical PID diagnosis is higher.[5]
Common symptoms of PID include lower abdominal pain, abnormal bleeding, dyspareunia, and vaginal discharge. In cases of TOA and severe PID, patients may also experience fever, bilateral pelvic pain (worse on one side), and right upper quadrant pain.[6] However, some cases go undiagnosed due to the mildness of symptoms or because both clinicians and patients may not recognize these symptoms as indicative of a severe condition.[5] Due to the potential for reproductive harm, a low threshold for the clinical diagnosis of PID is recommended, particularly in sexually active women.[10] Empiric treatment for PID is often initiated when a patient presents with pelvic or lower abdominal pain, other potential causes have been excluded, and one or more of the following clinical signs are evident—cervical motion tenderness, uterine tenderness, or adnexal tenderness.[5]
Clinical Examination
A comprehensive physical examination, including a detailed pelvic exam, is essential. In patients with clear signs of lower genitourinary infection, the pelvic exam typically reveals mucopurulent discharge, pelvic discomfort, and tenderness during cervical motion on bimanual examination. Additionally, patients may report symptoms such as fever, abnormal uterine bleeding, urinary complaints, or pain during intercourse. Pelvic tenderness, particularly when worsened on one side during palpation, along with adnexal mass and rectal discomfort, may suggest a TOA in a patient with suspected PID. Abdominal rigidity and signs of sepsis could indicate a ruptured TOA.[6][5][16]
When complications are suspected, laboratory tests or imaging studies can help guide treatment and management decisions, especially when assessing for a TOA. A bimanual exam is essential for detecting cervical, uterine, or adnexal tenderness, as well as masses or abscesses. A speculum exam can also confirm mucopurulent cervical discharge and cervical friability.[10][6][5] Therefore, the diagnosis of PID and TOA is primarily clinical, with additional tests reserved for complicated cases or when the diagnosis remains uncertain. A high index of suspicion and prompt initiation of treatment are critical to preventing long-term complications.[5][10]
Evaluation
Laboratory Studies
Urine pregnancy tests and urinalysis are essential to help rule out differential diagnoses.[10][6] Additional laboratory studies that should be performed to help assess for infectious etiologies and sepsis include a complete blood count, STI testing, blood cultures, and a wet prep of vaginal secretions. In cases where malignancy is suspected based on clinical features, tumor markers like cancer antigen 125 and alpha-fetoprotein should be considered.[7] Laboratory findings such as elevated C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), or positive results for gonorrhea and chlamydia can support a diagnosis of TOA.[5][10]
While no single clinical or laboratory finding is definitive for diagnosing PID, combining multiple diagnostic criteria can improve either sensitivity or specificity.[5] To improve the specificity of a PID diagnosis, the Centers for Disease Control and Prevention (CDC) recommends considering the following additional supportive findings:
- Oral temperature above 101 °F (38.3 °C)
- Mucopurulent cervical discharge or cervical friability on examination
- Large numbers of white blood cells evident on saline microscopy of vaginal fluid
- Increased serum ESR
- Elevated serum CRP
- Laboratory confirmation of cervical infection with N gonorrhoeae or C trachomatis [5]
Diagnostic Imaging Studies
