Introduction
The autonomic nervous system is a component of the peripheral nervous system that regulates involuntary physiologic processes including heart rate, blood pressure, respiration, digestion, and sexual arousal. It contains three anatomically distinct divisions: sympathetic, parasympathetic, and enteric.
The sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS) contain both afferent and efferent fibers that provide sensory input and motor output, respectively, to the central nervous system (CNS). Generally, the SNS and PNS motor pathways consist of a two-neuron series: a preganglionic neuron with a cell body in the CNS and a postganglionic neuron with a cell body in the periphery that innervates target tissues. The enteric nervous system (ENS) is an extensive, web-like structure that is capable of function independently of the remainder of the nervous system.[1][2] It contains over 100 million neurons of over 15 morphologies, greater than the sum of all other peripheral ganglia, and is chiefly responsible for the regulation of digestive processes.[3][4]
Activation of the SNS leads to a state of overall elevated activity and attention: the “fight or flight” response. In this process, blood pressure and heart rate increase, glycogenolysis ensues, gastrointestinal peristalsis ceases, etc.[5] The SNS innervates nearly every living tissue in the body. The PNS promotes the “rest and digest” processes; heart rate and blood pressure lower, gastrointestinal peristalsis/digestion restarts, etc.[5][6] The PNS innervates only the head, viscera, and external genitalia, notably vacant in much of the musculoskeletal system and skin, making it significantly smaller than the SNS.[7] The ENS is composed of reflex pathways that control the digestive functions of muscle contraction/relaxation, secretion/absorption, and blood flow.[3]
Presynaptic neurons of both the SNS and PNS utilize acetylcholine (ACh) as their neurotransmitter. Postsynaptic sympathetic neurons generally produce norepinephrine (NE) as their effector transmitter to act upon target tissues, while postsynaptic parasympathetic neurons use ACh throughout.[1][5] Enteric neurons have been known to use several major neurotransmitters such as ACh, nitrous oxide, and serotonin, to name a few.[8]
Structure and Function
Sympathetic Nervous System
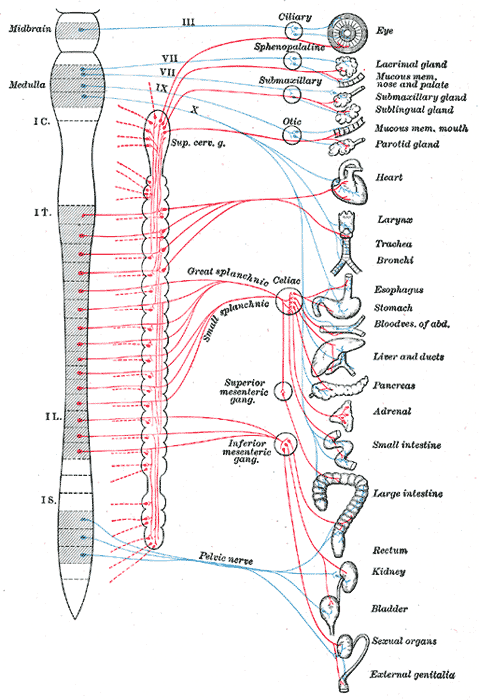
Sympathetic neurons have cell bodies located in the intermediolateral columns, or lateral horns, of the spinal cord. The presynaptic fibers exit the spinal cord through anterior roots and enter the anterior rami of T1-L2 spinal nerves and onto the sympathetic trunks via white rami communicantes. From here, the fibers may ascend or descend the sympathetic trunk to a superior or inferior paravertebral ganglion, respectively, pass to adjacent anterior spinal nerve rami via gray rami communicantes, or cross through the trunk without synapsing and continue through an abdominopelvic splanchnic nerve to reach prevertebral ganglia. Because of the central location of the sympathetic ganglia, presynaptic fibers tend to be shorter than their postsynaptic counterparts.[2][9]
Paravertebral ganglia exist as nodules throughout the sympathetic trunk, adjacent to the spinal column, where pre- and postganglionic neurons synapse. While the numbers may vary by individual, generally, there are three cervical, 12 thoracic, four lumbar, and five sacral ganglia. Of these, only the cervical have names of superior, middle, and inferior cervical ganglia. The inferior cervical ganglion may fuse with the first thoracic ganglion to form the stellate ganglion.[2][9]
All nerves distal to the paravertebral ganglia are splanchnic nerves. These convey afferent and efferent fibers between the CNS and the viscera. Cardiopulmonary splanchnic nerves carry the postsynaptic fibers destined for the thoracic cavity.
