Introduction
Around 6,000 years ago, laboratory medicine began with the analysis of human urine as uroscopy, which later became termed urinalysis. The word "uroscopy" derives from two Greek words: "ouron," which means urine and "skopeoa," which means to 'behold, contemplate, examine, inspect'. Ancient physicians spoke of urine as a window to the body's inner workings and reflected different diseases. For instance, Hindu civilizations recognized a "sweetness" in certain people's urine, which attracted black ants.[1] Hippocrates (460–355 BC) hypothesized that urine was a filtrate of the humors in the body, originating from the blood filtered through the kidneys. In Aphorisms, he described bubbles on the surface of fresh urine as a sign of long-term kidney disease and associated urinary sediment with fever.[2] Galen used the phrase "diarrhea of the urine" to describe excessive urination.[3] Theophilus Protospatharius, a seventh-century physician who wrote the first manuscript focused exclusively on urine called "De Urinis", determined heating urine would precipitate proteins, documenting proteinuria as a disease state.[4] Ismail of Jurjani, an eleventh-century physician, acknowledged food and aging altered urine composition and was the first to propose 24 hours urine collection.
By the late 12th-century, a French scholar named Gilles de Corbeil taught and classified 20 different types of urine, recording differences in urine sediment and color. De Corbeil also introduced the "matula," a glass vessel in which a physician could assess color, consistency, and clarity.[5] In 1630, Nicolas Fabricius de Peiresc, a French astronomer and naturalist, did the first microscopic description of urine crystals as "a heap of rhomboidal bricks." [6] Posteriorly, in the early-mid 1800s, Richard Bright, an English physician, pioneered the field of kidney research leading him to be ultimately recognized as the "father of nephrology." These few examples illustrate how urinalysis was the first laboratory test developed in the history of medicine, how it has been persistently used for several thousand years, and how it continues to be a formidable and cost-effective tool to obtain crucial information for diagnostic purposes.[7]
Specimen Requirements and Procedure
Urine is an unstable fluid; it changes composition as soon as it is eliminated through micturition.[7] Accurate collection, storage, and handling are crucial to maintaining the sample’s integrity.
Urine samples collected from the first void or “morning urine” are considered the best representative for testing. The urine accumulated overnight in the bladder is more concentrated, thus provides an insight into the kidneys’ concentrating capacities and allows for the detection of trace amounts of substances that may not be present in more diluted samples.[7][8] However, other types of urine specimens may be ordered according to specific purposes (randomly, 2-hours postprandial, 24-hour collection). Furthermore, urine should be ideally examined within the first hour after the collection due to the instability of some urinary components (cells, casts, and crystals). If not possible, the sample should be refrigerated at 4 degrees C for up to 24 hours, which will slow down the decomposition process. Any specimen older than 24 hours cannot be used for urinalysis.[7][8]
There are two methods to obtain a urine specimen: non-invasive and invasive techniques. Spontaneous voiding is the main non-invasive technique, although other strategies may be used in children who cannot yet control their voiding (i.e., bag urine). In contrast, urethral catheterization and suprapubic bladder puncture are the two invasive procedures described to date. The fundamental principle of either technique is to obtain a specimen without external contamination.
Non-invasive Techniques
Spontaneous voiding is the simplest and most commonly used method in clinical practice. Before collecting the sample, health personnel should be provided clear instructions to patients in order to minimize the chance of contamination from penile/vaginal microbiota (“clean-catch” method). Most urine collection kits include a sterile container with a lid and sterile moist towels to wipe the urethral area before collection; if not, cotton wool or toilet tissue and/or tap water with soap may be used. Traditionally, male patients are instructed to retract the foreskin and clean the glans of the penis before urinating. Consequently, females should clean the labia and urethral meatus as well before collection. Currently, it is under debate the need for these standard precautions, and even many areas no longer perform them.[7][8][9]
Subsequently, the patient should first void a small amount of urine into the toilet and afterward position the container mid-stream in the flow of urine. Approximately, only 15 mL to 30 mL of urine is sufficient for accurate analysis, so, in most cases, patients should be advised not to fill the containers to their full capacity. Finally, the container is closed with careful precautions not to contaminate its lid or rim, and the patient may finish urinating in the toilet, bedpan, etc. The sample must be labeled before or immediately following collection, and it should not be on the lid.[7][8]
Invasive Techniques
Invasive urine collection is warranted when patients cannot cooperate, have urinary incontinence or external urethral ulceration that increases contamination risk. Both of these techniques pose a risk for the inoculation of pathogens, thus causing urinary tract infections.
