Continuing Education Activity
Thoracic duct leaks are most often due to traumatic injury and iatrogenic injury. Surgery of the esophagus and the heart are the most common source of injury. Untreated high-volume leaks have a significant mortality rate. This activity reviews the evaluation and treatment of thoracic duct leaks and highlights the role of the interprofessional team in improving care for patients with this condition.
Objectives:
- Describe the etiology of thoracic duct leaks.
- Review the evaluation to diagnose and confirm a thoracic duct leak.
- Outline the management of low output and high output thoracic duct leaks.
- Explain the importance of collaboration and communication amongst the interprofessional team to ensure the appropriate selection of treatment in patients with thoracic duct leaks.
Introduction
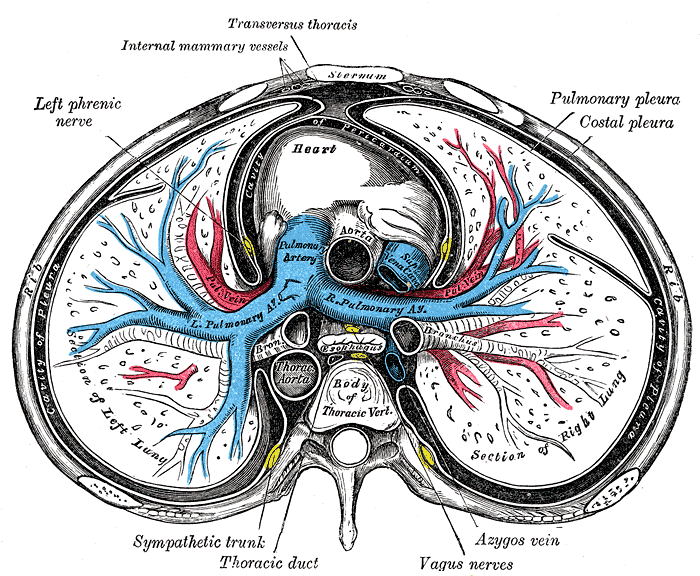
The thoracic duct is the largest lymphatic duct in the body,[1] with a typical length of 45 cm and a diameter of 2 to 5 mm. It drains lymph from the whole body except the right hemithorax, the right side of the head and neck, and the right upper limb. Chylothorax is the term used for thoracic duct leak and collection into the pleural space.
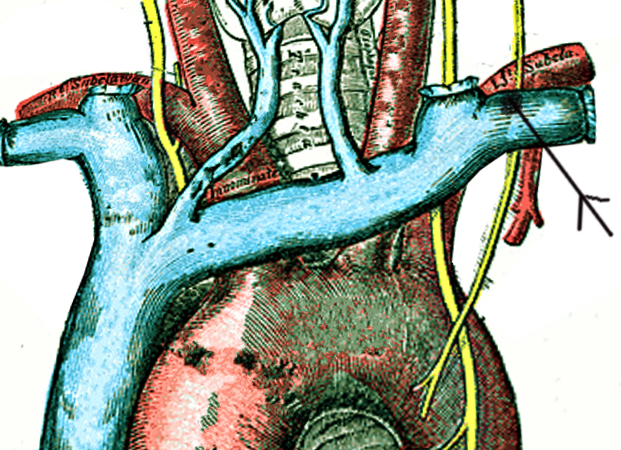
The thoracic duct typically starts at the second lumbar vertebra at the cisterna chyli, ending at the junction of the left subclavian and jugular veins. A total of 1.5 to 2 liters of lymph flows through the thoracic duct per day.[2][3] Any leak from this system results in significant morbidity with loss of lymph and secondarily causing respiratory distress.
Etiology
The etiology of thoracic duct leaks can be traumatic and non-traumatic. However, trauma-related etiologies are more common.
A. Traumatic causes include surgical and non-surgical
- Surgical causes: Iatrogenic injury while performing procedures on the esophagus, aorta, pleura, lung, vagotomy, spine surgery, and others. One of the leading reasons for the damage of the thoracic duct during surgical interventions is its anatomical variation.[1][4] A variation is seen in almost one-third of the population.[5]
- Non-surgical causes: Penetrating or non-penetrating trauma to the neck, thorax, upper abdomen, and occasionally due to straining or forceful vomiting and subclavian vein canalization are among the non-surgical causes.
