Introduction
Containing the fibularis longus muscle (FLM) and fibularis brevis muscle (FBM), common fibular and superficial fibular nerves, and branches of the anterior tibial and fibular arteries, the lateral leg compartment is one of the four compartments of the leg.[1][2] Structures with the term “peroneal” have been replaced with “fibular” for anatomical accuracy. The primary function of the FLM and FBM is foot and ankle eversion, with a secondary function of foot and ankle plantarflexion and maintenance of the foot transverse and lateral arches.[3][4] The anterior tibial and fibular arteries, and superficial fibular nerve provide neurovascular supply to the lateral leg compartment.[1][5] The lateral leg compartment is highly variable morphologically and may have variants such as the fibularis quartus (FQ) muscle, fibularis digit quanti (FDQ), variable insertions of the fibularis brevis tendon (FBT), and supernumerary fibularis muscle bellies.[6][7][8] Additionally, variations in the spitting of the common fibular nerve into the superficial fibular nerve and deep fibular nerve have been reported, as well as variations in the course and splitting of the superficial fibular nerve.[2][9][2] Due to the compact nature of the lateral leg compartment, it is prone to compartment syndrome, with 13.9% to 34.4% of leg pain being associated with chronic exertional compartment syndrome (CECS).[10] Additionally, patients may sustain acute compartment syndrome from direct trauma, inversion ankle sprains, prolonged surgical lithotomy position, and other general medical causes.[11][12][13][14] Compartment syndrome can have devastating consequences if not managed appropriately, including ischemic necrosis of the lateral leg compartment structures, causing leg dysfunction and potentially leg loss.[15] Therefore, it is important to be aware of the anatomical makeup of the lateral leg compartment, in order to understand potential pathologies and their implications on the function of the lower extremity.
Structure and Function
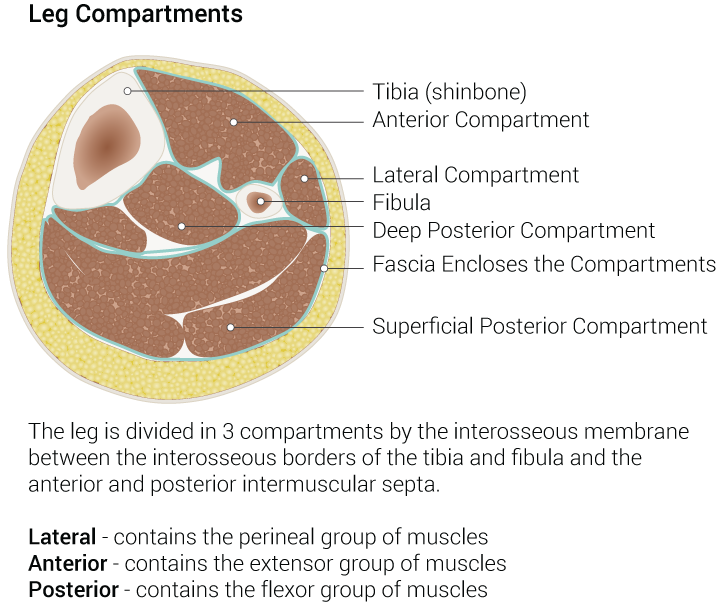
The muscles in the leg are divided into four compartments, the anterior, lateral, posterior superficial, and posterior deep, by intermuscular septa.[2] The lateral leg compartment is isolated from the other leg compartments by the deep (crural) fascia of the leg laterally, the fibula medially, the anterior intermuscular septa anteriorly, and the posterior intermuscular septa posteriorly.[1][16] [1]The lateral leg compartment is narrow and contains the FLM, FBM, the common and superficial fibular nerves, and branches of the anterior tibial artery and fibular artery.[1]
The primary function of the FLM and FBM is eversion of the foot and ankle, while their secondary function is ankle plantarflexion. The fibularis muscles act as antagonists to foot and ankle inversion, which is why they may be injured in inversion ankle sprains.[3] Additionally, the fibularis longus tendon’s (FLT’s) course along the plantar aspect of the foot, crossing the transverse and lateral arches of the foot, and inserting on the base of the first metatarsal, cause it to be important in maintaining the transverse and lateral arches, and in lowering the first metatarsal during foot pronation.[4]
The function of the superficial fibular nerve, anterior tibial artery, and fibular artery include providing a neurovascular supply to the lateral leg compartment.