Nerves that will innervate the abdominal and pelvic viscera pass through the paravertebral without synapsing, becoming abdominopelvic splanchnic nerves. These nerves include the greater, lesser, least, and lumbar splanchnic nerves. The presynaptic nerves finally synapse in prevertebral ganglia that are closer to their target organ. Prevertebral ganglia are part of the nervous plexuses that surround the branches of the aorta. These include the celiac, aorticorenal, and superior and inferior mesenteric ganglia. The celiac ganglion receives input from the greater splanchnic nerve, the aorticorenal from the lesser and least splanchnic nerves, and the superior and inferior mesenteric from the least and lumbar splanchnic nerves. The celiac ganglion innervates organs derived from the foregut: distal esophagus, stomach, proximal duodenum, pancreas, liver, biliary system, spleen, and adrenal glands. The superior mesenteric ganglion innervates the derivatives of the midgut: distal duodenum, jejunum, ileum, cecum, appendix, ascending colon, and proximal transverse colon. Lastly, the inferior mesenteric ganglion provides sympathetic innervation to the structures developed from the hindgut: distal transverse, descending, and sigmoid colon; rectum and upper anal canal; as well as the bladder, external genitalia, and gonads.[10][11][12] For more information, see the relevant StatPearls article, at this reference.[13]
The two-neuron general rule for SNS and PNS circuits has several notable exceptions. Sympathetic and parasympathetic postganglionic neurons that synapse onto the ENS are functionally part of a three-or-more neuron chain. The presynaptic sympathetic fibers that are destined for the adrenal medulla pass through the celiac ganglia and synapse directly onto chromaffin cells. These unique cells function as postganglionic fibers that secrete epinephrine directly into the venous system.[1][2][14]
Postganglionic sympathetic neurons release NE that acts on adrenergic receptors in the target tissue. The subtype of the receptor, alpha-1, alpha-2, beta-1, beta-2, or beta-3, and the tissues in which they express influences the affinity of NE for the receptor.[15] For more information, see the StatPearls articles related to adrenergic receptors, at the following references.[16][17][18]
As stated, the SNS enables the body to handle stressors via the “fight-or-flight” response. This reaction primarily regulates blood vessels. Vessels are tonically innervated, and in most cases, an increase in sympathetic signals leads to vasoconstriction and the opposite of vasodilation. The exceptions include coronary vessels and those that supply the skeletal muscles and external genitalia, for which the opposite reaction occurs.[2] This contradictory effect is mediated by the balance of alpha and beta receptor activity. In a physiologic state, beta-receptor stimulation increases coronary vessel dilation, but there is blunting of this effect by alpha-receptor-mediated vasoconstriction. In a pathologic state, such as in coronary artery disease, alpha-receptor activity is enhanced, and there is the muting of beta-activity. Thus, the coronary arteries may constrict via sympathetic stimulation.[19] Sympathetic activation increases heart rate and contractile force, which, however, increases metabolic demand and is thus detrimental to cardiac function in compromised individuals.[20]
The SNS is constantly active, even in non-stressful situations. In addition to the aforementioned tonic stimulation of blood vessels, the SNS is active during the normal respiratory cycle. Sympathetic activation complements the PNS by acting during inspiration to dilate the airways allowing for an appropriate inflow of air.[2][21]
Additionally, the SNS regulates immunity through the innervation of immune organs such as the spleen, thymus, and lymph nodes.[15][22] This influence may up- or down-regulate inflammation.[23] Cells of the adaptive immune system primarily express beta-2 receptors, while those of the innate immune system express those as well as alpha-1 and alpha-2 adrenergic receptors.[15][24] Macrophages activate by alpha-2 stimulation and are suppressed by beta-2 adrenergic receptor activation.
