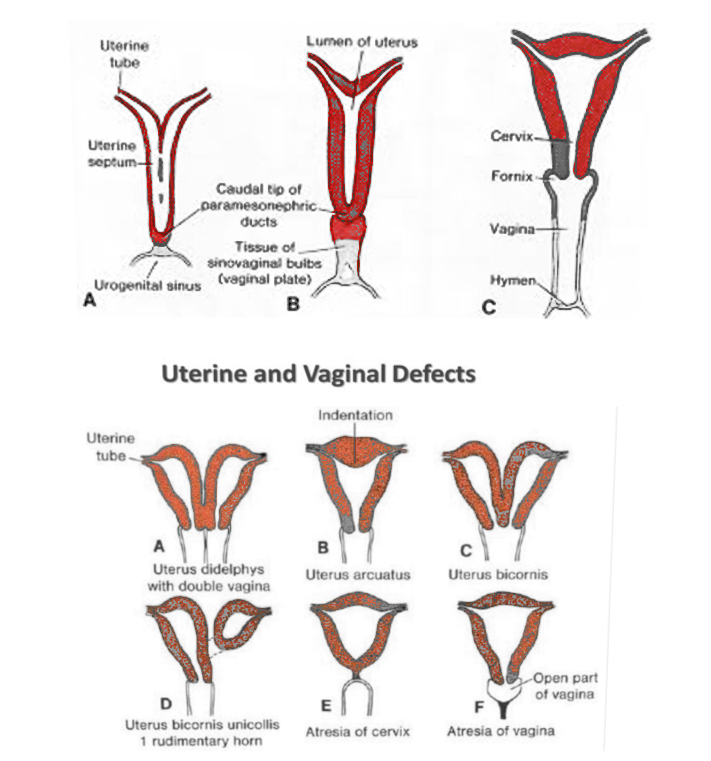
[1]
Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nature reviews. Genetics. 2003 Dec:4(12):969-80
[PubMed PMID: 14631357]
[2]
WITSCHI E. Embryology of the uterus: normal and experimental. Annals of the New York Academy of Sciences. 1959 Jan 9:75():412-35
[PubMed PMID: 13845458]
[3]
Robbins JB, Broadwell C, Chow LC, Parry JP, Sadowski EA. Müllerian duct anomalies: embryological development, classification, and MRI assessment. Journal of magnetic resonance imaging : JMRI. 2015 Jan:41(1):1-12. doi: 10.1002/jmri.24771. Epub 2014 Oct 7
[PubMed PMID: 25288098]
[4]
Guioli S, Sekido R, Lovell-Badge R. The origin of the Mullerian duct in chick and mouse. Developmental biology. 2007 Feb 15:302(2):389-98
[PubMed PMID: 17070514]
[5]
Warne GL, Kanumakala S. Molecular endocrinology of sex differentiation. Seminars in reproductive medicine. 2002 Aug:20(3):169-80
[PubMed PMID: 12428197]
[7]
Roly ZY, Backhouse B, Cutting A, Tan TY, Sinclair AH, Ayers KL, Major AT, Smith CA. The cell biology and molecular genetics of Müllerian duct development. Wiley interdisciplinary reviews. Developmental biology. 2018 May:7(3):e310. doi: 10.1002/wdev.310. Epub 2018 Jan 19
[PubMed PMID: 29350886]
[8]
de Ziegler D, Pirtea P, Galliano D, Cicinelli E, Meldrum D. Optimal uterine anatomy and physiology necessary for normal implantation and placentation. Fertility and sterility. 2016 Apr:105(4):844-54. doi: 10.1016/j.fertnstert.2016.02.023. Epub 2016 Feb 27
[PubMed PMID: 26926252]
[9]
Chandler TM, Machan LS, Cooperberg PL, Harris AC, Chang SD. Mullerian duct anomalies: from diagnosis to intervention. The British journal of radiology. 2009 Dec:82(984):1034-42. doi: 10.1259/bjr/99354802. Epub 2009 May 11
[PubMed PMID: 19433480]
[10]
Thomas DFM. The embryology of persistent cloaca and urogenital sinus malformations. Asian journal of andrology. 2020 Mar-Apr:22(2):124-128. doi: 10.4103/aja.aja_72_19. Epub
[PubMed PMID: 31322137]
[11]
Troiano RN, McCarthy SM. Mullerian duct anomalies: imaging and clinical issues. Radiology. 2004 Oct:233(1):19-34
[PubMed PMID: 15317956]
[12]
Morcel K, Camborieux L, Programme de Recherches sur les Aplasies Müllériennes, Guerrier D. Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome. Orphanet journal of rare diseases. 2007 Mar 14:2():13
[PubMed PMID: 17359527]
[13]
Sajjad Y. Development of the genital ducts and external genitalia in the early human embryo. The journal of obstetrics and gynaecology research. 2010 Oct:36(5):929-37. doi: 10.1111/j.1447-0756.2010.01272.x. Epub 2010 Sep 16
[PubMed PMID: 20846260]