Continuing Education Activity
Heavy metals are elements found in nature that accumulate in the environment mostly due to anthropogenic activities. Humans are exposed to them by occupational exposure or consuming foods that contain these elements. These substances can cause toxic effects that affect health and well-being. This activity outlines the evaluation and management of heavy metal toxicity and highlights the role of the interprofessional team in improving care for this condition in individual patients and communities at large.
Objectives:
Identify the etiologies of heavy metal toxicity.
Describe the clinical features that are useful in the evaluation of heavy metal toxicity.
Outline the management options available for heavy metal toxicity.
Summarize the importance of collaboration and communication amongst the interprofessional team to improve outcomes for individuals and communities affected by heavy metal toxicity.
Introduction
Heavy metals(HMs) are defined differently by different people. In health parlance, they are naturally occurring substances that accumulate and cause damage to the environment and living beings, including humans. They also include substances known as semimetals or metalloids that can have the same deleterious effects.
Humans are exposed to HMs through inhalation, ingestion, or contact with the skin. Environmental pollution with HMs can result in contamination of air, water, sewage, seawater, waterways, and can accumulate in plants, crops, seafood, and meat and indirectly affect humans. Some occupations have increased risk for particular HMs exposure and toxicity.
Some HMs can cause toxicity even at very low concentrations and are known as non-threshold HMs. Factors influencing the risk of toxicity include age, body weight, genetics, route of acquisition, duration of exposure, amount, health, nutritional status, and a combination of HMs. Some preparations used in complementary medicine can result in toxicity.[1]
Symptoms and signs of heavy metal(HM) toxicity vary with the substance and can be due to acute exposure to large amounts, or chronic exposure to repeated small quantities which can result in cumulative toxicity. Many body systems can be affected. Toxic exposure to two or more HMs can lead to more damage than a single HM.