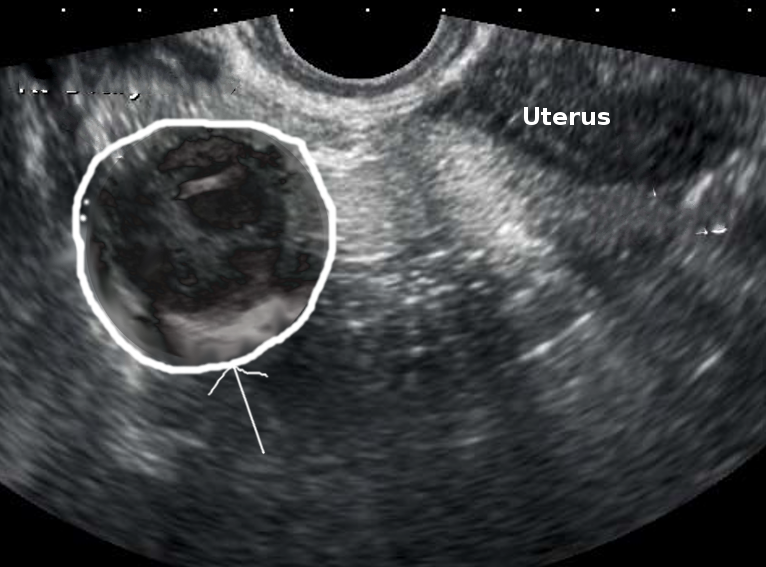
Diagnostic imaging studies, such as ultrasonography or computed tomography (CT), may be used when a TOA is suspected. Transabdominal and transvaginal ultrasonography are preferred as the initial imaging modalities to evaluate pelvic pain in reproductive-aged women (see Image. Right Tubo-Ovarian Abscess).[17] Ultrasonographic findings of a TOA may vary but typically include the loss of normal anatomical boundaries secondary to severe inflammatory changes, a heterogeneous complex, a fluid-filled irregular mass with septations, and loculated free fluid in the cul-de-sac.[17] However, several common inflammatory conditions, such as appendicitis and infectious enterocolitis, may produce ultrasonographic findings similar to those of a TOA, necessitating additional studies for confirmation.[18][16] Thickened, distended fallopian tubes are also more suggestive of a TOA.
If the initial ultrasonographic examination is inconclusive, CT is commonly used to further evaluate a suspected TOA.[16] A CT scan of a TOA may reveal a solid-cystic adnexal mass with thickened, irregularly enhancing walls and complex septated internal fluid. The mesosalpinx surrounding the TOA is often thickened, and the fallopian tube may appear dilated. Additional findings may include peritoneal free fluid, an enlarged and edematous ovary, periovarian fat stranding, and signs of reactive inflammation such as hydronephrosis, bowel wall thickening, and ileus.[19][16]
Magnetic resonance imaging (MRI) is recommended when ultrasonographic and CT findings require further differentiation.[17] MRI is also preferred when additional imaging is needed, as tubal changes associated with TOA are more clearly visualized using various MRI techniques. These include septal and thick-rim mucosal enhancement with intravenous (IV) gadolinium and restricted diffusion due to purulent tubal content on diffusion-weighted imaging.[19]
Treatment / Management
Historically, TOAs were managed with a total abdominal hysterectomy and bilateral salpingo-oophorectomy.[20] However, treatment has evolved significantly with the development of broad-spectrum antibiotics and advancements in imaging and drainage techniques. Studies utilizing these management strategies report success rates of 70% or higher. Daily monitoring of leukocytosis with complete blood counts is recommended to assess treatment response.[21][22][23]
Inpatient management and gynecological consultation are essential for any woman with a TOA. IV antibiotics are recommended as first-line therapy for unruptured TOAs, achieving effectiveness in 70% to 87% of cases.[8] Image-guide drainage or surgical intervention may be necessary based on clinical factors or the response to antibiotics.[7] Image-guided aspiration and laparoscopy can provide both diagnostic confirmation and treatment simultaneously. Laparoscopy remains the gold standard for confirming PID. While laparoscopy is a reliable tool for identifying salpingitis and offering a more accurate bacteriological diagnosis, its use is less frequent than more conservative methods due to its invasiveness and limited availability in some regions.[10][6]
Antibiotic Treatment Regimens for Tubo-Ovarian Abscess
Treatment of TOA typically involves IV antibiotics, which have demonstrated efficacy in randomized trials. Clinical improvement is usually expected within 24 to 48 hours, after which a transition to oral therapy may be considered. Inpatient monitoring for over 24 hours is recommended for patients with TOA.[5] The CDC recommends the following parenteral regimens:
