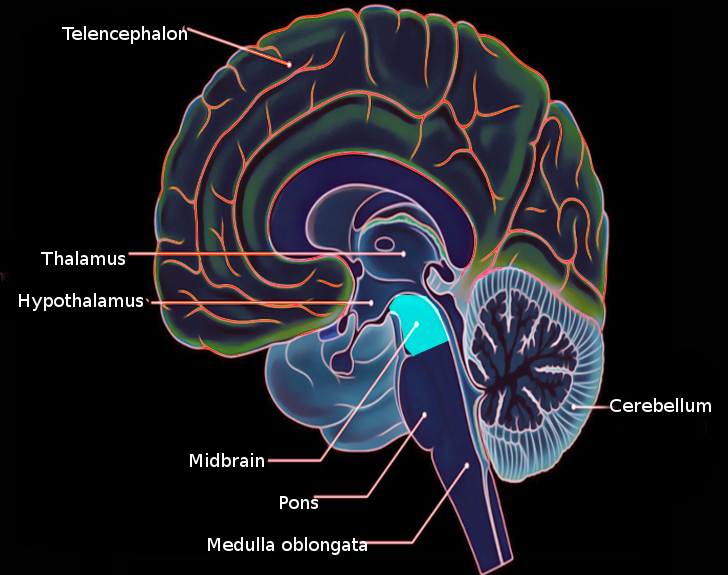
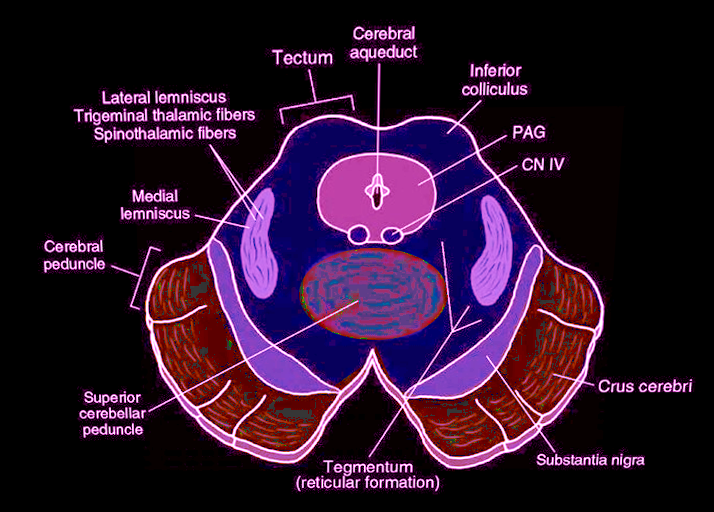
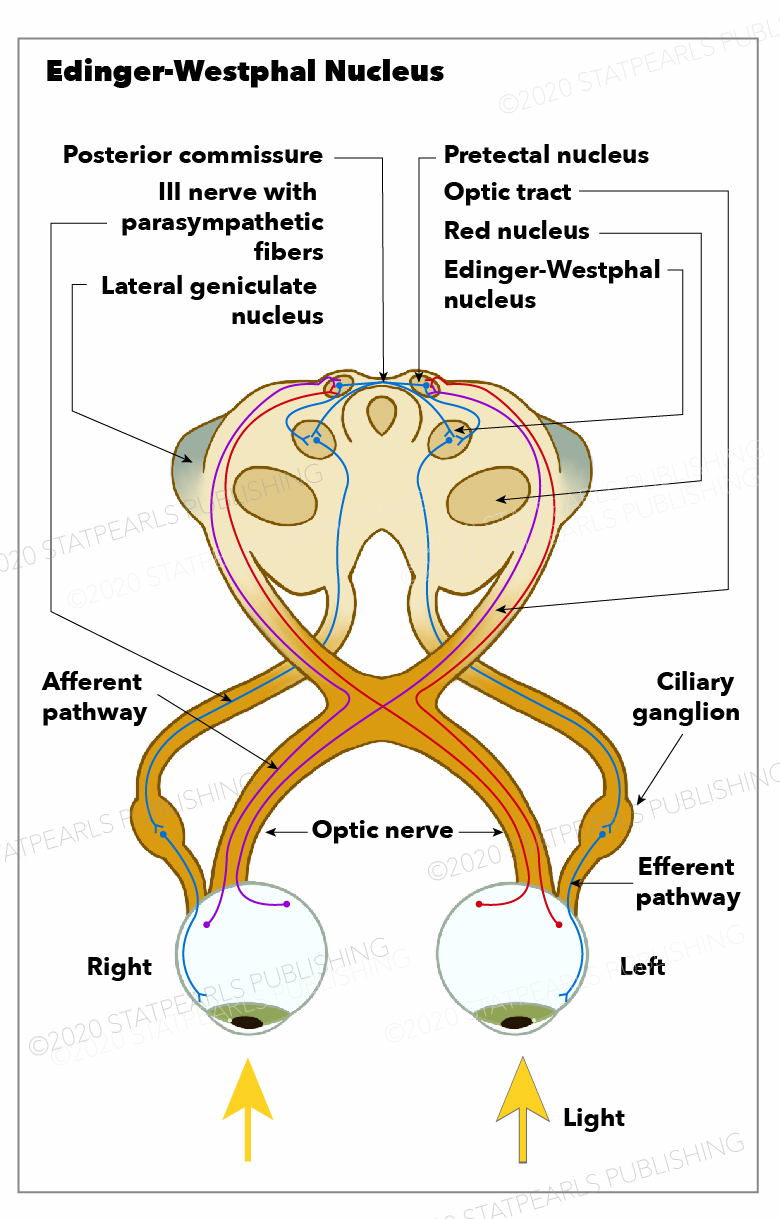
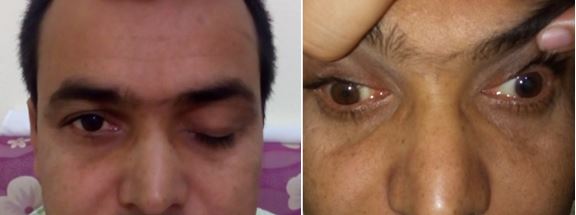
[1]
Dos Santos Júnior ED, Da Silva AV, Da Silva KR, Haemmerle CA, Batagello DS, Da Silva JM, Lima LB, Da Silva RJ, Diniz GB, Sita LV, Elias CF, Bittencourt JC. The centrally projecting Edinger-Westphal nucleus--I: Efferents in the rat brain. Journal of chemical neuroanatomy. 2015 Oct:68():22-38. doi: 10.1016/j.jchemneu.2015.07.002. Epub 2015 Jul 21
[PubMed PMID: 26206178]
[2]
Kozicz T, Bittencourt JC, May PJ, Reiner A, Gamlin PD, Palkovits M, Horn AK, Toledo CA, Ryabinin AE. The Edinger-Westphal nucleus: a historical, structural, and functional perspective on a dichotomous terminology. The Journal of comparative neurology. 2011 Jun 1:519(8):1413-34. doi: 10.1002/cne.22580. Epub
[PubMed PMID: 21452224]
Level 2 (mid-level) evidence
[3]
Che Ngwa E, Zeeh C, Messoudi A, Büttner-Ennever JA, Horn AK. Delineation of motoneuron subgroups supplying individual eye muscles in the human oculomotor nucleus. Frontiers in neuroanatomy. 2014:8():2. doi: 10.3389/fnana.2014.00002. Epub 2014 Feb 12
[PubMed PMID: 24574976]
[4]
Ryabinin AE,Tsivkovskaia NO,Ryabinin SA, Urocortin 1-containing neurons in the human Edinger-Westphal nucleus. Neuroscience. 2005;
[PubMed PMID: 16039794]
[5]
Cunha RP, Reiner A, Toledo CA. Involvement of urocortinergic neurons below the midbrain central gray in the physiological response to restraint stress in pigeons. Brain research. 2007 May 25:1147():175-83
[PubMed PMID: 17320052]
[6]
McDougal DH, Gamlin PD. Autonomic control of the eye. Comprehensive Physiology. 2015 Jan:5(1):439-73. doi: 10.1002/cphy.c140014. Epub
[PubMed PMID: 25589275]
[7]
Szabadi E. Functional Organization of the Sympathetic Pathways Controlling the Pupil: Light-Inhibited and Light-Stimulated Pathways. Frontiers in neurology. 2018:9():1069. doi: 10.3389/fneur.2018.01069. Epub 2018 Dec 18
[PubMed PMID: 30619035]
[8]
Pagida MA, Konstantinidou AE, Tsekoura E, Patsouris E, Panayotacopoulou MT. Immunohistochemical demonstration of urocortin 1 in Edinger-Westphal nucleus of the human neonate: colocalization with tyrosine hydroxylase under acute perinatal hypoxia. Neuroscience letters. 2013 Oct 25:554():47-52. doi: 10.1016/j.neulet.2013.08.054. Epub 2013 Sep 4
[PubMed PMID: 24012814]
Level 3 (low-level) evidence
[9]