Investigations include tests of urine, blood, skin, nail, and hair. Management includes preventing any further exposure, removal of the offending agent using chelating agents, supportive therapy, and patient education.
Prevention or minimizing exposure is a must, and laws exist to this effect. Public health measures include monitoring air, water, foods, and at-risk individuals, and environmental manipulation of soil, water, and sewage.
Etiology
HMs are dense, naturally occurring elements that cause health hazards by accumulation in the environment and living beings.
Some elements like Arsenic(As)share properties of both metals and non-metals, are known as semimetals or metalloids and listed as HM due to the toxic effects similar to metals.
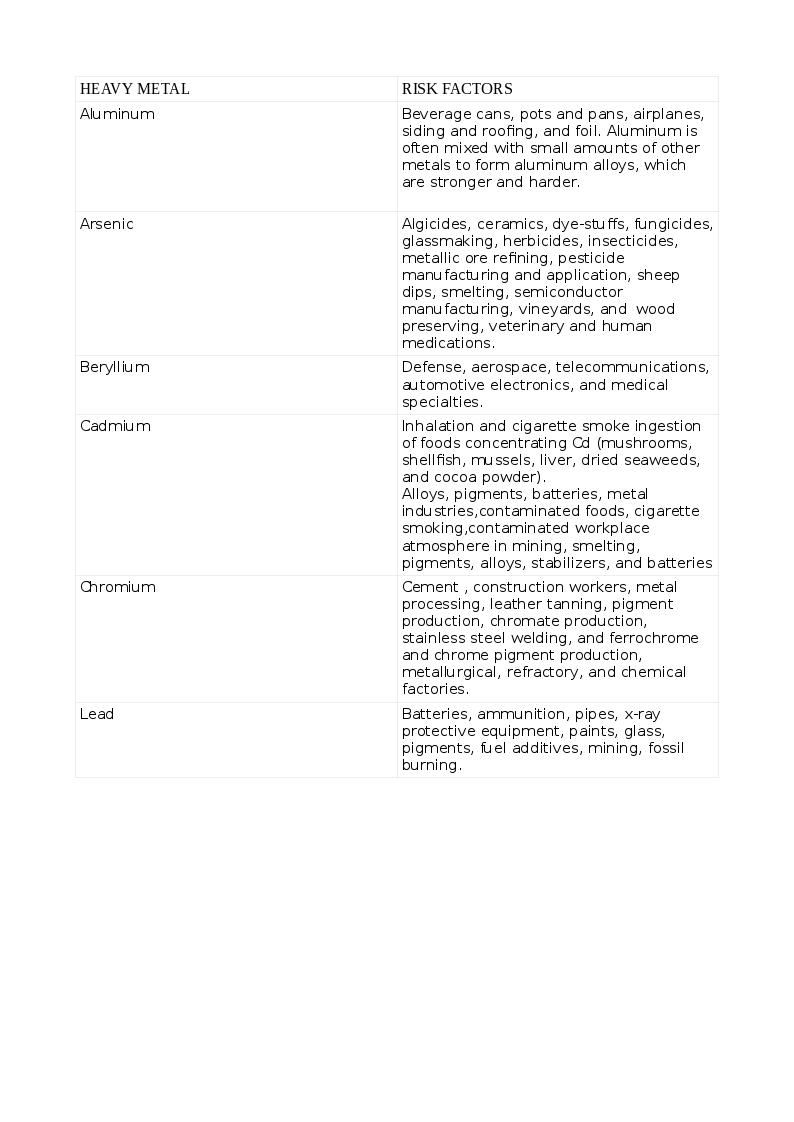
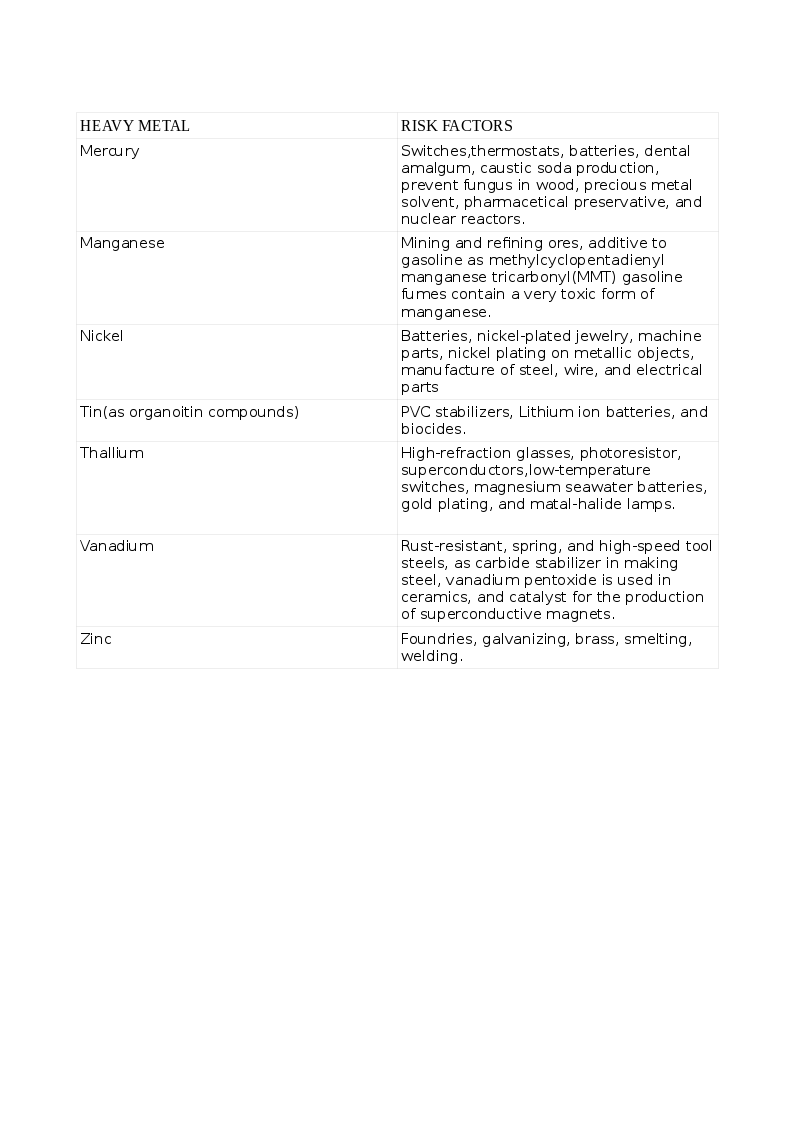
The common list of non-essential HM that cause toxicity includes Arsenic (As), Cadmium (Cd), Lead (Pb), Mercury (Hg), and those that are essential to humans in trace quantities for various cellular activities like Cobalt (Co), Copper (Cu), chromium (Cr), Iron (Fe), Manganese (Mn), Molybdenum (Mo), Nickel (Ni), Selenium (Se) and Zinc (Zn). Exposure to HMs can be natural or occupational. The various HMs and the risks for their toxicity appear in the tables. See Tables. Risk Factors for Heavy Metal Toxicity, 1 and 1A.
Toxicity can result from inhalation, ingestion, and skin contamination. Humans can acquire hMs as a result of activities harnessing nature like mining, fossil-burning, industrial, agriculture, consuming plants, seafood, and meat that have accumulated these HMs from contaminated soil, waterways, and seawater. Toxicity with multiple HMs can be more pronounced and cause tremendous morbidity. Some indigenous medicinal preparations contain toxic amounts of HMs. Children are more susceptible than adults. While acute toxicity results from short exposure to large doses, longterm exposure of smaller amounts results in chronic toxicity.
Industrial waste containing Hg is released into the waters. Aquatic microbes convert inorganic Hg (iHg) to organic Hg(CH3Hg). Fish consume this, and bigger fish consume the smaller fish. Humans consume the bigger fish and thus complete the food cycle and suffer HM toxicity.