Urethral catheterization involves a small French urinary catheter passed through the urethral meatus after the previous cleansing with proper equipment. Depending on the catheter, personnel may or may not need a sterile syringe. In cases where patients already have a urinary catheter placed, the specimen should never be taken from the catheter bag as it is considered contaminated.
Suprapubic needle aspiration of the bladder is both the most invasive and uncomfortable procedure of all previously mentioned and may generate false-positive results (protein, red and white cells) as a consequence of blood contamination. They are generally reserved for situations where samples may not be obtained or are persistently contaminated through previous methods, which usually occurs in small children. The main advantage is that, by bypassing the urethra, it minimizes the risk of obtaining a contaminated sample.
Before the procedure, trained personnel must identify the bladder by examination. If not distinguished, it is recommended to hydrate the patient and wait until correct identification or use ultrasound guidance if available. After proper cleaning with an antiseptic solution and anesthetizing the skin located approximately 5 cm above the pubic symphysis, a small needle (i.e., 22-gauge spinal needle x 10 cm in adults) is inserted approximately at 60 degrees at the point identified previously. The needle is directed slightly caudal or cephalic in adults or children, respectively, according to the anatomic location. Usually, the needle will enter the abdominal bladder after advancing it approximately 5 cm in adults. Finally, attempt to aspirate using a sterile syringe. If a sample is not obtained, advance the needle applying continuous suction on the syringe. If unsuccessful after an additional 5 cm in adults, withdraw the needle and repeat the procedure. If unsuccessful, personnel should seek help from a specialist or use ultrasound guidance if not previously done.[7][8][10]
Diagnostic Tests
A complete urinalysis consists of three components or examinations: physical, chemical, and microscopical.
- Physical examination describes the volume, color, clarity, odor, and specific gravity.
- Chemical examination identifies pH, red blood cells, white blood cells, proteins, glucose, urobilinogen, bilirubin, ketone bodies, leukocyte esterase, and nitrites.
- Microscopic examination encompasses the detection of casts, cells, crystals, and microorganisms.
Interfering Factors
The following factors may alter the results of a urine sample analysis:
- Light and Temperature: If exposed for a considerable period of time, bilirubin and urobilinogen may decompose due to their instability under these conditions. Additionally, room temperature favors the growth of microorganisms, such as bacteria.
- Bacterial Growth: Contamination of the sample or pathogenic bacteria may produce a variety of inaccurate results. For example, they may produce a false-positive blood reaction and affect the specimen's pH towards acidity or alkaline.
- Alkaline pH: This concentration may show false-positive results regarding the presence of protein.
- Glucose: If present in the sample, it may be metabolized by microorganisms and cause a decrease in the sample's pH.
- Contrast Agents: May produce false-positive results of specific gravity.
- Exercise: May alter the specific gravity and electrolyte concentration of the sample.
- Foods and Drugs: May alter the urine's color, odor, or pH value. Examples include, but are not limited to, red beets, blackberries, rhubarb, food coloring (e.g., aniline), ibuprofen, chloroquine, metronidazole, deferoxamine, nitrofurantoin, phenytoin, rifampicin, phenolphthalein, phenothiazines, and imipenem/cilastatin.
- Preservatives: Although used occasionally, they can alter the accuracy of the results. Some examples include:
- Thymol: May generate false-positive reactions for albumin.
- Formaldehyde: May cause false-positive results for leukocyte esterase, peroxidase reaction, urobilinogen, and glucose if strips are used.
- Hydrochloric Acid: Although used to preserve cell structures and determine steroid concentrations, it affects the sample's pH.
- Mercury Salts: May produce false-negative results for leukocyte esterase reaction.
- Boric Acid: While commonly used to preserve bacteria present in urine, this substance may reduce the sensitivity of the leukocyte reagent on dipsticks and alter initial pH values. Moreover, excessive concentrations may prevent bacterial growth in samples reserved for culture.[11][12][13]
Results, Reporting, and Critical Findings
Physical Examination
Only in a few instances, the color, odor, and/or appearance are of clinical significance; nonetheless, any abnormal finding should be noted.
