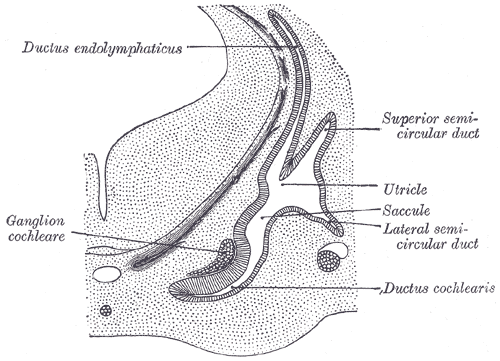
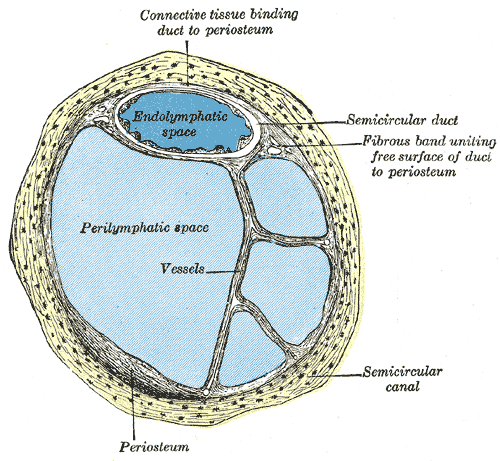
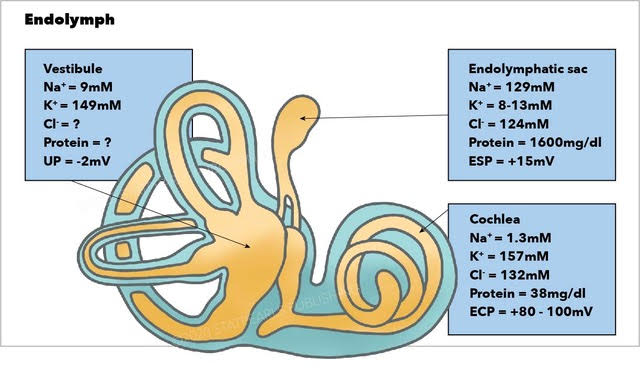
[1]
Couloigner V, Teixeira M, Sterkers O, Rask-Andersen H, Ferrary E. [The endolymphatic sac: its roles in the inner ear]. Medecine sciences : M/S. 2004 Mar:20(3):304-10
[PubMed PMID: 15067575]
[2]
Shibata T, Matsumoto S, Agishi T, Nagano T. Visualization of Reissner membrane and the spiral ganglion in human fetal cochlea by micro-computed tomography. American journal of otolaryngology. 2009 Mar-Apr:30(2):112-20. doi: 10.1016/j.amjoto.2008.07.012. Epub
[PubMed PMID: 19239953]
[3]
Köppl C, Wilms V, Russell IJ, Nothwang HG. Evolution of Endolymph Secretion and Endolymphatic Potential Generation in the Vertebrate Inner Ear. Brain, behavior and evolution. 2018:92(1-2):1-31. doi: 10.1159/000494050. Epub 2018 Nov 9
[PubMed PMID: 30415265]
[4]
Yu W, Zong S, Du P, Zhou P, Li H, Wang E, Xiao H. Role of the Stria Vascularis in the Pathogenesis of Sensorineural Hearing Loss: A Narrative Review. Frontiers in neuroscience. 2021:15():774585. doi: 10.3389/fnins.2021.774585. Epub 2021 Nov 19
[PubMed PMID: 34867173]
Level 3 (low-level) evidence
[5]
Coppens AG, Salmon I, Heizmann CW, Poncelet L. Dark-cell areas in the dog vestibular endorgans: an immunohistochemical study. Histology and histopathology. 2004 Oct:19(4):1227-35. doi: 10.14670/HH-19.1227. Epub
[PubMed PMID: 15375766]
[6]
Kurbel S, Borzan V, Golem H, Dinjar K. Cochlear potential difference between endolymph fluid and the hair cell's interior: a retold interpretation based on the Goldman equation. Medicinski glasnik : official publication of the Medical Association of Zenica-Doboj Canton, Bosnia and Herzegovina. 2017 Feb 1:14(1):8-15. doi: 10.17392/868-16. Epub
[PubMed PMID: 28165435]
[7]
Angelaki DE,Cullen KE, Vestibular system: the many facets of a multimodal sense. Annual review of neuroscience. 2008
[PubMed PMID: 18338968]
[8]
McPherson DR. Sensory Hair Cells: An Introduction to Structure and Physiology. Integrative and comparative biology. 2018 Aug 1:58(2):282-300. doi: 10.1093/icb/icy064. Epub
[PubMed PMID: 29917041]
Level 2 (mid-level) evidence
[9]
Kniep R, Zahn D, Wulfes J, Walther LE. The sense of balance in humans: Structural features of otoconia and their response to linear acceleration. PloS one. 2017:12(4):e0175769. doi: 10.1371/journal.pone.0175769. Epub 2017 Apr 13
[PubMed PMID: 28406968]
[10]
Iversen MM, Rabbitt RD. Wave Mechanics of the Vestibular Semicircular Canals. Biophysical journal. 2017 Sep 5:113(5):1133-1149. doi: 10.1016/j.bpj.2017.08.001. Epub
[PubMed PMID: 28877495]
[11]
Whitfield TT. Development of the inner ear. Current opinion in genetics & development. 2015 Jun:32():112-8. doi: 10.1016/j.gde.2015.02.006. Epub 2015 Mar 19
[PubMed PMID: 25796080]
Level 3 (low-level) evidence
[12]