When refurbishing old buildings, care is necessary when removing flaky loose paint. Sanding facilitates Pb and other toxins in the paint to be spread into the dust and atmosphere, so alternatives like suctioning or vacuuming should be used.
Epidemiology
HM toxicity is worldwide. However, the incidence and magnitude of the toxicities of individual HMs vary with the geographical location, natural soil content, habits, customs, location of industries, regulatory measures to contain pollution, healthcare facilities to detect HM toxicity, and individual factors like nutritional status, and genetics.
When an HM is released into the air, water, or soil, it can be absorbed by plants, crops, consumed by cattle and fish, and finally, end up in humans to complete the food chain.
Industrial and workplace exposure can result in HM toxicity by inhalation, ingestion, or skin contact.
The World Health Organization(WHO) has listed ten major pollutants, four of which are HMs.
Groundwater contaminated with HMs can be consumed by humans and result in chronic HM toxicity. An example is a high level of arsenic in the groundwater in Bengal, India, and neighboring Bangladesh, where the concentration of arsenic in the water is far above permissible limits.[2]
Time and again, there have been reports of HM toxicity occurring in epidemic proportions; this has been the result of a rather careless release of toxic industrial effluents into the air, soil, sea, and waterways.
Hunter-Russell syndrome occurred in a seed-packing factory in England due to the inhalation of methyl mercury, which was sprayed as an insecticide.
Industrial release of methyl mercury into rivers and seawater resulted in poisoning and deaths in Japan, infamously known as Minamata disease.
Another Japanese example is the accumulation of Cd in bones with resultant pains and fractures, the so-called "Itai-Itai (it hurts-it hurts) disease."[3]
An epidemic of mercury poisoning occurred in Iraq, where grains sprayed with insecticide were consumed.
A well-known case is that of groundwater contamination with chromium VI in California by a Gas Company that released its waste into the region's groundwater.
Epidemiological studies have shown the association of HM exposure and chronic disease like diabetes, kidney disease, degenerative neurological conditions, skin ailments, respiratory diseases, cardiovascular disease as well as cancer.[4][5][6]
Pathophysiology
Heavy metals generate metal-specific free radicals or reactive oxygen species(ROS) that cause oxidative stress to cells.[7]
These result in:
- DNA damage, impaired DNA repair, cell apoptosis, or carcinogenesis.
- Peroxidation of cell membrane lipids and cell damage
- Inactivation of the enzyme proteins.
- Prevention of protein folding.
- Protein aggregation.
- Conformational changes that affect their structure and function and cause cell damage.
Examples of free radicals include:
- Hydroxyl radical. (OH.)
- Superoxide. (.O2-)
- Hydrogen peroxide. (H2O2)
- Peroxyl radical. (ROO.)
- Nitric oxide. (NO)
NO can bind to ROS to form reactive nitrogen species, which are again harmful to cells.[8]
An HM, when ingested, is acted upon by gastric acid and undergoes oxidation. The oxidized HM has a high affinity for enzymes and other proteins to form strong and stable bonds. Commonly thio groups(SH) are attracted to HM binding. In this way, enzymes are inhibited, and products downstream are not produced, and biological functions impaired. An example is the glutathione reductase enzyme affected by arsenic restricting the removal of ROS. Inorganic As is toxic and is biotransformed to the end-metabolites monomethyl arsonic acid (MMA) and dimethyl arsinic acid (DMA), which are excreted in the urine and are a measure of chronic As toxicity. An intermediate, Monomethylarsonic acid (MMA III), accumulates in tissues and is a carcinogen.[9]
Heavy metals inhibit protein folding, cause misfolding, and aggregation, all of which damages cells and disrupts function.
Pb and CH3Hg are lipophilic and easily cross cell membranes, especially of the tissues high in lipids like the central nervous system(CNS). Here they are sequestered and remain for an indefinite period and difficult to remove by ordinary means and produce long-lasting neurotoxicity. Pb replaces Fe, Ca, Mg, and interferes with various cellular functions, including neural transmission.[10]
Pb acts as a non-competitive inhibitor of the N-methyl-d-aspartate receptor (NMDAR), especially in the hippocampus, and interferes with learning and memory. It inhibits several enzymatic steps in hemoglobin synthesis and causes anemia.
A metalloprotein enzyme(zinc-finger metalloprotein) is affected by an HM like Cd, which displaces the trace essential element Zn from the enzyme and inactivates it.
Cd and Pb replace Ca in bones and results in osteoporosis and fractures. Cd causes a Fanconi like syndrome in the proximal S1 segment of the renal tubule and results in losses of phosphate, calcium, amino acids, bicarbonate, and results in hypophosphatemia, low bicarbonate, and proximal tubular acidosis; increased mobilization of calcium from the bone which coupled with increased renal excretion stimulates the parathyroid hormone secretion, causing further bone loss. There is inhibition of the 1-alfa hydroxylase enzyme in the kidney by Cd and therefore decreased active vitamin D3 production and less intestinal calcium absorption to compound the problem. Cd inhibits the osteoblastic conversion of bone tissue stem cells and stimulates osteoclasts, acting at the level of the bone in enhancing bone loss.[11]
Mn accumulates in the mitochondria of neurons and glial cells disrupting ATP synthesis and increasing ROS and cell damage.