Color
- Normal: Yellow (light/pale to dark/deep amber)
- Associations:
- Amber: Bile pigments
- Brown/Black (Tea-colored): Bile pigments, cascara, chloroquine, fava beans, homogentisic acid (alkaptonuria), levodopa, melanin or oxidized melanogen, methemoglobin, methyldopa, metronidazole, myoglobin, nitrofurantoin, primaquine, rhubarb, riboflavin, senna
- Dark Yellow: Concentrated specimen (dehydration, exercise)
- Green/Blue: Amitriptyline, asparagus, biliverdin, cimetidine, clorets (breath mint), indicans, indigo carmine, indomethacin, methocarbamol, methylene blue, promethazine, propofol, pseudomonal UTI, triamterene
- Orange: Bile pigments, carrots, coumadin, nitrofurantoin, phenothiazines, phenazopyridine, rifampin, vitamin C
- Pink/Red: Beets, blackberries, chlorpromazine, food dyes, hematuria, hemoglobinuria, menstrual contamination, myoglobinuria, phenolphthalein, porphyrins, rifampin, rhubarb, senna, thioridazine, uric acid crystals.[7][14] A urine sample that turns red on standing suggests the presence of porphobilinogen, which is increased in acute porphyrias.
Appearance
- Normal: Clear or translucent
- Associations: Bacteria, blood clots, contrast media, a diet high in purine-rich foods, fecal contamination or material (i.e., gastrointestinal-bladder fistula), lipids such as chyluria (chylomicrons in the urine), lymph fluid, mucus, precipitation of cells (red blood cells (RBC), white blood cells (WBC), squamous and non-squamous epithelial cells), casts or crystals (calcium phosphate, calcium oxalate, uric acid), pyuria, semen, small calculi, talcum powder, vaginal creams or secretions, yeast or non-specific/normal.[7][14]
Odor
- Not routinely reported
- Normal: "Urinoid"
- Associations:
- Cystine Decomposition: Sulfuric smell
- Dehydration/Prolonged Room Temperature: Strong smell
- Diabetes Mellitus: Honey
- Diabetic Ketoacidosis: Fruity/sweet
- Gastrointestinal-bladder Fistula: Fecal smell
- Maple-syrup Urine Disease: "Burnt sugar."
- Prolonged Bladder Retention: Ammoniacal
- Urinary Tract Infection: Pungent or fetid
- Medications and Diet: Onions, garlic, asparagus[7][14]
Specific Gravity (USG)/Osmolality (O)
The urinary specific gravity (USG) and osmolality are of special importance because they indicate the kidney's capacity to dilute or concentrate urine. USG is defined as the ratio between the density of urine and the density of an equal volume of pure distilled water. Normal values are lab-dependent since there are multiple methods to calculate this parameter (hydrometer, dipstick reagent pad, refractometer, and harmonic oscillation or urinometry). As it depends primarily on mass, it is not a truly reliable measure for quantifying the exact number of solute particles. Thus, USG is commonly used to rapidly estimate screen urine concentration, employing the term hyposthenuric and hypersthenuric depending on whether the USG is diminished, or elevated. Isosthenuria connotes urine with a fixed specific gravity and portends renal disease. Conversely, osmolality is a measure of the sum of all dissolved particles in urine. It is more reliable and accurate than USG for evaluating kidney function. Urine osmolality ranges from 50-1200 mOsmol/kg; the key is to always compare to serum osmolality to establish a pathological condition. Both parameters directly correlate; for example, a USG of 1.010 approximates to a urine osmolality of 300 mOsm/kg.[7][15]
- Normal: USG = 1.002-1.035 (usually 1.016 to 1.022). O = 50-1200 mOsm/kg (usually 275-900 mOsm/kg) [Both parameters are lab dependent]
- Variations according to the patient’s diet, health, hydration status, and physical activity.
- Associations:
- High Values: Contrast media, dehydration, decreased renal blood flow (shock, heart failure, renal artery stenosis), diarrhea, emesis, excessive sweating, glycosuria, hepatic failure, syndrome of inappropriate antidiuretic hormone (SIADH)
- Low Values: Acute tubular necrosis, acute adrenal insufficiency, aldosteronism, diuretic use, diabetes insipidus, excessive fluid intake (psychogenic polydipsia), impaired renal function, interstitial nephritis, hypercalcemia, hypokalaemia, pyelonephritis
- False Elevation: Dextran solutions, intravenous (IV) radiopaque contrast media, proteinuria
- False Depression: Alkaline urine[7][11][14][15]
Volume
- Normal: 0.5 to 1.5 cc/kg/hour or 600 and 2,000 mL daily in adults (typically 1,000– 1,600 mL/day)
- Associations:
- Anuria (less than 100 cc/day) and oliguria (less than 500 cc/day): Severe dehydration from vomiting, diarrhea, hemorrhage or excessive sweating; renal disease, renal obstruction, renal ischemia secondary to heart failure or hypotension
- Polyuria (greater than 2,500 - 3,000 cc/day): Alcohol or caffeine consumption, diabetes mellitus, diabetes insipidus, diuretics, increased water intake, saline or glucose intravenous therapy[7]
Foam
- Not routinely reported
- Normal: Appears upon agitation and dissipates readily on standing
- Associations: Proteinuria, bile pigments, retrograde ejaculation, medications (phenazopyridine, etc.), non-specific/unexplained[7][11][14]
Chemical Examination
pH
Urine pH is a vital piece of information and provides insight into tubular function. Normally, urine is slightly acidic because of metabolic activity. A urinary pH greater than 5.5 in the presence of systemic acidemia (serum pH less than 7.35) suggests renal dysfunction related to an inability to excrete hydrogen ions. On the contrary, the most common cause of alkaline urine is a stale urine sample due to the growth of bacteria and the breakdown of urea releasing ammonia. Determination of urinary pH is helpful for the diagnosis and management of urinary tract infections and crystals/calculi formation.[7][11][14]
- Normal: 4.5 to 8 (usually 5.5 to 6.5)
- Associations:
- High Values (alkaline): Stale/old urine specimens (most common), hyperventilation, presence of urease-producing bacteria, renal tubular acidosis, vegetarian diet, vomiting.