Embryology
The formation of a human limb is a complex process embryologically, with numerous molecular signaling pathways and factors being involved. Limbs arise from limb buds, which are small buds of undifferentiated lateral plate mesoderm cells surrounded by ectoderm cells. The lateral plate mesoderm cells give rise to the connective tissues of the limb, such as bone, cartilage, muscle, and tendon. Cells that migrate from the somites to the lateral plate mesoderm also gives rise to muscle cells. The limb buds are located bilaterally, on two distinct levels of the anterior-posterior (AP) axis. The limb buds located by the cervical-thoracic transition give rise to the upper limbs (forelimbs), while the limb buds located by the lumbar-sacral transition give rise to the lower limbs (hindlimbs). Specific genes, Pitx1 and Hox, have been found to determine the identity of limbs; whether they will be forelimbs or hindlimbs. Pitx1 has been found in the lateral plate mesoderm, and regulates Tbx4 expression, helping to form a hindlimb identity. Hox4 and Hox5 regulate Tbx5 expression and help to form a forelimb identity. Hox genes also contribute to the development of the interlimb region, including the vertebral column.[17]
Signaling regions in the limb buds control the growth of the lateral plate mesoderm in three different positional planes: dorso-ventral (from the back of the hand to the palms), AP (from the thumb to the 5th digit), and the proximo-distal (from the shoulder to the fingertips).[17]
- Dorso-ventral growth: dorsal growth is controlled by the dorsal ectoderm, which produces Wnt7a, while ventral growth is controlled by the ventral ectoderm, which produces bone morphogenic proteins (BMPs).
- AP growth: controlled by the mesoderm cells by the posterior aspect of the limb bud, known as the zone of polarizing activity (ZPA), which produces the Sonic hedgehog protein (Shh) controlling AP growth. Additionally, Hox genes are involved in AP growth of the forelimbs, with Hox5 contributing to anterior forelimb growth and Hox9 contributing to posterior forelimb growth.
- Proximo-distal growth: controlled by the apical ectodermal ridge (AER), which produces fibroblast growth factors (FGFs). The apical ectodermal ridge is where the dorsal and ventral ectoderm meets and is a thickening of the ectoderm.
Blood Supply and Lymphatics
The popliteal artery branches into the anterior tibial artery and the tibiofibular trunk, with the tibiofibular trunk branching into the fibular artery and the posterior tibial artery. The anterior tibial and fibular arteries provide blood supply to the lateral leg compartment structures.[1]
The blood supply of the lateral leg compartment is described below going proximally to distally[1]:
- The anterior tibial artery gives off the superior lateral fibular artery and the inferior lateral fibular artery, which supply the proximal fibularis muscles.
- The fibular artery gives off branches which supply the distal portions of the fibularis muscles.
- Small branches arise from the anterior tibial artery and fibular artery to supply the proximal fibularis tendons, while small branches of the anterior tibial artery supply the distal fibularis tendons.
