Introduction
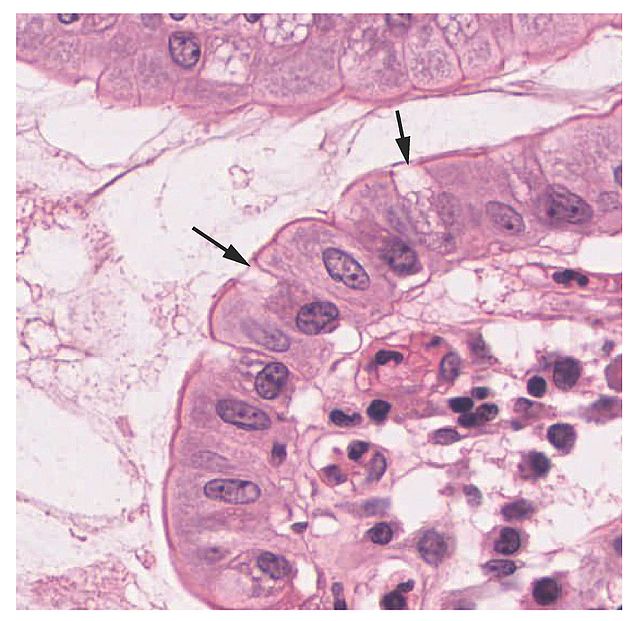
Goblet cells arise from pluripotent stem cells and derive their name from their goblet, cup-like appearance (see Image. Histology Showing Goblet Cells). The primary function of goblet cells is to secrete mucin and create a protective mucus layer. Goblet cells are also thought to be involved with immunoregulation. Samples of goblet cells can be preserved through cryopreservation and analyzed with light microscopy. Additionally, goblet cells exhibit a complex cytoskeletal architecture and may have different glycosylation patterns. As a result, different localized goblet cells may have slightly altered functionalities. Clinically, goblet cells are associated with respiratory diseases and inflammatory bowel diseases.
Structure
Formed by mucin granules, goblet cells derive their name from their goblet, cup-like appearance.[1] At the base of the intestinal crypt, pluripotent stem cells secrete many substances, such as trefoil peptide and mucin. Goblet cells originate from these pluripotent stem cells and form the intestinal mucus layer that protects epithelial cells.[2] Goblet cells anchor to the inner mucus layer of the colon. This mucus layer system separates bacteria from epithelial cells in a stratified and organized filter.[1]
Stem cells essentially give rise to all epithelial cells.[2] Goblet cells, comprising the intestinal surface epithelium, are renewed continuously from stem cells at the crypt base. Normal cell turnover is between 3 to 7 days. Notch signaling controls the differentiation pathway for the enterocyte lineage, which is the primary cell lineage.[1] When gamma-secretase becomes inhibited, Notch signaling is blocked, thus, preventing the Notch cytoplasmic component from entering the nucleus.[2] This sequence inhibits transcriptional signaling and shifts differentiation in the secretory pathway. Goblet cells are the default result of this shift, assuming no further changes in signaling arise. Spdef, a transcription factor, is essential for full goblet cell maturation.[1]
Function
Goblet cells are intestinal mucosal epithelial cells that serve as the primary site for nutrient digestion and mucosal absorption.[2] The primary function of goblet cells is to synthesize and secrete mucus.[1] As the primary secretory cell in the superficial epithelium of large airways, goblet cells secrete mucin glycoproteins, the major macromolecular components of mucus.[3] Different types of goblet cells can be identified based on location and function.[1]
Colonic and small intestinal crypt goblet cells secrete upon stimulation. Examples of stimulation include responses to endocytosis or acetylcholine. Surface colonic goblet cells constantly secrete to maintain the inner mucus layer. These goblet cells contrast with the upper part of the colonic crypt goblet cells that can secrete by rapid compound exocytosis.[1]
Goblet cells play an important role in maintaining intestinal homeostasis. Recent studies have shown that goblet cells can act as antigen importers and may serve as a regulator of innate immune function. Goblet cells help maintain homeostasis by providing bicarbonate for proper mucin unfolding in the small intestine. Goblet cells can also form a line of defense at the intestinal mucosa and have a common secretory role. Small intestinal goblet cells uptake antigens through cholinergic agonists regulated by muscarinic receptor 4.[1]
Tissue Preparation
Preserving the original structure of mucus is an essential aspect of histological fixation. Tissue fixation significantly impacts the intestinal mucus layer visualization, and due to its unstable structure, the histological fixation of intestinal mucus is difficult. In piglets, the original state of the tissue samples was preserved, and mucus position between epithelium and intestinal content was preserved in cryo-preservation with liquid nitrogen, except for partial preservation observed in jejunal samples. However, chemical fixation in piglets did not find samples with sufficiently preserved mucus. Cryo-preservation followed by AB-PAS staining would then enable the evaluation of mucus thickness in the colon of pigs.[4] Additionally, mucus thickness can be indirectly quantified through the number of intestinal goblet cells.[5]
Histochemistry and Cytochemistry
Intermediate filaments, microtubules, and microfilaments compose the goblet cell cytoskeleton. Although actin plays a significant functional role, the contribution of actin is minimal in number.[6]