- Low Values (acid): Cranberry juice, dehydration, diabetes mellitus, diabetic ketoacidosis, diarrhea, emphysema, high protein diet, starvation, potassium depletion, medications (methionine, mandelic acid, etc.), and a possible predisposition to the formation of renal or bladder calculi.[7][11][14][15]
Proteins
Proteinuria is another critical finding. In normal conditions, the glomerular capillary wall is permeable to molecules of less than 20,000 Daltons. Most of the small fraction of filtered proteins are reabsorbed and metabolized by the proximal tubule cells. Thus, proteins are normally present in urine in trace amounts. From the total urinary proteins, approximately one-third of the total is albumin, another third is a protein secreted by the tubular cells called Tamm–Horsfall glycoprotein, and the rest is made up of plasma proteins such as globulins. Proteinuria can be classified into a transient or persistent, with the first one typically been a benign condition (i.e., orthostatic proteinuria due to prolonged standing). For the latter, persistent proteinuria can my categorized as a glomerular pattern, a tubular pattern, and an overflow pattern. The first occurs when proteins that are not normally filtered (i.e., albumin, transferrin) pass by a damaged glomerular capillary wall. Thus, this pattern may be seen with low serum albumin, secondary generalized edema, and high serum lipids as in nephrotic syndrome. Usually, protein excretion is greater than 3.0 g/day to 3.5 g/day. The tubular pattern results from the tubular cells' inability to reabsorb filtered proteins. Consequently, small serum proteins are typically seen in the microscopic examination, and proteinuria is not relatively high (approximately 1 g/day to 2 g/day). Finally, overflow proteinuria occurs when excessive concentrations of small proteins in plasma are filtered, and tubular cells reabsorption's capacity is surpassed, which occurs in conditions such as rhabdomyolysis (myoglobin) and multiple myeloma (Bence Jones light chains). This phenomenon harms tubular cells, and they may be seen on microscopic examination. Qualitative assessment of minimal amounts of proteinuria serves as a marker for glomerular injury and risk of progression of renal disease. Normal albumin excretion is less than or equal to 29 mg/g creatinine. It is best to express albuminuria per gram of creatinine. According to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines, albuminuria can be classified into three stages: A1 (less than 30 mg/g creatinine; normal to mildly increased), A2 (30 mg/g to 300 mg/g creatinine; moderately increased, formerly termed as "microalbuminuria"), and A3 (greater than 300 mg/g creatinine; severely increased).[7][14][16][17]
Normal: Proteinuria less than or equal to 150 mg/day (typically albuminuria less than 30 mg/day) or 10 mg/dL
- Associations:
- Albuminuria of 30 mg/day to 300 mg/day is an indicator of early renal disease, glomerular injury, and risk of progression of renal disease
- Other Associations: Multiple myeloma, congestive heart failure, Fanconi syndrome, Wilson disease, pyelonephritis, and physiological conditions (strenuous exercise, fever, hypothermia, emotional distress, orthostatic proteinuria, and dehydration)
- False-positive: Alkaline or concentrated urine, phenazopyridine, quaternary ammonia compounds
- False-negative: Acid or dilute urine, primary protein is not albumin[7][11][14][15]
Blood Cells
- Dipstick test for blood detects primarily the peroxidase activity of erythrocytes, but myoglobin and hemoglobin can also catalyze this reaction. Thus, a positive test result indicates hematuria, myoglobinuria, or hemoglobinuria.