Nerves
The common fibular nerve normally splits into the superficial fibular nerve and the deep fibular nerve within the fibular tunnel, which is formed laterally by the deep fascia, medially by the fibular head, and anteriorly and posterior by the origin of the fibularis longus off the fibular head and fibular shaft.[13][18][13]
The superficial fibular nerve runs within the FLM, providing a motor function to the FLM and FBM. Approximately 12cm above the ankle joint, the superficial fibular nerve pierces through the deep (crural) fascia of the leg and splits into the medial dorsal cutaneous nerve (MDN) and intermediate dorsal cutaneous nerve (IDN).[5] The MDN and IDN provide sensory input for the lateral leg and dorsum of the foot, except between the first web-space.[2][9][5][9]
The deep peroneal nerve supplies the muscles of the anterior leg compartment.[2]
Muscles
There are normally two muscles in the lateral leg compartment, the FLM and FBM.[7] The FLM is the more superficial and longer of the two muscles. It originates on the superolateral surface of the fibular head, proximal two-thirds of the fibular shaft, the lateral tibial condyle, and the intermuscular septum.[3] The FBM is located deep and medial to the FLM, and it is shorter than the FLM. The FBM originates on the lower two-thirds of the lateral fibular shaft and the anterior intermuscular septum. The muscle fibers of the FLM descend vertically and become the FLT along the midpoint of the lateral leg compartment. The muscle fibers of the FBM also descend vertically and become the FBT. THE FLT and FBT run together in the common fibular synovial sheath along the posterior aspect of the lateral malleolus, within the retro-malleolar groove. The FLT runs posterior to the FBT in the common fibular synovial sheath. The common fibular synovial sheath spans 4cm proximal and 1cm distal to the lateral malleolus. The common fibular synovial sheath is secured within the retro-malleolar groove by the superior fibular retinaculum. As the common fibular synovial sheath travels behind and under the lateral malleolus, it eventually splits into separate synovial sheaths for the FLT and FBT at the fibular trochlea (a bony prominence of the calcaneus). The inferior fibular retinaculum originates on the lateral sinus tarsi and attaches to the fibular trochlea and lateral calcaneus, and it secures the tendon synovial sheaths at the fibular trochlea. Its attachment to the fibular trochlea forms two separate compartments for the FLT and FBT, with the FBT synovial tendon sheath running in the compartment superior to the fibular trochlea, and the FLT synovial tendon sheath running in the compartment inferior to the fibular trochlea.
The FBT inserts onto the lateral aspect of the tuberosity at the base of the 5th metatarsal.[7]
The FLT travels inferolateral along the calcaneus, and when it reaches the cuboid bone, the FLT tendon may have an os peroneum (a fibrocartilaginous nodule) that articulates with the cuboid, forming the peroneocuboid joint. The presence of this joint is variable. Distal to the cuboid, the FLT makes a sharp turn, passing between the calcaneal tuberosity and the base of the 5th metatarsal, traversing the cuboid tunnel anteromedially, and entering the plantar aspect of the foot. From there, the FLT inserts onto the base of the first metatarsal, with its synovial tendon sheath ending right before it inserts. Variable insertions of the FLT include the medial cuneiform, the plantar aspect of the base of the 2nd- 5th metatarsals, and the first dorsal interosseous muscle.[18]
Physiologic Variants
Musculoskeletal Variants
FBM Insertion Variations
While the origin of the FBM is consistent, variations in its insertion exist:[7]
- Type I: One single distal attachment exists, at the lateral aspect of the tuberosity on the base of the 5th metatarsal. This is the most common, with a prevalence of 70%.
- Type II: Two distal attachments exist, one with the main tendon, and one accessory band. The main tendon always inserts on the lateral aspect of the tuberosity on the base of the 5th metatarsal, with variation in the insertion of the accessory band.
- Type IIa: The accessory band inserts onto the dorsal aspect of the base of the 5th metatarsal. This accessory band inserts onto the same location as the fibularis tertius tendon, but the two structures are separate.
- Type IIb: The accessory band further splits into a medial and lateral band, with the medial band inserting onto the middle of the 5th metatarsal, and the lateral band inserting onto the dorsal surface of the base of the 5th metatarsal.
- Type IIc: The accessory band further splits into a medial and lateral band, with the medial band fusing with the fibularis tertius tendon, and the lateral band inserting on the dorsal aspect of the base of the 5th metatarsal. This is the rarest type of FBT insertion variant.
Fibularis Digit Quanti (FDQ)
Originating as a small slip from the FBT, the FDQ is a small muscle with a wide range of reported prevalence, from 15.5% to 59.7%. The FDQ is innervated by the superficial fibular nerve. The FDQ has three different insertions, which are used to classify the muscle variant:[7]
- Type I: the FDQ inserts on the base of the fifth digit proximal phalanx.
- Type II: the FDQ inserts on the extensor aponeurosis of the fifth digit.
- Type III: the FDQ inserts onto one of the extensor digitorum longus tendons.