The majority of postganglionic sympathetic neurons are noradrenergic, and also release one or more peptides such as neuropeptide Y or somatostatin. NE/neuropeptide Y neurons innervate blood vessels of the heart, thus regulating blood flow,[25] while NE/somatostatin neurons of the celiac and superior mesenteric ganglia supply the submucosal ganglia of the intestine and are involved in the control of gastrointestinal motility. The thinking is that these peptides serve to modulate the response of the postsynaptic neuron to the primary neurotransmitter.[1]
Peptides also have associations with cholinergic sympathetic postganglionic neurons. These neurons are most commonly found innervating sweat glands and precapillary resistance vessels in skeletal muscle and produce vasoactive intestinal polypeptide along with ACh. Calcitonin gene-related peptide, a potent vasodilator, has also been discovered in paravertebral sympathetic neurons.[26][27][28][29]
Parasympathetic Nervous System
Parasympathetic fibers exit the CNS via cranial nerves (CN) III, VII, IX, and X, as well as through the S2-4 nerve roots. There are four pairs of parasympathetic ganglia, and they are all located in the head. CN III, via the ciliary ganglion, innervates the iris and ciliary muscles of the eye. CN VII innervates the lacrimal, nasal, palatine, and pharyngeal glands via the pterygopalatine ganglion, as well as the sublingual and submandibular glands via the submandibular ganglion. CN IX innervates the parotid glands via the otic ganglion.[4] Every other presynaptic parasympathetic fiber synapses in a ganglion near or on the wall of the target tissue; this leads to the presynaptic fibers being significantly longer than the postsynaptic. The location of these ganglia gives the PNS its name: “para-” means adjacent to, hence, “parasympathetic.”[2]
The vagus nerve, CN X, makes up about 75% of the PNS and provides parasympathetic input to most of the thoracic and abdominal viscera, with the sacral parasympathetic fibers innervating the descending and sigmoid colon and rectum. The vagus nerve has four cell bodies in the medulla oblongata. These include the following[2][4][30][31]:
- Dorsal nucleus: provides parasympathetic output to the viscera
- Nucleus ambiguus: produces motor fibers and preganglionic neurons that innervate the heart
- Nucleus solitarius: receives afferents of taste sensation and that from viscera, and lastly
- Spinal trigeminal nucleus: receives information of touch, pain, and temperature of the outer ear, the mucosa of the larynx, and part of the dura
Additionally, the vagus nerve conducts sensory information from baroreceptors of the carotid sinus and the aortic arch to the medulla.[32]
As mentioned in the introduction, the vagus nerve is responsible for the “rest and digest” processes. The vagus nerve promotes cardiac relaxation in several aspects of function. It decreases contractility in the atria and less so in the ventricles. Primarily, it reduces conduction speed through the atrioventricular node. It is by this mechanism that carotid sinus massage acts to limit reentry in Wolff-Parkinson-White syndrome.[2] The other key function of the PNS centers around digestion. Parasympathetic fibers to the head promote salivation, while those that synapse onto the ENS lead to increased peristaltic and secretory activity.[4][33] The vagus nerve also has a significant effect on the respiratory cycle. In a nonpathological state, parasympathetic nerves fire during expiration, contracting and stiffening airways to prevent collapse. This function has implicated the PNS in the onset of postoperative acute respiratory distress syndrome.[2][21]
Due to the expansive nature of the vagus nerve, it has been described as an ideal “early warning system” for foreign invaders as well as for monitoring the body’s recovery. Up to 80% of vagal fibers are sensory and innervate nearly all major organs. Parasympathetic ganglia have been found to express receptors for interleukin-1, a key cytokine in the inflammatory immune response.[34] This, in turn, activates the hypothalamic-pituitary-adrenal axis and SNS, leading to the release of glucocorticoids and NE, respectively.[2] Studies have correlated inhibited vagal action through vagotomy and cholinergic inhibitors with significantly reduced, if not eliminated, allergic, asthmatic, and inflammatory responses.[7]