Different glycosylation patterns may exist in the goblet cells of the large intestine, but they present in morphologically homogeneous cell populations. Due to these different patterns, researchers have suggested that goblet cells have various functional subclasses. One example of such a subclass is in carboxylated and sulfated goblet cells documented to secrete mucin within an acidic component.[7]
Microscopy, Light
Goblet cells are visible through laser scanning confocal microscopy (LSCM).[8] Some tissues for light microscopy are dehydrated in graded ethanols and embedded in plastic or resin. Methods for staining tissues are the periodic acid-Schiff (PAS), mucicarmine stains, and iron hematoxylin.[9] In one study, researchers used nine different locations and three different depths to scan goblet cells. LSCM can determine the size, shape, and reflectivity of goblet cells. Due to their balloon-like appearance and size, goblet cells were reportedly easily differentiated from their surrounding cells. Through a non-invasive manner, LSCM remains a promising method for the quantification of goblet cells.[8]
A different study examined goblet cell carcinoids through light microscopy on paraffin sections. These sections were then mounted with Entellan and not counterstained to better visualize the product. Light microscopy was able to identify goblet cells in the submucous and muscle layer of the appendix.[10]
Microscopy, Electron
Electron microscopy is an option for identifying goblet cells. Sections for electron microscopy can be stained with uranyl acetate and lead citrate.[9] One study analyzed goblet cells with paraffin sections 40 to 60 micrometers in size.[10] Another study stained goblets cells en bloc with 1% uranyl acetate in 0.1 M sodium acetate buffer, followed by dehydration in graded ethanols, and finally embedded in resin mixtures.[9]
Pathophysiology
Mucus hypersecretion due to goblet cell hyperplasia is a symptom of asthma and chronic obstructive pulmonary disease. Hyperplasia refers to areas where the excessive proliferation of goblet cells occurs in an area they are normally present, such as large airways. As a result of this goblet cell proliferation, the usual protective role of goblet cell mucin secretion transforms into a pathophysiological role. The proliferation of goblet cells can also contribute to respiratory disease through changes in volume and biophysical features of airway mucus.[5]
Other pathologies may also present with goblet cell metaplasia due to altered size, shape, number, and distribution. A hallmark of chronic lung disease, goblet cell metaplasia lacks curative treatment. In this disease, mucin-secreting goblet cells accumulate in the airway and invoke mucus hypersecretion. This blockage affects epithelial cells, immune cells, and other types of cells in the airways. Inflammatory pathways may attenuate or worsen the condition. Timing for goblet cell metaplasia may last from a few weeks to decades. Smoking cigarettes, inhaling biomass fuels, chronic bronchitis, environmental allergens that trigger asthma, and mutations of the cystic fibrosis transmembrane conductance regulator may cause lung diseases with goblet cell metaplasia.[11]
Clinical Significance
Chronic infections can cause goblet cell depletion and thus cause immunologic implications; goblet cell secretion of mucus encourages pathogen elimination and safeguards the protective mucus layers. Besides chronic infections, parasitic intestinal infections can significantly affect these cells. Some studies have also implicated goblet cells to play a role in cystic fibrosis.
Two major inflammatory bowel diseases that may involve goblet cells are Crohn disease and ulcerative colitis. In ulcerative colitis, goblet cell number and size drastically decrease. Mucin, the main product of goblet cells, plays an essential role in maintaining protective mucus barriers, and the breakdown of these barriers is thought to eventually result in colitis.[12]
Pools of superfluous amounts of extracellular mucin characterize mucinous carcinoma. Mucinous carcinoma is a tumor where 50% of the tumor mass comes from mucin. This carcinoma leads to high levels of MUC2 goblet cell mucin. MUC2 becomes upregulated and, thus, increases the binding of the goblet cell lineage transcription factor to the MUC2 promoter.[12]