Salgado-Lopez L, Leonel LCP, Aydin SO, Peris-Celda M. Surgical Anatomy of the Labyrinthine and Subarcuate Arteries and Clinical Implications. World neurosurgery. 2020 Sep:141():e880-e887. doi: 10.1016/j.wneu.2020.06.083. Epub 2020 Jun 19
[PubMed PMID: 32565373]
[13]
Sugita M, Masutani H, Moriguchi M, Matsunaga K, Nakai Y. Distribution of arteries from brain stem to inner ear around the internal auditory canal. Acta oto-laryngologica. Supplementum. 1991:486():45-52
[PubMed PMID: 1842877]
[14]
Hansen JM, Qvortrup K, Friis M. Vestibular tributaries to the vein of the vestibular aqueduct. Acta oto-laryngologica. 2011 Jan:131(1):9-13. doi: 10.3109/00016489.2010.511260. Epub 2010 Oct 19
[PubMed PMID: 20958133]
[15]
Tassinari M, Mandrioli D, Gaggioli N, Roberti di Sarsina P. Ménière's disease treatment: a patient-centered systematic review. Audiology & neuro-otology. 2015:20(3):153-65. doi: 10.1159/000375393. Epub 2015 Mar 31
[PubMed PMID: 25832807]
Level 1 (high-level) evidence
[16]
Salt AN,Plontke SK, Endolymphatic hydrops: pathophysiology and experimental models. Otolaryngologic clinics of North America. 2010 Oct
[PubMed PMID: 20713237]
[17]
Minor LB, Schessel DA, Carey JP. Ménière's disease. Current opinion in neurology. 2004 Feb:17(1):9-16
[PubMed PMID: 15090872]
Level 3 (low-level) evidence
[18]
Shulman A, Goldstein B. Brain and inner-ear fluid homeostasis, cochleovestibular-type tinnitus, and secondary endolymphatic hydrops. The international tinnitus journal. 2006:12(1):75-81
[PubMed PMID: 17147045]
[19]
Domínguez P,Manrique-Huarte R,Suárez-Vega V,López-Laguna N,Guajardo C,Pérez-Fernández N, Endolymphatic Hydrops in Fluctuating Hearing Loss and Recurrent Vertigo. Frontiers in surgery. 2021
[PubMed PMID: 34136529]
[20]
Baráth K, Schuknecht B, Naldi AM, Schrepfer T, Bockisch CJ, Hegemann SC. Detection and grading of endolymphatic hydrops in Menière disease using MR imaging. AJNR. American journal of neuroradiology. 2014 Jul:35(7):1387-92. doi: 10.3174/ajnr.A3856. Epub 2014 Feb 13
[PubMed PMID: 24524921]
[21]
Foster CA, Breeze RE. Endolymphatic hydrops in Ménière's disease: cause, consequence, or epiphenomenon? Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2013 Sep:34(7):1210-4. doi: 10.1097/MAO.0b013e31829e83df. Epub
[PubMed PMID: 23921917]
[22]
Tyrrell JS, Whinney DJ, Ukoumunne OC, Fleming LE, Osborne NJ. Prevalence, associated factors, and comorbid conditions for Ménière's disease. Ear and hearing. 2014 Jul-Aug:35(4):e162-9. doi: 10.1097/AUD.0000000000000041. Epub
[PubMed PMID: 24732693]
[23]
Tyrrell J, White MP, Barrett G, Ronan N, Phoenix C, Whinney DJ, Osborne NJ. Mental Health and Subjective Well-being of Individuals With Ménière's: Cross-sectional Analysis in the UK Biobank. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2015 Jun:36(5):854-61. doi: 10.1097/MAO.0000000000000732. Epub
[PubMed PMID: 25730447]
Level 2 (mid-level) evidence
[24]
Havia M, Kentala E, Pyykkö I. Prevalence of Menière's disease in general population of Southern Finland. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2005 Nov:133(5):762-8