Fibularis Quartus (FQ)
The FQ is a common accessory muscle in the ankle, with a prevalence of 4.3 to 21.7%. Possible origins of the FQ are the distal FBM (most common) and the distal fibula. The possible insertions of the FQ are the retrofibular trochlea of the calcaneus (most common), the base of the 5th metatarsal, and the cuboid bone. The FQ assists with supporting the lateral edge of the foot during eversion, as well as lateral foot and ankle stabilization. The presence of an FQ can increase a patient’s risk of FBT degeneration through stenosis of the lateral leg compartment, which may cause lateral ankle instability. On the contrary, the FQ can be used to reconstruct a ruptured superior fibular retinaculum. Therefore, it is important for clinicians to be aware of the presence of the FQ, as it may be a cause of chronic lateral ankle instability, as well as be used in surgical reconstruction.[6] In patients with a symptomatic FQ, orthotics can be utilized, and if those are ineffective, surgical ablation of the FQ can be performed.[19]
Supernumerary Fibularis Muscle Bellies
Although a very rare occurrence, patients may also have the presence of double fibularis muscle bellies, each with their own tendon. The FLM can have its normal origin, then divide into one large superficial muscle belly, with its normal FLM and FLT structure and insertion. It can also have a smaller deep muscle belly, which became a tendon after 8.6cm, and inserted into the lateral calcaneus directly inferior to the lateral malleolus. The point where the FLM split into two muscle bellies was 22.1cm proximal to the lateral malleolus. Additionally, the FBM originated in its normal location, and split into two muscle bellies, with one being superficial and lateral, and one being deep and medial. The split occurred 8.2cm proximal to the lateral malleolus. The superficial and lateral muscle belly followed the normal anatomy of the FBM and FBL, while the deep and medial muscle belly had a tendinous insertion on the lateral calcaneus, 1.4cm inferior to the lateral malleolus. There was 1.4cm of space between the insertions of the deep muscles of the FLT and FBT on the calcaneus. In conclusion, supernumerary fibularis muscles are an anatomic variant that can be found, and it is important to be aware of their occurrence while performing reconstruction surgeries involving the lateral leg compartment.[8]
Neural Variants:
It has been reported that the common fibular nerve may split into the superficial and deep fibular nerve within the popliteal fossa, not by the head of the fibula in the fibular tunnel. While their anatomical splitting is altered, the two branches maintain innervation to their usual structures.[2]
The superficial fibular nerve may not be located in the lateral leg compartment in some patients, but rather, it may be found in the anterior leg compartment in approximately 28% of Indian patients.[20]
The superficial fibular nerve may have variations in where it pierces through the dorsal (crural) fascia and variations in its splitting into the MDN and IDN. There are three variations which have been reported in the literature:[9]
- Type 1: The superficial fibular nerve pierces through the dorsal (crural) fascia then splits into the MDN and IDN. This is the normal splitting pattern.
- Type 2: The superficial fibular nerve bifurcates into the MDN and IDN, and the MDN and IDN individually pierce through the dorsal (crural) fascia.
- Type 3: The superficial fibular nerve pierces through the dorsal (crural) fascia, and courses along the normal MDN pathway, never splitting to form the IDN.
Surgical Considerations
Compartment Syndrome Surgery
Patients who have acute compartment syndrome or CECS that has not responded to conservative treatment require surgical intervention to relieve the symptoms associated with their condition and to prevent progressive damage. Patients with CECS of their lateral compartment have high surgical success rates at an average of two years following surgery, with a systematic review finding that 90% of patients had successful lateral compartment surgery. However, the success rate of surgical intervention for CECS as a whole (with different compartments being affected), was lower at 66%. Therefore, surgical intervention in patients who have lateral compartment CECS is more efficacious than surgical intervention in other lower leg compartments.[10]
Surgical Techniques:[10]
- Compartment-Specific Open Fasciotomy
- 86% of all surgeries for CECS were compartment-specific open fasciotomies, with the anterior compartment being the most commonly released compartment.
- Fasciotomy with Partial Fasciectomy
- 12% of all CECS operations were a fasciotomy with a partial fasciectomy.
- Endoscopic Fasciotomy
- 2% of all CECS surgeries were done endoscopically, with no significantly better outcomes over the open techniques.