- Ceftriaxone (1 g IV every 24 h) + doxycycline (100 mg orally/IV every 12 h) + metronidazole (500 mg orally/IV every 12 h)
- Cefotetan (2 g IV every 12 h) + doxycycline (100 mg orally/IV every 12 h)
- Cefoxitin (2 g IV every 6 h) + doxycycline (100 mg orally/IV every 12 h) [5]
Doxycycline should be administered orally whenever possible to avoid the discomfort associated with IV administration. Since oral and IV forms of doxycycline and metronidazole exhibit similar absorption, oral therapy is generally preferred for stable patients. Upon clinical improvement, treatment is transitioned to oral doxycycline and metronidazole, with a total therapy duration of at least 14 days.[5]
The CDC recommends the following alternative parenteral regimens, supported by limited data, for use when the preferred antibiotic regimen is contraindicated, such as in cases of patient allergy to specific components:
- Ampicillin-sulbactam (3 g IV every 6 h) + doxycycline (100 mg orally/IV every 12 h)
- Clindamycin (900 mg IV every 8 h) + gentamicin (2 mg/kg body weight as loading dose IV or intramuscular (IM), followed by 1.5 mg/kg every 8 h or daily dosing at 3-5 mg/kg) [5]
If patients show clinical improvement within 24 to 48 hours, a transition to oral antibiotics is appropriate to complete at least 14 days of treatment. Oral regimens should include clindamycin or metronidazole alongside doxycycline to ensure adequate anaerobic coverage. Percutaneous drainage or surgical intervention should be considered if no improvement is observed within 72 hours.[24][8][13] In patients with a worsening clinical condition suggestive of TOA rupture, such as increasing pain or signs of sepsis, laparoscopy should be promptly considered.[5][16]
Procedural Therapeutic Approaches for Tubo-Ovarian Abscess
TOA management typically begins with antimicrobial therapy, reserving invasive procedures for suspected rupture or cases unresponsive to antibiotics after 72 hours.[20] Approximately 25% to 30% of patients fail to respond adequately to antibiotics, necessitating image-guided drainage or surgical intervention.[13] Studies have shown that TOAs measuring greater than or equal to 5.5 cm are more likely to require invasive therapy compared to those less than or equal to 5 cm.[8][25] The incidence of antibiotic treatment failure is also higher in older women and those with elevated white blood cell counts (>16,000/µL), elevated CRP and ESR levels, and temperatures more than 38 °C.[13][8]
Therapeutic procedures for draining TOAs more than 3 cm may include percutaneous catheter drainage or surgical approaches.[26] While surgery was historically the recommended treatment approach, recent evidence suggests that image-guided drainage is equally effective.[13] Neither procedure is currently favored over the other, so the choice should depend on clinician preference and clinical factors, such as reproductive status.[25][13][7]
Image-guided percutaneous catheter drainage: When selecting percutaneous catheter drainage for a TOA, the route and technique depend on the clinician's preference and clinical factors, such as the patient's body habitus, as well as the size and location of the TOA. Common routes for percutaneous catheter drainage include transabdominal, transgluteal, transrectal, transvaginal, transperineal, and transvesicular, with studies showing effectiveness for each. Transabdominal and transgluteal routes are generally recommended due to their sterility compared to other methods.[26] Please see StatPearls' companion resource, "Percutaneous Abscess Drainage," for more information.