[PubMed PMID: 16274806]
[25]
Hietikko E, Kotimäki J, Okuloff A, Sorri M, Männikkö M. A replication study on proposed candidate genes in Ménière's disease, and a review of the current status of genetic studies. International journal of audiology. 2012 Nov:51(11):841-5. doi: 10.3109/14992027.2012.705900. Epub 2012 Aug 30
[PubMed PMID: 22934933]
[26]
Huppert D, Strupp M, Brandt T. Long-term course of Menière's disease revisited. Acta oto-laryngologica. 2010 Jun:130(6):644-51. doi: 10.3109/00016480903382808. Epub
[PubMed PMID: 20001444]
[27]
Nakashima T, Pyykkö I, Arroll MA, Casselbrant ML, Foster CA, Manzoor NF, Megerian CA, Naganawa S, Young YH. Meniere's disease. Nature reviews. Disease primers. 2016 May 12:2():16028. doi: 10.1038/nrdp.2016.28. Epub 2016 May 12
[PubMed PMID: 27170253]
[28]
Gonçalves DU, Felipe L, Lima TM. Interpretation and use of caloric testing. Brazilian journal of otorhinolaryngology. 2008 May-Jun:74(3):440-6
[PubMed PMID: 18661020]
[29]
Fife TD, Satya-Murti S, Burkard RF, Carey JP. Vestibular evoked myogenic potential testing: Payment policy review for clinicians and payers. Neurology. Clinical practice. 2018 Apr:8(2):129-134. doi: 10.1212/CPJ.0000000000000430. Epub
[PubMed PMID: 29708189]
[30]
Gibson WP. The Clinical Uses of Electrocochleography. Frontiers in neuroscience. 2017:11():274. doi: 10.3389/fnins.2017.00274. Epub 2017 May 19
[PubMed PMID: 28634435]
[31]
Basura GJ, Adams ME, Monfared A, Schwartz SR, Antonelli PJ, Burkard R, Bush ML, Bykowski J, Colandrea M, Derebery J, Kelly EA, Kerber KA, Koopman CF, Kuch AA, Marcolini E, McKinnon BJ, Ruckenstein MJ, Valenzuela CV, Vosooney A, Walsh SA, Nnacheta LC, Dhepyasuwan N, Buchanan EM. Clinical Practice Guideline: Ménière's Disease. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2020 Apr:162(2_suppl):S1-S55. doi: 10.1177/0194599820909438. Epub
[PubMed PMID: 32267799]
Level 1 (high-level) evidence
[32]
Monzani D, Barillari MR, Alicandri Ciufelli M, Aggazzotti Cavazza E, Neri V, Presutti L, Genovese E. Effect of a fixed combination of nimodipine and betahistine versus betahistine as monotherapy in the long-term treatment of Ménière's disease: a 10-year experience. Acta otorhinolaryngologica Italica : organo ufficiale della Societa italiana di otorinolaringologia e chirurgia cervico-facciale. 2012 Dec:32(6):393-403
[PubMed PMID: 23349559]
[33]
Pullens B, Verschuur HP, van Benthem PP. Surgery for Ménière's disease. The Cochrane database of systematic reviews. 2013 Feb 28:2013(2):CD005395. doi: 10.1002/14651858.CD005395.pub3. Epub 2013 Feb 28
[PubMed PMID: 23450562]
Level 1 (high-level) evidence
[34]
Greenberg SL, Nedzelski JM. Medical and noninvasive therapy for Meniere's disease. Otolaryngologic clinics of North America. 2010 Oct:43(5):1081-90. doi: 10.1016/j.otc.2010.05.005. Epub
[PubMed PMID: 20713246]