Hg in the inorganic form can inhibit catechol o-methyl transferase(COMT), the enzyme catalyzing the degradation of catecholamines, by inactivating its cofactor S-adenosyl methionine resulting in unexplained hypertension. Omega-3 fatty acids and Se retard Hg toxicity.[12] Genetic polymorphisms in the gene for COMT may explain the differences in the neurobehavioral effects of Hg toxicity.[13]
History and Physical
Heavy metal toxicity is in general underdiagnosed, and a high index of suspicion is needed.
A history of occupational exposure is vital.
When taking a medication history, attention to alternative medicine intake is easily overlooked. Many medications in Chinese, Ayurveda, homeopathy, and Siddha systems contain heavy metals in significant quantities that, when taken over time, may cause toxicity.
Living in older homes is a risk factor for lead toxicity by way of water pipes and flaking paint and dust.
Living in a neighborhood that has HM contamination(in industrial belts)is a definite risk factor. A parent who suffers exposure at work may contaminate his family when he gets home.
Heavy metal exposure during the antenatal period may yield clues to problems in newborn and infancy.
Young children are more vulnerable to HM toxicity. A previously active and healthy child may present with poor play, distractibility, falling grades at school, abdominal pain, anemia, and delay or retarded development.
Accidental consumption is more common, but in some cases, the cause is suicidal and will prompt urgent mental health consultation.
Exposure to more than one HM will cause profound symptoms than one HM alone.
Acute exposure is usually dramatic in presentation. Inhalation of HMs causes respiratory symptoms, topical contamination, skin lesions, and ingestion mimics acute gastroenteritis or dysentery.
Chronic exposure is more difficult to detect and needs a high index of suspicion. Depending on the organ system involved, the symptoms will vary. Typical findings may be present only in selected cases, while the majority may be non-specific.
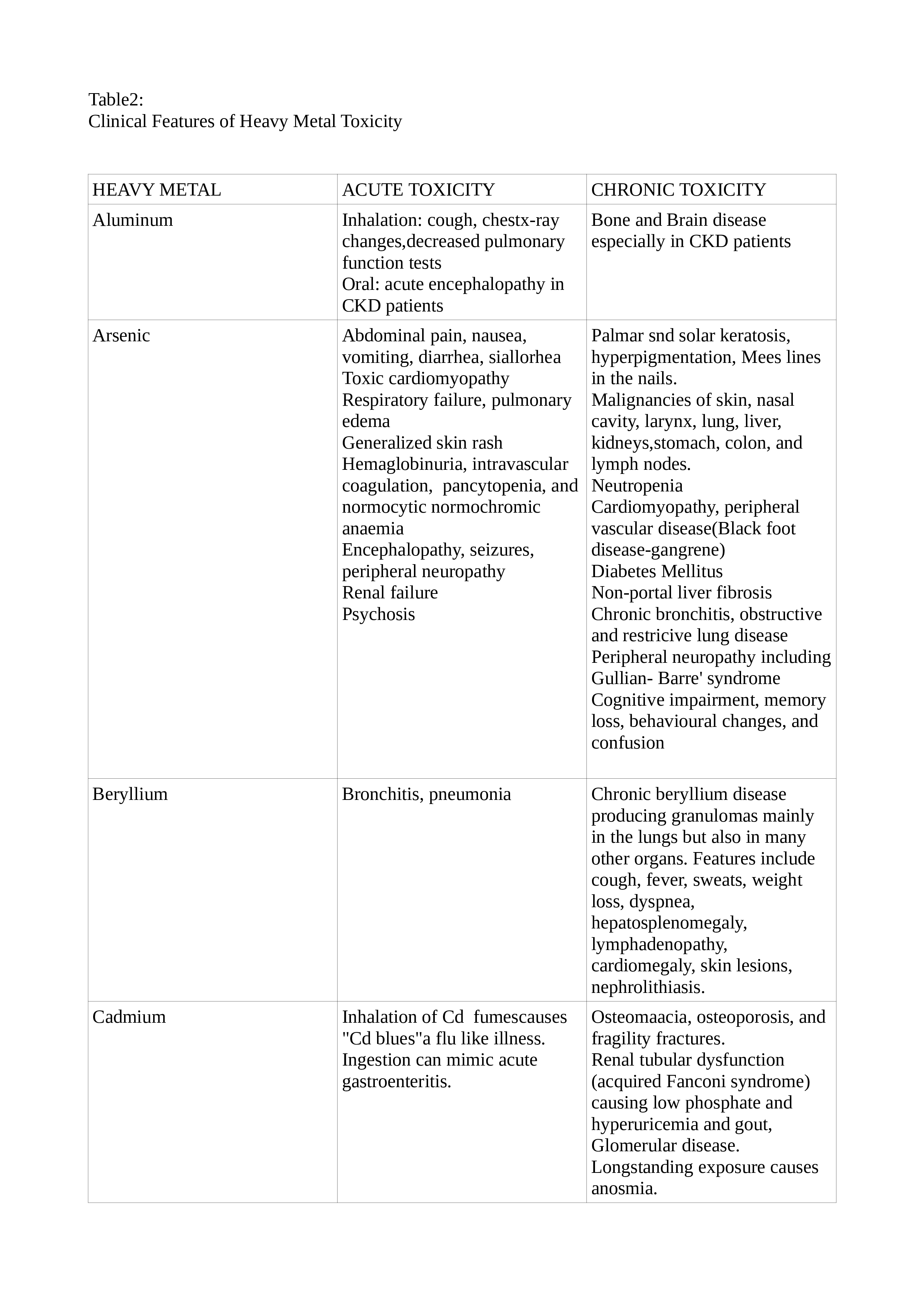
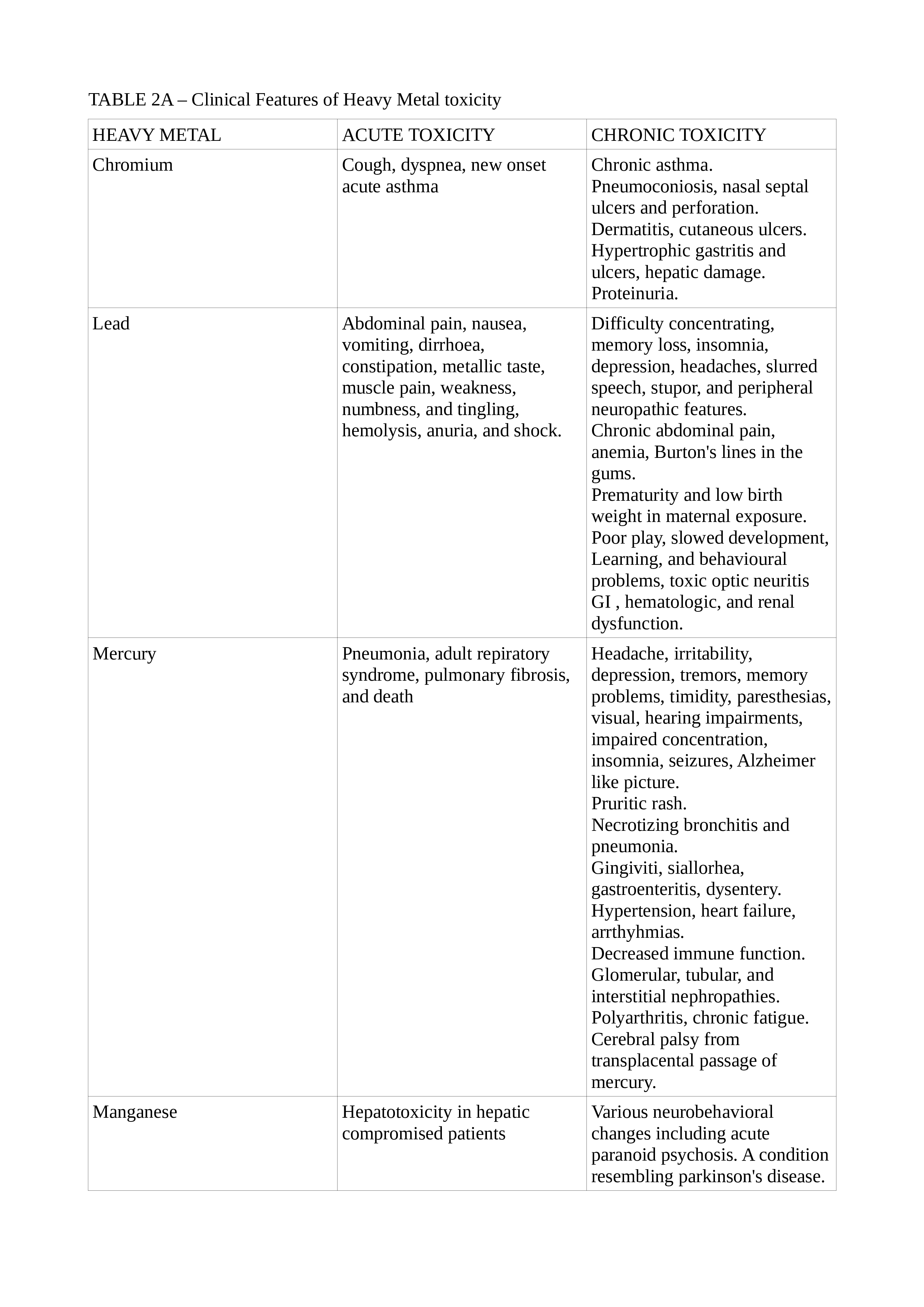
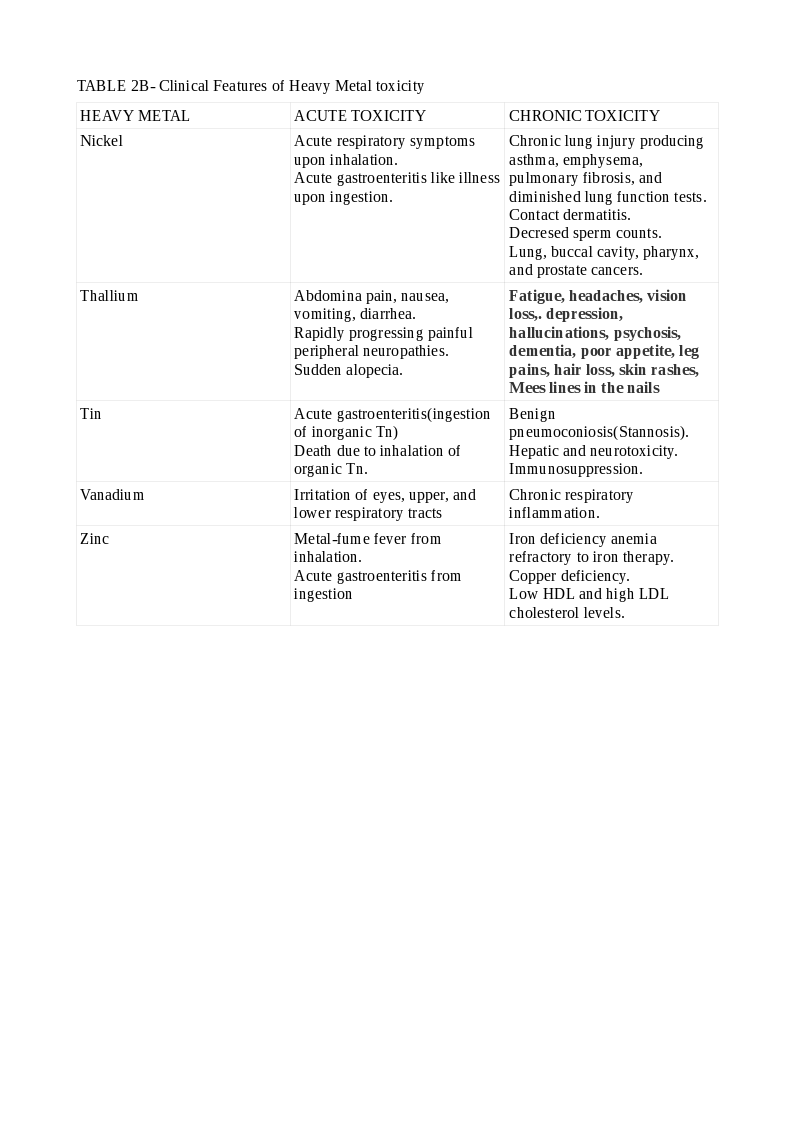
Lead toxicity may cause a blue line in the gingiva, mercury toxicity may present with Mees lines in the nails, while chromium (Cr) toxicity may present as cutaneous ulcers that appear punched out with thickened raised edges involving the extensor surface of the forearms and web spaces of the hands. See Tables. Clinical Features of Heavy Metal Toxicity, 2, 2A, and 2B.[11][14][15][16][17][18][19][20]
Evaluation
The examination of blood, urine, hair, nail, and tissues may be utilized in confirming HM toxicity.
The patient should refrain from seafood for at least 48 hours before the tests. If gadolinium or iodine-contrast was used, the waiting time increases to 96 hours.