Surgical intervention: A ruptured TOA or deterioration of a patient's condition suggestive of sepsis requires emergent surgical treatment for washout and evaluation of the peritoneal cavity. Both laparoscopic and laparotomy approaches may be used, though laparotomy is more commonly preferred due to the extensive adhesions and anatomical distortion often associated with TOAs. Laparoscopy is preferred if a surgeon with advanced minimally invasive surgical skills is available.[25][27][8][26] Surgical management is also recommended for postmenopausal women due to the increased risk of an occult malignancy.[7] Copious irrigation, microbial cultures, and excision of the abscess cavity should be performed. For patients who have completed childbearing, a total abdominal hysterectomy with bilateral salpingo-oophorectomy may be considered, while salpingo-oophorectomy is preferred for those desiring fertility conservation.[8] A closed suction drain should be placed to monitor postsurgical drainage.[26]
Other Treatment Considerations
Women should refrain from sexual activity until treatment is completed, symptoms have resolved, and partners are treated. Testing for STIs, including gonorrhea, chlamydia, HIV, and syphilis, is recommended, though the value of testing for M genitalium remains uncertain. Retesting for gonorrhea and chlamydia is advised 3 months posttreatment or at the next medical visit.[5]
Differential Diagnosis
The differential diagnosis for TOA often includes:
- Renal stone
- Appendicitis
- Cholecystitis
- Inguinal hernia
- Obturator hernia
- Bowel obstruction
- Diverticulitis
- Inflammatory bowel disease
- Pelvic inflammatory disease
- Ovarian torsion
- Ectopic pregnancy
- Ruptured ovarian cyst
- Pyelonephritis
- Cystitis [7]
Prognosis
The prognosis for TOA is generally favorable with prompt and appropriate treatment. Most patients experience clinical improvement within 24 to 48 hours of receiving parenteral antibiotics, followed by oral therapy.[5] Approximately 70% of patients achieve resolution of the TOA with antibiotic treatment alone.[13]
Reproductive implications of TOAs, such as ectopic pregnancy and infertility, can be significant. One study found that only 7.5% of patients with a TOA reported subsequent pregnancy.[5][1] However, early recognition and timely intervention, including more invasive treatments, can improve fertility outcomes. Pregnancy rates range from 32% to 63% in patients treated with antibiotics and laparoscopic drainage.[25]
Complications
Complications of TOA include:
- Chronic pelvic pain
- Sepsis
- Distortion of the pelvic anatomy
- Increased risk of ectopic pregnancy
- Infertility
- Recurrent PID
- Peritoneal adhesions [25][5]
Complications associated with TOA treatment include:
- Septic shock
- Bacteremia
- Allergic reaction
- Bowel injury
- Hemorrhage [28]
Enhancing Healthcare Team Outcomes
Effective management of a TOA requires an interprofessional healthcare team approach due to its complex presentation, which can be confused with conditions such as appendicitis, ureteral stones, cystitis, or obturator hernia. Delayed treatment can result in severe morbidity, making timely diagnosis and intervention crucial. Physicians, advanced practitioners, nurses, pharmacists, and other healthcare professionals must collaborate closely to improve patient-centered care, safety, and outcomes.
In many cases, patients with TOA first present to the emergency department, making the triage nurse's role crucial. Nurses must quickly recognize symptoms of TOA, ensure prompt admission, and notify the physician or advanced practitioner immediately. A gynecologist should be consulted when TOA is suspected to ensure appropriate management. Radiologists and interventional radiologists are critical in confirming the diagnosis and performing therapeutic drainage through imaging. Once diagnosed, swift coordination among all involved disciplines is essential to prevent complications and optimize patient outcomes.
Nurses are also instrumental in patient education, particularly regarding prevention. They should educate patients about risk factors such as unprotected sex and multiple sexual partners, encouraging safe sex practices and condom use to reduce the risk of conditions that may lead to TOA. Pharmacists and infectious disease specialists contribute by recommending an appropriate antibiotic regimen and promoting adherence to prescribed antibiotics, which is essential for the resolution of the abscess and for preventing long-term sequelae.
Effective communication among healthcare team members is crucial for improving patient outcomes. Clear and consistent communication ensures that all healthcare professionals are aligned in their approach, reducing the risk of complications such as infertility, pelvic thrombophlebitis, and chronic pelvic pain. In the event of a TOA rupture, which constitutes a surgical emergency, prompt and coordinated action is necessary to prevent life-threatening sepsis and death. By collaborating and staying informed, the interprofessional team can deliver comprehensive, patient-centered care, optimizing both short-term and long-term health outcomes for patients with TOA.