B. Non-traumatic can be further categorized into malignant and non-malignant etiologies.
- Malignant causes: Lymphomas and non-lymphomatous causes include primary lung cancers, mediastinal tumors, sarcomas, and leukemia.
- Non-malignant causes: It can be due to benign tumors, cirrhosis of the liver, thoracic aortic aneurysm, amyloidosis, sarcoidosis, protein-losing enteropathy, tuberous sclerosis, and in developing countries, tuberculosis.[6]
Among the above, surgical and tumors are the most frequent causes of chylothorax.[5]
Epidemiology
Thoracic duct leak most often is observed after esophagectomy in about 3% and up to 6% of pediatric patients undergoing cardiac surgeries. Approximately 3% of the patients with blunt thoracic trauma and 1.3% of penetrating thoracic trauma demonstrate thoracic duct leaks.[7]
Pathophysiology
The thoracic duct is more like a vein histologically with the primary function of transporting chyle from the gastrointestinal tract into a systemic venous system. It carries almost 4 liters of chyle daily, most of which originates in the digestive tract. The flow rate can be up to 100 ml/kg/day, depending on the diet consumed. A combination of intrathoracic and intraabdominal pressures and arterial pulsations helps in maintaining lymph flow in the thoracic duct.
Chyle is rich in chylomicrons, triglycerides, cholesterol, fat-soluble vitamins and also contains albumin in high concentrations (12-14 g/L). Thoracic lymph also contains lymphocytes, which account for 95% of the cell content, and this keeps chyle mostly sterile. Electrolytes and other enzymes levels resemble that of the plasma.[8][9]
Because of its unique contents, a thoracic duct leak can have multiple effects such as mechanical compression on the heart leading to tamponade, volume loss, pleural effusion, and pulmonary atelectasis, all of which can occur in an acute event.[10]
In the chronic phase, with depletion of chyle, depression of immunity is observed due to a fall in cellular and humoral immunity attributed to lymphocyte and immunoglobulin depletion. Also, patients may have severe malnutrition with loss of absorbed fat. Hence complications can occur in up to 50% of patients.[10][11]
History and Physical
Symptom onset depends on the etiology, with traumatic or surgical causes presenting earlier compared to the other etiologies.
Typically, patients present with dyspnea due to pleural effusion. Chronically leaking chyle also results in malnourishment and susceptibility to infections, which is seen in non-traumatic causes (e.g., malignancy). However, in surgical and traumatic causes, the symptom onset may be immediate if the volume is more than 500 ml/day.
Examination reveals decreased breath sounds and also dullness to percussion in areas of lymph collection.
Evaluation
Blood investigations: Complete blood count, serum glucose, total protein, triglycerides, albumin, and LDH are done routinely.
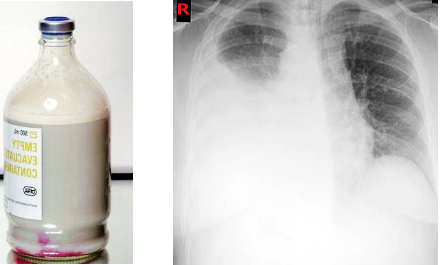
Imaging: A chest radiograph may show pleural effusion. Usually, it is unilateral in up to 78% of patients, and it involves the right hemithorax more commonly.[12]
The varied course of the thoracic duct and its site of leak dictates the side of pleural effusion. Hence, injury to the duct below the 5th thoracic vertebrae results in pleural effusion on the right side, and damage above this level occurs in a left-sided pleural effusion.[13] Diseases can also disrupt multiple tributaries to the thoracic duct that can produce pleural effusion unilaterally or bilaterally.