In industrial workers, blood and urine testing for heavy metal exposure are necessary to monitor safe permissible limits and specimens should be obtained at the end of the workweek; for example, if Friday is the last day of the workweek, the test specimens should be obtained at the end of that day to affect actual levels of exposure.
Special metal-free tubes for blood or acid-washed containers for urine will maximize accuracy.
The level of HMs in the blood or urine will reflect current exposure and will not accurately reflect body stores from chronic exposure. Symptoms may not correlate with blood levels, and profound symptoms may be apparent in individuals with HM levels only slightly above normal. Sometimes a chelation challenge is necessary to strengthen the diagnosis and is the most accurate method of establishing the diagnosis of HM toxicity.
Ancillary tests like hemogram to detect anemia, liver, and renal function tests are often helpful. Hypophosphatemia, hyperphosphaturia, hypercalciuria, proteinuria with low molecular weight proteins like beta-2 microglobulin, elevated alkaline phosphatase, and bone markers, decreased serum 1,25 dihydroxy vitamin D3 and increased intact PTH are all useful in documenting Cd-induced bone disease.[3]
An abdominal X-ray may show a radioopaque foreign body that contains heavy metal. A chest X-ray may show changes due to inhalation exposure. These changes may be amplified in CT scans of the chest and will help differentiate sarcoidosis, infective, and malignant diseases causing similar clinical features.