- Normal: Negative (usually) or less than or equal to 5 RBCs per mL (lab-dependent value)
- Associations:
- Hematuria: Renal calculi, glomerulonephritis, pyelonephritis, tumors, trauma, anticoagulants, strenuous exercise, exposure to toxic chemicals
- Hemoglobinuria: Hemolytic anemias, RBC trauma, strenuous exercise, transfusion reactions, severe burns, infections (i.e., malaria)
- Myoglobinuria: Muscle trauma eg, rhabdomyolysis, prolonged coma, convulsions, drug abuse, extensive exertion, alcoholism/overdose, muscle wasting diseases
- False-positive: Dehydration, exercise, hemoglobinuria, menstrual blood, myoglobinuria
- False-negative: Captopril, elevated specific gravity, acid urine, proteinuria, vitamin C[7][11][14][15]
Glucose
Glycosuria occurs when the filtered load of glucose exceeds the tubular cells' ability to reabsorb it, which normally happens at a glucose serum concentration of around 180 mg per dL. Furthermore, nitrites are not normally found in urine, and it is highly specific for urinary tract infection. However, due to its low sensitivity, a negative result does not rule out infection.[14]
- Normal: Negative
- Associations: Diabetes mellitus, Cushing syndrome, Fanconi syndrome, glucose infusion, pregnancy.
- Glucosuria with normal plasma glucose without other features of Fanconi syndrome is due to a benign condition referred to as renal glycosuria and is due to a mutation in the sodium-glucose linked transporter 2
- False-positive: Ketones, levodopa
- False-negative: Elevated specific gravity, uric acid, vitamin C[7][11][14][15]
Bilirubin (conjugated)
- Normal: There is no bilirubin in normal urine
- Associations: Liver dysfunction, biliary obstruction, congenital hyperbilirubinemia, viral or drug-induced hepatitis, cirrhosis
- False-positive: medications such as phenazopyridine that have a similar color at the low pH of the reagent pad
- False-negative: stale/old urine specimens, chlorpromazine, selenium [7][11][14][15]
Urobilinogen
- The degradation product of bilirubin metabolism from bacteria in the intestine
- Normal: 0.1 mg/dL to 1 mg/dL in random samples or up to 4 mg/daily
- Associations:
- Elevation: Hemolysis, liver disease (cirrhosis, hepatitis), sickle cell disease, thalassemia
- Decrease: Antibiotic use, bile duct obstruction
- False-positive: Elevated nitrite levels, phenazopyridine, porphobilinogen, sulfonamides, and aminosalicylic acid
- False-negative: Prolonged exposition to daylight, formaldehyde, high levels of nitrites[7][11][14][15]