Postganglionic parasympathetic neurons release ACh that acts on muscarinic and nicotinic receptors, each with various subunits: M1, M2, and M3, and N1 and N2, with “M” and “N” standing for muscarine and nicotine, respectively.[5] The postganglionic ACh receptors and those on the adrenal medulla are N-type, while the parasympathetic effectors and sweat glands are M-type.[2] As in sympathetic neurons, several peptides, such as vasoactive intestinal peptide (VIP), Neuropeptide Y (NPY), and calcitonin gene-related peptide (CGRP) are expressed in, and released from, parasympathetic neurons.[27][28][35][36] For more information, see the StatPearls article on cholinergic receptors, here.[37]
Enteric Nervous System (ENS)
The ENS is composed of two ganglionated plexuses: the myenteric (Auerbach) and the submucosal (Meissner). The myenteric plexus sits in between the longitudinal and circular smooth muscle of the GI tract, while the submucosal plexus is present within the submucosa. The ENS is self-contained, functioning through local reflex activity, but often receives input from, and provides feedback to, the SNS and PNS. The ENS may receive input from postganglionic sympathetic neurons or preganglionic parasympathetic neurons.[1][38]
The submucosal plexus governs the movement of water and electrolytes across the intestinal wall, while the myenteric plexus coordinates the contractility of the circular and longitudinal muscle cells of the gut to produce peristalsis.[39]
Motility is produced in the ENS through a reflex circuit involving the circular and longitudinal muscles. Nicotinic synapses between interneurons mediate the reflex circuits.[39] When the circuit activates by the presence of a bolus, excitatory neurons in the circular muscle and inhibitory neurons in the longitudinal muscle fire producing a narrow section of bowel proximal to the bolus; this is known as the propulsive segment. Simultaneously, excitatory neurons in the longitudinal muscle and inhibitory neurons in the circular muscle fire producing the “receiving segment” of the bowel in which the bolus will continue. This process repeats with each subsequent section of the bowel.[40]
The ENS maintains several similarities to the CNS. As in the CNS, enteric neurons can be bipolar, pseudounipolar, and multipolar, between which neuromodulation via excitatory and inhibitory communication.[1] Likewise, ENS neurons use over 30 neurotransmitters that are similar to those of the CNS, with cholinergic and nitrergic transmitters being the most common.[39]
While much of this discussion has focused on the efferent functions of the ANS, the afferent fibers are responsible for numerous reflex activities that regulate everything from heart rate to the immune system. Feedback from the ANS is usually processed at a subconscious level to produce reflex actions in the visceral or somatic portions of the body. The conscious sensation of the viscera is often interpreted as diffuse pain or cramps that may correlate with hunger, fullness, or nausea. These sensations most commonly result from sudden distention/contractions, chemical irritants, or pathological conditions such as ischemia.[41]
Surgical Considerations
Horner syndrome is a mild, rare condition often presenting with unilateral ptosis, miotic, but a reactive pupil, and facial anhidrosis secondary to sympathetic nerve damage in the oculosympathetic pathway.[46] This damage may have a central cause such as infarction of the lateral medulla, or peripheral such as from damage secondary to thoracic surgery or from partial/total resection of the thyroid gland.[46][47] More centralized lesions tend to correlate with a constellation of symptoms that include Horner syndrome.[46] For more information, please see the associated StatPearls articles, here.[48][49]
Hyperhidrosis is a common disease characterized by excessive sweating, primarily of the face, palms, soles, and/or axilla. While the cause of primary hyperhidrosis is not fully understood, it has been attributed to increased cholinergic stimulation. Treatment can be either clinical or surgical.[50] Treatment on the clinical side centers on anticholinergic agents such as topical glycopyrrolate or oral oxybutynin, or less commonly, alpha-adrenergic agonists such as clonidine, calcium channel blockers, or gabapentin.[50][51] The most common and permanent surgical technique is the resection, ablation, or clipping of the thoracic sympathetic chain. While permanent, the procedure may lead to compensatory hyperhidrosis in a small number of individuals. These hyperhidrosis symptoms are the same if not more severe than prior to the procedure due to possible overcompensation by the hypothalamus. Research has demonstrated that surgical reconstruction of the sympathetic chain can reduce this compensatory response.[52]