Yamaguchi K. Development of the human oculomotor nuclear complex: Centrally-projecting Edinger-Westphal nucleus. Neuroscience letters. 2017 Apr 12:646():8-14. doi: 10.1016/j.neulet.2016.11.040. Epub 2016 Nov 21
[PubMed PMID: 27884738]
Level 3 (low-level) evidence
[10]
Korosi A, Schotanus S, Olivier B, Roubos EW, Kozicz T. Chronic ether stress-induced response of urocortin 1 neurons in the Edinger-Westphal nucleus in the mouse. Brain research. 2005 Jun 7:1046(1-2):172-9
[PubMed PMID: 15885665]
[11]
Viau V, Sawchenko PE. Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress: rapid publication. The Journal of comparative neurology. 2002 Apr 15:445(4):293-307
[PubMed PMID: 11920708]
Level 2 (mid-level) evidence
[12]
Agarwala S, Ragsdale CW. A role for midbrain arcs in nucleogenesis. Development (Cambridge, England). 2002 Dec:129(24):5779-88
[PubMed PMID: 12421716]
[14]
Párraga RG, Ribas GC, Andrade SE, de Oliveira E. Microsurgical anatomy of the posterior cerebral artery in three-dimensional images. World neurosurgery. 2011 Feb:75(2):233-57. doi: 10.1016/j.wneu.2010.10.053. Epub
[PubMed PMID: 21492726]
[15]
Ciceri EF, Klucznik RP, Grossman RG, Rose JE, Mawad ME. Aneurysms of the posterior cerebral artery: classification and endovascular treatment. AJNR. American journal of neuroradiology. 2001 Jan:22(1):27-34
[PubMed PMID: 11158883]
[16]
Sun BL, Wang LH, Yang T, Sun JY, Mao LL, Yang MF, Yuan H, Colvin RA, Yang XY. Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Progress in neurobiology. 2018 Apr-May:163-164():118-143. doi: 10.1016/j.pneurobio.2017.08.007. Epub 2017 Sep 10
[PubMed PMID: 28903061]
[17]
Mehta VS, Chandra PS, Singh PK, Garg A, Rath GK. Surgical considerations for 'intrinsic' brainstem gliomas: proposal of a modification in classification. Neurology India. 2009 May-Jun:57(3):274-81. doi: 10.4103/0028-3886.53272. Epub
[PubMed PMID: 19587467]
[18]
Sciacca S, Lynch J, Davagnanam I, Barker R. Midbrain, Pons, and Medulla: Anatomy and Syndromes. Radiographics : a review publication of the Radiological Society of North America, Inc. 2019 Jul-Aug:39(4):1110-1125. doi: 10.1148/rg.2019180126. Epub
[PubMed PMID: 31283463]
[19]
Shepherd TM, Hoch MJ. MRI-Visible Anatomy of the Brainstem. Neuroimaging clinics of North America. 2022 Aug:32(3):553-564. doi: 10.1016/j.nic.2022.04.003. Epub
[PubMed PMID: 35843662]
[20]
Kitagawa H, Sasaki Y, Ishihara K, Kawakami M. Heartworm migration toward right atrium following artificial pulmonary arterial embolism or injection of heartworm body fluid. Nihon juigaku zasshi. The Japanese journal of veterinary science. 1990 Jun:52(3):591-9