Ketone Bodies
- Products of body fat metabolism
- Normal: Negative
- Associations: Uncontrolled diabetes mellitus (diabetic ketoacidosis), pregnancy, carbohydrate-free diets, starvation, febrile illness.
- False-positive: Acid urine, elevated specific gravity, mesna, phenolphthalein, some drug metabolites (e.g., levodopa, captopril)
- False-negative: Stale/old urine specimens.
- Remember: Reagent strips do not detect beta-hydroxy-butyric acid, only acetoacetic acid and acetone[7][11][14][15]
Nitrites
- Products originating from the reduction of urinary nitrates
- Normal: Negative
- Associations: Urinary tract infection (UTI) from a nitrate reductase-positive bacteria (E. coli, Proteus, Enterobacter, Klebsiella, Streptococcus faecalis and Staphylococcus aureus)
- False-positive: Contamination, exposure of dipstick to air, pigmented materials, phenazopyridine
- False-negative: elevated specific gravity, elevated urobilinogen levels, nitrate reductase-negative bacteria, acid urine, vitamin C, urine with less than 4 hours of bladder resting, absent dietary nitrates
- Remember: A negative result does not rule out UTI[7][11][14][15]
Leukocyte Esterase
- An enzyme present in certain WBCs (except lymphocytes)
- Normal: Negative
- Associations: Inflammation of the urinary tract, sterile pyuria (balanitis, urethritis, tuberculosis, bladder tumors, nephrolithiasis, foreign bodies, exercise, glomerulonephritis, corticosteroids, and cyclophosphamide), fever, glomerulonephritis, pelvic inflammation
- False-positive: Contamination, highly pigmented urine, strong oxidizing agents, Trichomonas
- False-negative: Elevated specific gravity, glycosuria, ketonuria, proteinuria, some oxidizing drugs (cephalexin, nitrofurantoin, tetracycline, gentamicin), vitamin C[7][14][15]
Microscopic examination
Casts
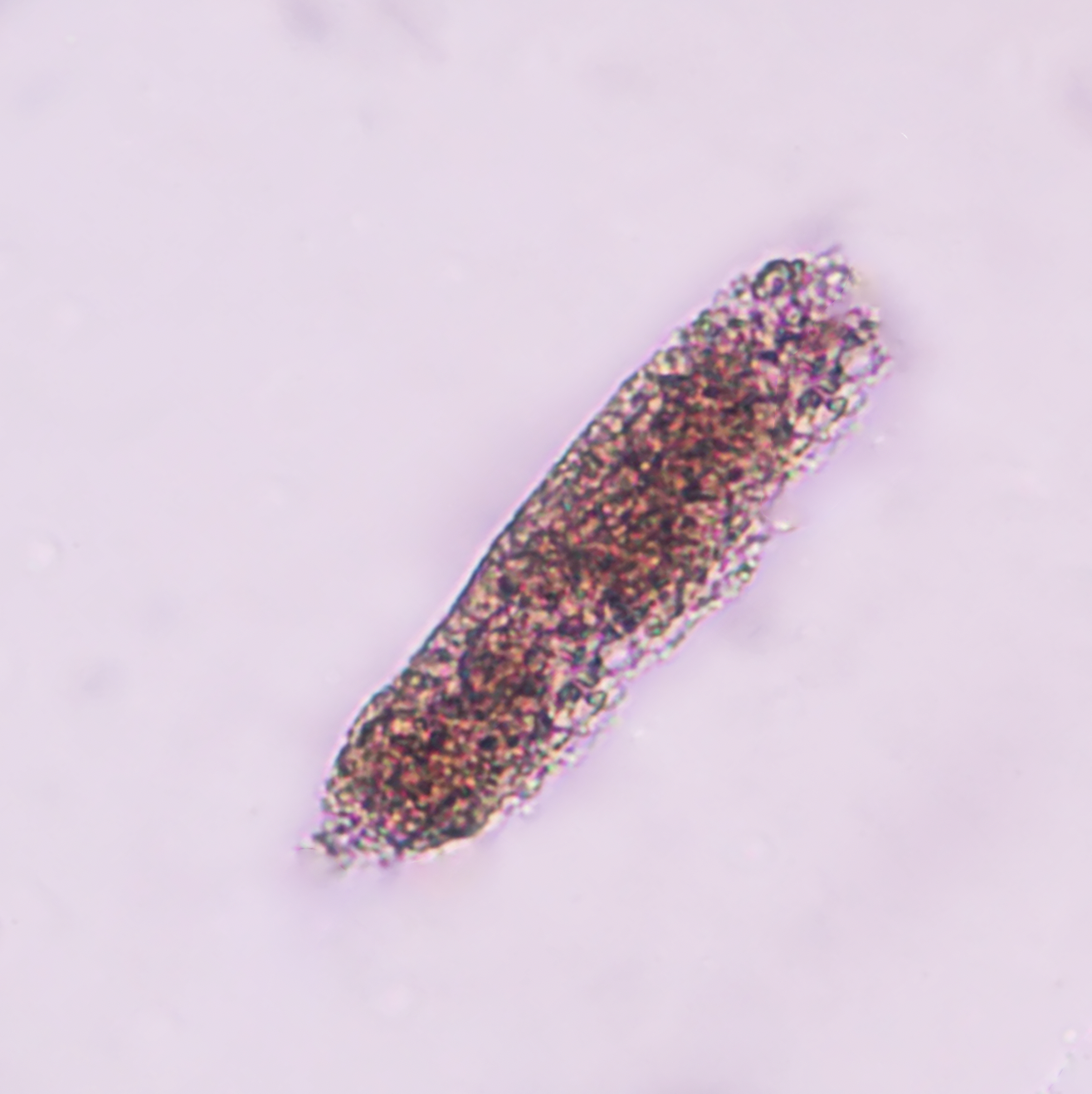
Casts are a coagulum composed of the trapped contents of tubule lumen and Tamm-Horsfall mucoprotein. They originate in the lumen the distal convoluted tubule or collecting duct with pH alterations or long periods of urinary concentration or stasis. The casts preserve the cylindrical shape of the tubule in which they were formed. Only a few hyaline or finely granular casts may be seen under normal physiological conditions. Cellular casts can dissolve within 30 to 10 minutes depending on the pH of the urine sample, thus promptly testing is mandatory for appropriate testing.