Suspicion should arise when a pleural effusion is recurrent or persistent, more so when it is milky, turbid, or serosanguinous on aspiration. However, the classically described appearance of milky or opalescent aspirate is not universal and is seen in only up to 50% of patients with a thoracic duct leak.[14]
Pleural fluid analysis: Fluid appearance should be noted. The white cell count typically has a lymphocyte predominance with a nucleated cell count greater than 70%. Also, lymphocytes found would be a polyclonal population of T cells.[13]
Electrolyte and protein content is similar to the plasma. LDH content is usually low, and a high level signifies a malignant etiology. Glucose levels are similar to plasma, and a level less than 60 mg/dL, indicates either an infection or a malignant pleural effusion. Triglyceride levels >110mg/dL and cholesterol <200 mg/dL is typically found in patients suspected to have chylothorax. A triglyceride level >240 mg/dL has a sensitivity and specificity of >95%.[15]
The definitive diagnostic test is the detection of chylomicrons in the fluid by lipoprotein electrophoresis, though not performed routinely due to its high expense and availability. Chylomicrons can also be detected by cytological analysis of the obtained fluid with Sudan III, although this is a sensitive test but not specific. Hence it is usually combined with fluid analysis to increase its accuracy.[4]
After confirming the presence of chylothorax, patients require additional evaluation to find the etiology for the leak, and that includes computed tomography (CT) of the chest and abdomen. Though at times, CT may not provide the site of the leak and also in the presence of thoracic duct anomalies, CT may not yield much information. Lymphangiography is a dye study where a dye (methylene blue) is injected in the subcutis of digits and is carried by the pedal lymphatics into the central system. Also, lipiodol contrast can be injected into the lymphatic vessel, and the probable site of the leak and anatomical variation can be visualized using fluoroscopy.
Lymphoscintigraphy is the injection of a technetium 99-labeled agent into the dorsum of the foot bilaterally. Later, imaging is done with single-photon emission computerized tomography (SPECT)/CT, which gives a good visualization of the thoracic duct.
Recent studies suggest that near-infra-red fluorescence is nonionizing imaging and an easy-to-use method to detect thoracic duct leaks in open surgery or thoracoscopic interventions. Yet, no application to percutaneous sclerotherapy has been described, and hence it can also be a useful tool for percutaneous sclerotherapy.[16]
Treatment / Management
Management includes addressing the leak and also the etiology of the leak after diagnosis. Thoracic duct leak is classified into low output if the volume is < 1 liter and high volume if > 1 liter.
Low output chylothorax - Drainage of the fluid in symptomatic patients along with dietary control measures and concomitantly treating the underlying cause is the goal of treatment. Octreotide is often used to reduce the number of leaks and to avoid surgery.
High output chylothorax - Most commonly seen post-surgically. Though conservative management is tried initially, many end up needing an intervention such as thoracic duct ligation or embolization.[17]
Ancillary measures include complete bowel rest, initiation of parenteral nutrition, and also octreotide/somatostatin and etilefrine therapy for patients awaiting surgery.[4] The timing of surgery is debated, with some advocating the immediate postoperative day and others suggesting initial conservative management for five days and then surgery in failed cases.[18]
Alternatively, non-surgical treatment, including thoracic duct embolization or disruption, may be utilized in high-risk patients.[10][19][20] Because of the high success rate and very low morbidity and mortality, thoracic duct embolization has become the first line of treatment in recent years for the treatment of chylothorax.[21] Embolization of the thoracic duct is a percutaneous technique that normally includes pedal or intranodal lymphangiography,[22] thoracic duct transabdominal catheterization, and glue embolization.