A beryllium lymphocyte proliferation test (BeLPT) has been described. It can identify prior sensitization to the element. About one in ten of sensitized subjects will develop chronic beryllium disease.[21]
A Chronic Arsenic Intoxication Diagnostic Score(CAsIDS) has been developed. This evaluation takes into account the bone As load and several clinical features in quantifying the risk for chronic As toxicity.[22]
The detection of HM pollution of water-bodies has been revolutionized by real-time biosensors that are quick, accurate, and less labor-intensive and can aid in controlling community HM toxicity.[23]
Toxicogenomic technologies show promise in decoding many of the links between HMs and carcinogenicity.[24]
Treatment / Management
The patient is removed from toxic exposure. The HM may be hastened out of the body by gastric lavage, activated charcoal, and skin decontamination. Supportive care can be in the form of intravenous fluids, oxygen, ventilatory, and circulatory support as needed. In severe cases, hemodialysis, plasma exchanges, and extracorporeal membrane oxygenation (ECMO) may be necessary.
Specific therapy to remove the HM is by the administration of chelating agents, which are metal-binding ligands specific for each metal forming a ring-structure called a chelate. It is more effective when used in combination with antioxidants.[25]
The properties of an ideal chelating agent are:
- High solubility
- High cell permeability
- Ability to bind most toxic HMs and form non-toxic compounds
- Highly effective by oral and parenteral routes
- A high rate of elimination
However, no such ideal chelator exists, and the search is on. Due to several drawbacks of chelating agents, research has focussed on plant products to serve such functions. These naturally occurring phytochelatins may offer a cheaper and safer alternative, especially in economically downtrodden nations where the problem is manifold.[26]
In treating Pb toxicity, DMSA (meso-2,3-dimercaptosuccinic acid) was superior to dimercaprol, also called British anti-Lewisite (BAL) plus calcium ethylene diamine tetraacetic acid (CaEDTA). CaEDTA resulted in increased Pb accumulation in various organs, including the brain.
BAL was ineffective, but a combination of deferasirox and deferiprone was extremely effective in Cd toxicity.[11]
In As toxicity, the benefits of chelation exceed the side-effects and prevents acute renal failure. 2,3-di-sulfanyl-1-propane sulfonic acid (DMPS) or DMSA are the therapeutic choices. They are more soluble in water than the BAL, and oral administration is effective.
Sodium diethyldithiocarbamate is useful and effective in Ni toxicity and is superior to D-penicillamine and dimercaprol (BAL).
DMSA is very effective in Hg toxicity. BAL is contraindicated in this instance because it increases the levels of both iHg and CH3Hg in the brain. Selenium and vitamin E can aid in the treatment of Hg toxicity.[27]
Extracellular Zn chelation is by CaEDTA. Since this does not cross the blood-brain barrier, intracellular chelators like 1-hydroxy pyridine-2-thione (pyrithione), with a lower affinity for zinc and N, N, N’, N’-tetrakis-(2-pyridyl methyl) ethylene diamine penta-ethylene (TPEN), having a high affinity for zinc have been developed.
Differential Diagnosis
The nervous system involvement may masquerade as dementia, depression, degenerative disease, or peripheral nerve lesions.
- Renal affliction may resemble tubular, interstitial, or glomerular pathology.
- More general symptoms of fatigue, poor appetite, loss of weight, and anemia may lead to confusion with chronic infections or malignancy.
The physical examination findings are not always classical but require diligent investigation:
- Cr VI induced cutaneous ulcers may be misdiagnosed as chronic arterial or infective ulcers.[28]
- CrVI and/or Cd along with Ni can cause nasal septal perforation and confused with a host of other causes like infective, traumatic, and connective tissue problems.[29]
- Acute arsenic toxicity may resemble ciguatera poisoning.[30]
- Chronic mercury toxicity may be confused with pheochromocytoma.[31]
- Lead toxicity may be confused with porphyria.[32] It may also be confused with ADHD in children.
Prognosis
The emphasis should be on prevention. In certain circumstances(industrial exposures, for instance), total prevention is impossible, and minimization of contamination should be the goal. Workers at risk for exposure to HMs should take adequate precautions. Management should provide them without any hindrance. Health authorities should supervise the smooth conduct of these measures, and authorities should enforce the law strictly. With minimal exposure and early detection, the prognosis is good. Delayed diagnosis and serious toxicity translate to a bad prognosis. Care of the ill in specialized centers with facilities for testing and treatment may have an edge in managing difficult and serious cases.
The prognosis of HM toxicity at the community level depends on many factors and include:
1) Proper discharge of effluents from factories.
2) Periodic inspection of soil and groundwater and measures to remove excess HM like:
- Excavation and removal.