- Red Blood Cell Casts
- Normal: Absent
- Associations: Glomerulonephritis, vasculitis, intrinsic renal disease (tubulointerstitial nephritis, acute tubular injury/necrosis), strenuous exercise (see image attached)[7][14][15]
- White Blood Cell Casts
- Normal: Absent
- Associations: Pyelonephritis, interstitial nephritis, glomerulonephritis, renal inflammatory processes (see image attached)[7][14][15]
- Epithelial Cell Casts
- Normal: Absent
- Associations: Acute tubular injury/necrosis, interstitial nephritis, glomerulonephritis, eclampsia, nephritic syndrome, transplant rejection, heavy metal ingestion, renal disease[7][14][15]
- Granular Casts
- Normal: Absent
- Associations: Glomerular or tubular disease, pyelonephritis, advanced renal disease, viral infections, stress/exercise, non-specific[7][14][15]
- Waxy (broad) Casts
- Normal: Absent
- Associations: Advanced renal failure (dilated tubules with decreased flow)[7][14][15]
- Hyaline Casts
- Normal: Up to 5 casts/low-power field
- Associations: Normal finding in concentrated urine, fever, exercise, diuretics, pyelonephritis, chronic renal disease[7][14][15]
- Fatty Casts
- Normal: Absent
- Associations: Heavy proteinuria (nephrotic syndrome), renal disease, hypothyroidism, acute tubular necrosis, diabetes mellitus, severe crush injuries[7][14][15]
Cells
- Red Blood Cell
- Normal: 0-5 cells/high-power field
- Associations: UTI, inflammation[7][14][15]
- White Blood Cell
- Normal: 0-5 cells/high-power field
- Associations: UTI, inflammation[7][14][15]
- Eosinophil
- Normal: Absent
- Associations: Interstitial nephritis, acute tubular necrosis, UTI, kidney transplant rejection, hepatorenal syndrome[7][14][15]
- Epithelial cell
- Squamous, transitional, or renal tubular cells
- Type of cell encountered depends on the location of the disease process
- Normal: Less than or equal to 15-20 squamous epithelial cells/high-power field
- Associations:
- Squamous (most common): Contamination
- Transitional: Normal, UTI
- Renal Tubular: Heavy metal poisoning, drug-induced toxicity, viral infections, pyelonephritis, malignancy, acute tubular necrosis[7][14][15]
- Bacteria, Fungi, or Parasites
- Normal: Absent
- Associations: UTI, contamination[7][14][15]
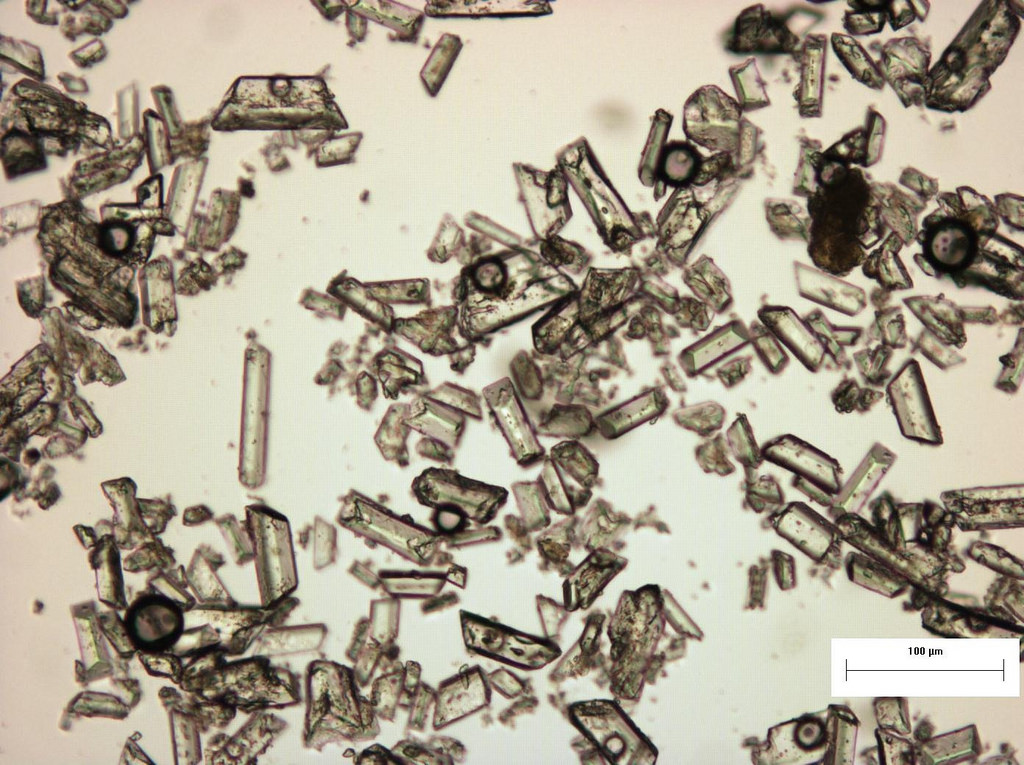
Crystals
End products of metabolism are found highly concentrated in the urine and can precipitate in the form of crystals. The presence of crystals is not necessarily associated with pathological states, although several types of crystals are associated with certain diseases. For example, cholesterol crystals are seen in polycystic renal disease and nephrotic syndrome and polycystic renal disease; leucine and tyrosine crystals are associated with severe liver disease.[7][14]
- Uric Acid
- Yellow to orange-brown, diamond- or barrel-shaped crystals
- Normal: Absent
- Associations: Acid urine, hyperuricosuria, uric acid nephropathy, normal (see image attached)[7][14]
- Calcium Oxalate
- Most commonly encountered crystal in human urine
- Refractile square "envelope" shape
- Normal: Absent
- Associations: Ethylene glycol poisoning, acid urine, hyperoxaluria, normal (see image attached)[7][14]
- Amorphous Phosphate (Calcium and magnesium Phosphate)
- Normal: Absent
- Associations: Alkaline urine, decreased urine volume, a diet rich in calcium, prolonged immobilization, overactive parathyroid glands, bone metastases, normal[7][14]
- Triple Phosphate (Struvite)
- "Coffin lid" appearance crystals
- Normal: Absent
- Associations: Alkaline urine, decreased urine volume, UTI from urease-producing bacteria (Proteus, Klebsiella)[7][14]
- Cysteine
- Colorless crystals with a hexagonal shape