An alternative route to approach the thoracic duct is by retrograde transvenous approach or even direct ultrasound-guided puncture in the neck followed by embolization.[23] Thoracic duct embolization is usually performed by the percutaneous transabdominal method, and the supply route is blocked using N-butyl cyanoacrylate that is well mixed with lipiodol.[24] The success rate of thoracic duct embolization is almost 70%.[4] Postoperative complications of this procedure include chronic diarrhea, lower extremity edema, and abdominal ascites.[21]
In post-pneumonectomy patients with chylothorax, the leak is managed without chest tube drainage if there is no evidence of a mediastinal shift. A drain may be a necessity if there is a contralateral mediastinal shift, and further evaluation for thoracic duct ligation or embolization should be carried out. Thoracic fistulas are usually treated by direct suture ligation of the thoracic duct with or without adjuncts, including biologic glues or sclerosing agents.[25] If the chylothorax is detected after three weeks postoperatively, then better results are obtained when the sinus tract dilatation technique is used with cavity visualization and clipping of the leaking channel.[26]
Efficacy of conservative management depends on etiology and volume of pleural fluid drainage, with benign conditions such as infection or sarcoidosis. [27]
Pleurodesis: In patients who are not surgical candidates, pleurodesis is an option and is done by installing substances like talc or chemicals such as bleomycin and tetracycline through a catheter into the chest drainage. In surgical pleurodesis with abrasion, pleurectomy can be done during thoracotomy or thoracoscopically, depending on the fitness of the patient. It is observed that surgical pleurodesis is more effective compared to medical pleurodesis.[12][28]
Thoracic duct ligation and thoracic duct embolization are done through catheterization and embolization or disruption of prominent retroperitoneal lymphatics. After every thoracic duct ligation, it is advisable to confirm by looking at their frozen section tissues.[25]
Shunts: In patients with failed definitive procedures, a pleuroperitoneal or pleurovenous shunt may be performed.[29]
Bypass: Terminal thoracic duct bypass is said to be a novel procedure that improves the condition and also cures patients suffering from thoracic leaks due to central conducting lymphatic anomalies.[30]
Differential Diagnosis
Differential diagnosis includes:
- Milky appearing fluid may be due to cholesterol effusion, empyema, and tube feed or lipid leak.
- Non-milky fluid- Exudative effusion due to bacterial/tubercular or atypical cases of pneumonia. Pancreatitis, radiation therapy, acute respiratory distress syndrome (ARDS), hypothyroidism, and lupus pleuritis are other causes of non-milky fluid collection.
Prognosis
A large portion of patients improve with conservative management and hence have a good prognosis. Patients with a malignant etiology or in whom the chyle leak cannot be controlled have a poor prognosis. A mortality rate of almost 50% is seen in these patients when their chylothorax is not treated.[10]
Complications
Complications of chylothorax are due to persistent loss of chyle, which contains considerable amounts of fat and fat-soluble vitamins, proteins, electrolytes, and immune system components such as T-lymphocytes and immunoglobulins resulting in impaired immunity and malnutrition.[31]
Postoperative and Rehabilitation Care
- Care of intercostal drainage tube (ICD) or indwelling catheter and accurate measurement of the chyle output.
- Adequate replenishment of electrolytes, with monitoring of serum lymphocyte counts, albumin, total protein, and weight should be considered.
- Oral or enteral low-fat diet.
Deterrence and Patient Education
Patients with a low output thoracic duct leak who are managed conservatively should be counseled for strict dietary suggestions and frequent monitoring of blood parameters such as electrolytes, leukocyte count, and proteins. Adequate hygiene of the ICD should be maintained. Infection is a possibility, and in such cases, computed tomography may be indicated.
Pearls and Other Issues
The most common cause for thoracic duct leak is surgical, typically after surgeries on the esophagus, lung, or heart.
Most are low output leaks and can be managed conservatively with a low-fat diet, and in symptomatic patients, thoracocentesis or sometimes a chest tube may be needed.
- Chyle leak should be suspected in the post-surgical period when there is a serosanguinous fluid or milky fluid in the drain.
- A triglyceride level >110 mg/dL in the fluid is indicative of a chyle leak.
- CT scan is useful in diagnosing a chyle leak.
- Lymphangiogrpahy and lymphoscintigraphy have a higher sensitivity in diagnosing a chyle leak.
Enhancing Healthcare Team Outcomes
Thoracic duct leaks can result in significant morbidity and mortality if not recognized, more so in the intraoperative period where most of the injuries occur. An interprofessional team approach is beneficial for patients with thoracic duct leaks. Close cooperation between the critical care team is required in the immediate postoperative period to manage electrolyte imbalance and diminished immunity. A cardiothoracic surgeon is needed to assess the need for drainage of the chyle leak. Teamwork involving interventional radiologists in an early period may be needed for a probable intervention such as thoracic duct embolization or disruption. Nursing involvement in the care of the ICD should start immediately after the procedure.