- In-situ fixation including amending the soil
- Phytoremediation(growing certain plants to contain HMs).[33][34]
- Soil treatment using asymmetrical alternating current electrochemistry.[35]
- Nanomediation as a method to remove toxic HMs from the soil and water.[36][37]
- A novel method of HM removal from wastewater has been developed that is rapid, highly effective, and feasible. It involves a metal-organic framework/polydopamine composite and may offer solutions to vast areas of polluted waterways, including seawater.[38]
3) Regular testing of seawater to prevent contamination of fishing areas with HMs, and inspection of seafood.
There are three official agencies involved in the regulation of HMs:
- The Food and Drug Administration(FDA) sets the HM limits in foods.
- The Centers for Disease Control(CDC) oversees Pb testing in at-risk children.
- The Environmental Protection Agency(EPA):
- Evaluates the effects of HM exposures
- Regulates industrial emissions
- Establishes maximum HM contaminant levels
Complications
The chelating agents used as a treatment in HM toxicity are not devoid of side-effects.
Some metal ions are redistributed to other tissues like the brain and thereby increase neurotoxicity.
Others chelate essential trace elements and produce a deficiency state while yet others can cause hepatotoxicity.
Consultations
Diagnosis and management of heavy metal toxicity requires strong interdisciplinary collaboration and consultations from an array of specialists depending on the toxicity and presentation
- Primary care (family medicine, internal medicine, pediatrics)
- Emergency medicine (EM): adult, pediatric
- Clinical toxicology
- Laboratory medicine
- Community medicine
- Occupational health
- Clinical psychology/psychiatry/child and adolescent psychiatry
- Clinical pharmacy
- Nursing
- Other subspecialties (nephrology, neurology, gastroenterology, dermatology, endocrinology, and critical care)
- Environmental engineering
Deterrence and Patient Education
Patients and their families should be wary of HM content in many substances like common foods, health foods, and complementary medicine preparations. Particular care is warranted with the care of young children, especially when the family is occupying an older home.
Industrial workers and others at risk should receive proper timely and periodic screening for toxicity from HMs and cooperate with the health authorities. They should take appropriate precautions to prevent or minimize exposure. Health education, in this regard, should be reinforced from time to time.
Industries and factories should understand that they have a social responsibility for proper disposal of wastes and cooperate with the health and other civic authorities.
Pearls and Other Issues
- Heavy metal (HM) toxicity is underestimated in the community.
- The teaching of HM toxicity is underrepresented in the medical curriculum.
- Heavy metals can cause harm to the environment and living beings, including humans, by direct and indirect means.
- Young children are affected more than adults.
- Affected pregnant women can pass on the toxic HMs to the developing fetus and produce much harm.
- The toxicity of HMs can be acute and chronic.
- Although classical symptoms and signs for each HM exist, they are not present in every case, and a high index of suspicion is necessary for a correct diagnosis.
- Heavy metals can affect the nervous, pulmonary, cardiovascular, renal, skin, reproductive, and skeletal systems in varying degrees.[39]
- A good history, physical examination, and appropriate laboratory testing of blood, urine, and other tissues can aid with the diagnosis.
- The levels of heavy metals in the blood and urine reflect current exposure and not the true tissue levels; a chelation challenge may be needed.
- Heavy metals cause harm to proteins like enzymes, DNA, membrane lipids, and disrupt normal cellular functions.
- Many body-systems can be involved and can mimic other diseases that affect those systems.
- If detected and treated early, the prognosis is good.
- Apart from the removal of the offending HMs and the patient from the toxic environment, supportive care, and chelation therapy are the mainstays.
- Occupational and health regulations require strict enforcement and environmental engineering measures taken to reduce HM toxicity at the community level.
Enhancing Healthcare Team Outcomes
In individual patients presenting with HM toxicity, the primary care physician (PCP) or the EM physician is usually the first contact health provider.
The PCP/EMP should have a high index of suspicion of HM as the etiology, especially if the clinical picture does not improve with treatment for usual conditions.
PCP often needs consultation from the clinical toxicologist who is an expert in these conditions and will advise on the tests and treatments.
The laboratory personnel should be provided with a good history so that they will select the specific containers to collect blood, urine, and other tissue specimens to enhance the accuracy of the tests. They will also guide the clinician in the proper preparation of the patient before the tests to avoid confounders from misleading the diagnosis. [Level 5]
In selected cases, care from other specialties/subspecialties may be needed to treat specific organ dysfunction, as discussed under consultations.
The clinical pharmacist plays a vital role in guiding the dosing and monitoring of chelation therapy.
One should not forget the role played by skilled nurses in the care of patients affected by HM toxicity from primary care in the ambulatory clinic through tertiary-care for complicated cases and in the occupational health clinics.
Finally, the roles of the occupational health consultant, community medicine physician, and environmental engineer are vital to control HM toxicity in the soil, water, industrial segment, and provide for a healthier society.
Proper coordination among all the above providers is essential to reduce the burden and prevent morbidity and mortality from HM toxicity.