- Normal: Absent
- Associations: Cystinuria[7][14]
- Sulfur
- Normal: Absent
- Associations: Antibiotics containing sulfa[7][14]
Clinical Significance
Urinalysis is an ancient diagnostic screening test that has stood the test of time and is still useful in clinical laboratories since it plays a critical role in the health assessment process.[11][13] For some, a urinalysis is considered as the most common, simple, and relevant screening exam that provides clinicians with valuable information about the general health status of a patient, including hydration, urinary tract infection, diabetes mellitus, and liver or renal disease.[8]
Quality Control and Lab Safety
Multiple reagent strips and tablets are used for several semiquantitative and qualitative tests, which allow the analysis of diverse parameters such as glucose, albumin, specific gravity, hydrogen ions, electrolytes, leukocytes, leukocyte esterase, nitrite, ketones, blood, bilirubin, urobilinogen, and heme. Most reagent strips are narrow bands of plastic 4 mm to 6 mm wide and 11 cm to 12 cm long with a series of absorbent pads. Each pad contains reagents for different reactions, so various tests can be carried out simultaneously. The reagent strip method comprises multiple complex chemical reactions. A color change on the pad demonstrates a reaction which can be compared to a color chart provided by the manufacturer for result interpretation. When using this method, it is essential to test the urine promptly, understand the advantages and limitations of each test, and establish controls.[7]
Reagent strips are designed to react progressively, modifying color for positive reactions along the strip at specific periods. The fundamental principle corresponds to read the strip at the specified time from the manufacturer to obtain accurate results. These times are established on the label of the bottle containing the particular strip. Furthermore, the reagent trips should never be stored in alternative containers because they have a relatively short shelf life. Expired strips may produce inaccurate results, the expiration date is also located on the bottle.[11]
Additionally, some computerized urine analyzers are available for reading reagent strips. They show the analysis on a small screen and print them out to include in the patient’s records. These analyzers comprise greater accuracy, convenience, simplicity, and time savings. However, they may not be able at many facilities due to financial limits.[11]
Enhancing Healthcare Team Outcomes
A urinalysis is a valuable test commonly used in clinical practice. Depending on the technique and hospital, most samples are usually collected by nurses or phlebotomists who must determine whether or not the specimen meets the minimum requirements for proper analysis. They should be familiar with each method of collection and educate patients for appropriate sampling in the outpatient setting. In addition, as many conditions may alter the sample analysis (food, drugs, exercise, intercourse, room temperature, daylight, etc.), adequate recording and communication between the interprofessional team ensure discarding possible false-positive or false-negative results. Accurate collections provide key information for screening multiple systemic diseases and monitor treatment progress.
Finally, careful consideration of current guidelines must be done at all times. For example, the American Academy of Pediatrics no longer recommends performing routine screening urinalysis for asymptomatic children and adolescents.[18] However, in adults, it is an excellent cost-effective screening test in primary care to screen for certain diseases.