Clinical Significance
Due to the extensive nature of the autonomic nervous system, it can be affected by a wide range of conditions. Some of these include[53][54][55]
- Inherited
- Amyloidosis
- Fabry disease
- Hereditary sensory autonomic neuropathy
- Porphyrias
- Acquired
- Diabetes mellitus
- Uremic neuropathy/chronic liver diseases
- Vitamin B12 deficiency
- Toxin/drug-induced: alcohol, amiodarone, chemotherapy
- Infections: Botulism, Chagas disease, HIV, leprosy, Lyme disease, tetanus
- Autoimmune: Guillain-Barre, Lambert-Eaton myasthenic syndrome, rheumatoid arthritis, Sjogren, systemic lupus erythematosus
- Neurological: multiple system atrophy/Shy-Drager syndrome, Parkinson disease, Lewy body dementia
- Neoplasia: Brain tumors, paraneoplastic syndromes
Likewise, autonomic neuropathy can present in nearly any system. Orthostatic hypotension is the most common autonomic dysautonomia, but numerous other, less understood, findings may present[53]
- Cardiovascular
- Fixed heart rate
- Postural hypotension
- Resting tachycardia
- Gastrointestinal
- Dysphagia
- Gastroparesis; nausea, vomiting, abdominal fullness
- Constipation
- Genitourinary
- Pupillary
- Absent/delayed light reflexes
- Decreased pupil size
- Sexual
- Erectile dysfunction
- Retrograde ejaculation
- Sudomotor
- Anhidrosis
- Gustatory sweating
- Vasomotor
- Cold extremities (due to loss of vasomotor responses)
- Edema (due to loss of vasomotor tone and increased vascular permeability)
The most prevalent symptoms of orthostatic hypotension are lightheadedness, tunnel vision, and discomfort in the head, neck, or chest. It may present concomitantly with supine hypertension due to increased peripheral resistance, which induces natriuresis, exacerbating orthostatic hypotension. There are numerous other, more benign stimuli that may either lower blood pressure (standing, food, Valsalva, dehydration, exercise, hyperventilation, etc.) or raise blood pressure (lying supine, water ingestion, coffee, head-down tilt, hypoventilation, etc.).[53]
Orthostatic hypotension evaluation is commonly done through orthostatic testing via repeated blood pressure and heart rate readings in supine and standing positions, but also through the use of the tilt-table test. However, the advantage of this latter test is minimal over the orthostatic test, with the main benefit being safety and convenience to the patient.[53]
Patients with dysautonomia are prone to hypotension during anesthesia[56]. This issue may be appropriately managed with low doses of phenylephrine, an alpha-1 agonist. Likewise, supine hypertension may be controlled with transdermal or IV nitrates.[53][57][58]
The sympathetic nervous system is well known to play a role in nociception. There are suggestions that the ANS has a regulatory inhibitory effect on pain, the loss of which creates a positive feedback circuit leading to hyperexcitability of nociceptive nerve fibers. The fact that the effect of sympathetic blocks often persists beyond the duration of the anesthetic agents administered supports this hypothesis.[59] Local sympathetic nerve blocks have been used to treat a variety of less-common pain conditions including complex regional pain syndrome, phantom limb pain, and herpetic pain. Likewise, visceral pain is treatable through a more central approach through a celiac plexus block. Due to the wide array of functions performed by the ANS, blocks are reserved for intractable pain, uncontrolled by more conventional analgesics.[59] See the related StatPearls articles for more information, here.[60][61][62]
Most conditions related to the ENS are congenital in origin and present during early childhood.[44] Enteric neurons function to relax intestinal smooth muscle. Their absence leaves the bowel tonically contracted, obstructing the bowel. Presenting complaints often consist of gastroesophageal reflux, dyspeptic syndromes, constipation, chronic abdominal pain, and irritable bowel syndrome. A notable life-threatening disorder of the ENS is Hirschsprung disease. This condition is a failure of embryologic ENS cells to colonize the distal bowel. When the ENS is missing (aganglionosis) or maldeveloped, children experience early constipation, vomiting, eventual growth failure, and possible death.[3][44] Studies have identified six genes in a causal relationship with Hirschsprung disease.[44] Down syndrome is the most common genetic disorder that predisposes an individual to Hirschsprung disease despite the fact that no genes related to ENS development have been identified on chromosome 21.[3]