[PubMed PMID: 2385039]
[21]
Alzheimer's Association. 2016 Alzheimer's disease facts and figures. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2016 Apr:12(4):459-509
[PubMed PMID: 27570871]
[22]
Mavroudis IA, Manani MG, Petrides F, Petsoglou C, Njau SN, Costa VG, Baloyannis SJ. Dendritic and spinal alterations of neurons from Edinger-Westphal nucleus in Alzheimer's disease. Folia neuropathologica. 2014:52(2):197-204
[PubMed PMID: 25118905]
[23]
Sheikh Hassan M, Osman Sidow N, Adam BA, Adani AA. Superior alternating hemiplegia (Weber's syndrome)- Case report. Annals of medicine and surgery (2012). 2022 May:77():103674. doi: 10.1016/j.amsu.2022.103674. Epub 2022 Apr 28
[PubMed PMID: 35638077]
Level 3 (low-level) evidence
[24]
Basile GA, Quartu M, Bertino S, Serra MP, Boi M, Bramanti A, Anastasi GP, Milardi D, Cacciola A. Red nucleus structure and function: from anatomy to clinical neurosciences. Brain structure & function. 2021 Jan:226(1):69-91. doi: 10.1007/s00429-020-02171-x. Epub 2020 Nov 12
[PubMed PMID: 33180142]
[25]
Cacciola A, Milardi D, Basile GA, Bertino S, Calamuneri A, Chillemi G, Paladina G, Impellizzeri F, Trimarchi F, Anastasi G, Bramanti A, Rizzo G. The cortico-rubral and cerebello-rubral pathways are topographically organized within the human red nucleus. Scientific reports. 2019 Aug 20:9(1):12117. doi: 10.1038/s41598-019-48164-7. Epub 2019 Aug 20
[PubMed PMID: 31431648]
[26]
Paidakakos NA, Rokas E, Theodoropoulos S, Dimogerontas G, Konstantinidis E. Posttraumatic Benedikt's syndrome: a rare entity with unclear anatomopathological correlations. World neurosurgery. 2012 Dec:78(6):715.e13-5. doi: 10.1016/j.wneu.2012.03.028. Epub 2012 Apr 3
[PubMed PMID: 22484069]
[27]
Cheng G, Yang Y, Wang Y, Tan H, Zhang S. Deep brain stimulation of the thalamic ventral intermediate nucleus for Benedikt's syndrome mainly present as tremor: a long-term case observation. Acta neurochirurgica. 2018 Jul:160(7):1349-1353. doi: 10.1007/s00701-018-3526-8. Epub 2018 Mar 30
[PubMed PMID: 29600395]
Level 3 (low-level) evidence
[28]
Bandt SK, Anderson D, Biller J. Deep brain stimulation as an effective treatment option for post-midbrain infarction-related tremor as it presents with Benedikt syndrome. Journal of neurosurgery. 2008 Oct:109(4):635-9. doi: 10.3171/JNS/2008/109/10/0635. Epub
[PubMed PMID: 18826349]