Many different incision locations and techniques are used. It is imperative that incisions are placed in locations that provide access to the fascial compartment that is being treated, and that incisions minimize iatrogenic neurovascular injury. It has been shown that while performing a fasciotomy of the anterior and lateral compartments, ultrasound can be utilized to locate the intermuscular septum separating the anterior and lateral compartments, providing a good location for surgeons to place their incision directly over the septum, in order to prevent damage to neurovascular structures in the area. Limitations of US include that it is not effective in patients with increased BMIs, especially patients who are obese, and the accuracy of US is heavily operator-dependent. Therefore, when properly trained individuals are utilizing US to locate the intermuscular septum between the anterior and lateral leg compartments, it can be an effective guide on the surgical incision placement.[21]
With a complication rate of 13%, it is important to be aware of the complications of surgical intervention for compartment syndrome. They include:[12][10][12]
- Hemorrhage
- Nerve damage and altered sensation
- Infection
- Damage to the venous and arterial structures
- Deep vein thrombosis (DVT)
- Skin breakdown
- Nerve entrapment
- Chronic pain
Patients, especially athletes, should be warned that recurrence of compartment syndrome is possible. Recurrence rates between 0% and 44.7% have been reported. Recurrence may also result from an incomplete fascial release, excessive scarring, or inadequate rehabilitation. A second-time fasciotomy should be open and more extensive than the first one.[10]
Surgical Precautions
Well-leg compartment syndrome (WLCS) occurs in patients who have been in the lithotomy position for a prolonged time. It is an acute compartment syndrome in the anterior and lateral compartments.[12][13][12] WLCS occurs in 1/3500 patients who have surgery performed in the lithotomy position, and its early detection can be limb-saving.[12] It may be caused by hip flexion and knee flexion leading to impaired venous drainage and increased lower limb venous pressure, similar to the hip flexion and knee flexion that horseback riders find themselves in, which also causes compartment syndrome.[15] When surgery must be performed in the lithotomy position, the hip and knee should not be flexed more than 90 degrees, the hip shoulder not be abducted more than 45 degrees, and the hip should be in neutral rotation. Additionally, when the surgical duration is expected to exceed four hours, the lithotomy position should be relieved for a few minutes every two hours, to prevent excessive lower leg compression.[12]
It is also important to be careful when performing surgical operations that utilize lower limb compression stockings for DVT prophylaxis. Acute compartment syndrome of the lateral compartment has been reported in a patient who had compression stockings on his leg during a surgical procedure for the patient’s foot and ankle. The patient returned two days following the operation with symptoms of acute compartment syndrome and needed surgical intervention. Therefore, proper fitting compression stockings should be utilized for DVT prophylaxis to minimize the risk of acute compartment syndrome.[22]
When surgical interventions are being performed for conditions around the fibular head and popliteal region, it is important to be aware of the anatomically variable splitting of the common fibular nerve, in order to avoid iatrogenic injury.[2]
Clinical Significance
Compartment Syndrome
Due to the narrow make-up of the lateral leg compartment, it is prone to compartment syndrome.[1] Typically, compartment syndrome in the leg occurs in the anterior compartment or in multiple compartments; it is usually not isolated to the lateral compartment.[23] Compartment syndrome is the increase of pressure within one of the four fascial compartments within the lower leg, decreasing capillary perfusion to structures within the compartment, causing tissue ischemia.[11][24] Muscle and neural tissue are sensitive to ischemia, which is reflected in the early occurrence of neuromuscular symptoms such as pain (usually very serve and out of proportion, especially with passive inversion of the ankle), paresthesia, compartment firmness, and weakness.[15] Vascular symptoms such as pallor, pulselessness, and edema can also occur.
The 5 P’s mnemonic can be used to remember the symptoms of compartment syndrome:
- Pain
- Pallor
- Paresthesia
- Pulselessness
- Paralysis
When ischemia occurs for a prolonged time, necrosis can occur, resulting in the replacement of muscle with fibrous scar tissue (Volkmann’s Ischemic Contracture). Rhabdomyolysis may also occur, which can cause acute renal injury and cardiac arrhythmias.[15] Therefore, swift diagnosis and treatment of compartment syndrome are essential to prevent irreversible damage. The diagnosis of compartment syndrome is a clinical diagnosis, utilizing an adequate history, physical examination, intracompartmental pressure (ICP), ultrasound (US), and magnetic resonance imaging (MRI). Surgical intervention for patients who need it should not be delayed by the lack of ICP or MRI availability, as “time is muscle.”[25]
Classification of Compartment Syndrome
Acute Compartment Syndrome
- Traumatic causes, which are defined as experiencing a direct contact injury to the lateral aspect of the leg, include crush injuries and contusions.[11]
- Atraumatic causes include non-contact inversion ankle sprains (which may cause the FLM to tear and hematoma formation, which compresses the lateral compartment), horse-back riding (when the height of the stirrups is too high, the ankle goes into more dorsiflexion than usual, which increases lateral compartment pressure), snowboarding (when utilizing boots that are too large, which can cause the lower leg to come into frequent contact with the higher part of the boot). [11][26][15][27][15]
- General medical causes: diabetes mellitus may cause an accumulation of fluid in muscle tissue, leading to idiopathic muscle infarction and increase of compartment pressure, which causes an acute compartment syndrome. Hypothyroidism, nephrosis, influenza virus-induced myositis, bleeding disorders, leukemic infiltration, and ganglion cysts of the tibiofibular joint (proximal) have all been reported to contribute to acute compartment syndrome.[14]
- Acute compartment syndrome needs prompt surgical treatment to relieve elevated compartment pressures and prevent muscle necrosis.
Chronic (Exertional) Compartment Syndrome (CECS)[25]
- Patients often have an insidious exercise-induced lateral leg pain, which may be associated with weakness, tightness, and cramping. Pain is usually relieved with rest. Patients are typically young and active, although CECS has also been reported in individuals performing daily activities like walking. To help diagnose CECS, patients typically have an ICP performed while resting, and then immediately after exercising on a treadmill.
- Approximately 36% of all CECS cases are isolated in the lateral compartment, with the other 64% involving multiple compartments, with the anterior and lateral compartments being the most commonly involved together (42% of total CECS cases).
- When patients have lateral compartment CECS, two-thirds of them have it bilaterally.
- Conservative treatment with rest, physical therapy, and cryotherapy are typically tried when CECS is diagnosed. If that fails, surgical intervention is recommended.
Muscular Injury
Injuries to the lateral leg compartment muscles normally involve the distal tendons of the fibularis muscles, with a prevalence of 11-37% in cadavers, which makes it a common occurrence. Additionally, in patients who have surgical correction of ankle instability, 30% of them have tears in the distal fibularis tendons. The FBM is more likely to be injured than the FLM, with tears of the proximal fibularis longus musculotendinous junction being a very rare occurrence. They can occur from an inversion ankle sprain mechanism, or from direct trauma to the proximal lateral leg compartment. Tears of the proximal fibular longus musculotendinous junction are commonly associated with lateral leg compartment syndrome, with the majority of the published case-studies reporting both conditions simultaneously. Therefore, it is important to keep lateral leg compartment syndrome on the differential diagnosis if a proximal fibularis longus musculotendinous tear is suspected. If patients have clinical symptoms of compartment syndrome with a proximal fibularis longus musculotendinous tear, the majority of them need to be corrected surgically. In other cases, patients may complain of numbness or tingling along the distribution of the common fibular nerve and superficial fibular nerve, along with swelling in the lateral leg compartment, which may be caused by compression of the nerves secondary to hemorrhage of the damaged structures, or from the traction of the nerves. Without the presence of other symptoms of lateral leg compartment syndrome, patients with these symptoms may be treated conservatively with a controlled ankle motion (CAM) boot and physical therapy, and achieve good outcomes. It is very important to use sound clinical judgment when managing patients with proximal fibularis longus musculotendinous junction injuries.[3]
Although rarely reported in the literature, patients may also have ruptures of the origin of their EPM, which may cause lateral compartment syndrome and isolated deep fibular nerve palsy. Patients with isolated deep fibular nerve palsy can present with drop foot since the deep fibular nerve normally supplies the tibialis anterior, which performs ankle dorsiflexion. Typically caused by lumbar disc herniation, if patients present with drop foot, along with symptoms of a proximal EPM tear, it is important to keep lateral compartment syndrome causing deep fibular nerve palsy on the differential diagnosis.[13]
Procedural Considerations
In patients who have pathologies of the dorsum of the foot and need anesthetization of the dorsal aspect of the foot through the superficial fibular nerve, it is important for clinicians to be aware of the anatomically variable splitting of the common fibular nerve in the popliteal fossa, so the superficial fibular nerve can be targeted appropriately.[2]